A few precepts
- •
To be of value, a combination must be more effective, show reduced toxicity, or cost significantly less than each component.
- •
Statistical significance should support biological significance, i.e. a 10% reduction of morbidity/mortality, even if significant statistically, may not be worth routinely raising the cost of treatment form $100/day to thousands of dollars/day. Alternatively, a marked reduction of mortality/morbidity (e.g. 50%) may be well worth additive costs.
- •
A superior combination might not take hundreds of patients to demonstrate it. On the other hand, too many case series consist of very small numbers insufficiently powered to be conclusive, and (excepting cryptococcosis) almost all are historical series. Case series may prompt randomized controlled trials, but are rarely sufficient in themselves to change medical practice. Randomized controlled trials should be designed to answer the most critical questions, and should have criteria for entry and for outcome evaluation clearly defined in the protocol.
For systemic mycoses, the potential of combinations began with the appearance of a second major antifungal drug in the 1970's. Flucytosine (5FC) has a limited spectrum against Candida species, Cryptococcus neoformans, and a few other fungal pathogens. Because of toxicity and limited clinical responses to amphotericin B, flucytosine was evaluated in combination with amphotericin B in the treatment of cryptococcal meningitis1. Reduced toxicity and increased efficacy of the combination in this relatively small benchmark study led to the adoption of combined amphotericin B/flucytosine as the standard therapy for cryptococcal meningitis. Just as important, it led to the formation of the Mycoses Study Group (MSG). This National Institutes of Health supported collaborative was formed at the time of the dramatic expansion of HIV/AIDS. The rise of invasive fungal infections (IFI) prompted aggressive development of the azole class of antifungal agents and more recently, the echinocandins. The MSG set about the evaluation of novel antifungal regimens for prevention and treatment of cryptococcosis, the endemic mycoses of North America, systemic candidiasis, and most recently, invasive mold infection. New treatments for cryptococcosis and histoplasmosis have substantially reduced the mortality of these two diseases. In the case of histoplasmosis, this was aided by the development of Histoplasma antigen detection, which decreased the time for diagnosis from weeks to days. As treatment for HIV improved, the incidence and mortality of cryptococcosis and endemic fungal infections also decreased.
As the acute IFI of AIDS came under control, largely through controlling HIV itself, improvements in chemotherapy of malignancies (particularly hematological), antibodies targeted against a number of inflammatory mediators (such as tumor necrosis factor) and a rapid increase in solid organ transplantation opened a new era in IFI. Candidemia increased in high risk groups, but was reduced by use of fluconazole prophylaxis, though Candida glabrata has emerged as an increasing problem in hospitalized patients in the US and Europe. Most recently, the center stage has been taken over by mold infections. Of these, invasive aspergillosis (IA) accounts for about 70–80% of mold infections. The zygomycetes (Mucorales) account for about 10–12%, and the remainder are divided among Scedosporium and a series of less common molds. Until the development of voriconazole (VOR), the mortality of IA has remained a distressingly high 65–85%, depending in part on the underlying disease, the level of immune suppression, and virulence of the pathogen. VOR, licensed as a first line treatment, (and unlicensed as prophylaxis), has led to successful outcomes in IA of 52% versus 32% for amphotericin B (AMB), a superior result2. However, VOR has no effects on Mucorales. A newer antifungal azole, posaconazole, is effective in prophylaxis of IA in patients with hematological malignancies and in rescue of patients with mucormycosis, histoplasmosis, aspergillosis, coccidioidomycosis, cryptococcosis, and other mycoses3. However, both VOR and posaconazole have pharmacokinetic problems, and VOR has significant toxicities. Add to this the recent appearance of three very potent and very similar echinocandins, with great potency in candidiasis, no efficacy in cryptococcosis, and some efficacy in IA4. There is now a much broader range of drugs and drug classes available than a few decades ago.
At present, patients are still suffering and dying with IFI, the most common ones now being the molds. The obvious question becomes, if one of these drugs is effective against fungus “X,” will two drugs be more effective? Can we reduce the morbidity and mortality of IFI with combination antifungal therapy? Can we do it as primary therapy and/or salvage therapy? Recent medical literature has shown what one drug can do in a number of well done studies on each of these new antifungals. We want to do better by our patients. We need to do better. Can we get there with combined antifungals?
Dr Kibbler and I have essentially the same pool of evidence on which to base our assessment. He is led to the conclusion that there is value in combined antifungal therapy. My assessment is also yes – but only for cryptococcal meningitis. Multiple studies of cryptococcal meningitis have given us a clear roadmap of what we should accomplish to show benefit of combinations against other mycoses. However, despite a lot of in vitro and mouse studies, and a very few adequate human studies, there remains uncertainty of the significant benefit of combined antifungal therapy for candidiasis and molds. If one adds the costs and potential toxicities of sustained combined therapy, there may more even more negatives than simple inefficacy. Multicenter studies are costly, and in general evaluate only two regimens. In the case of combined antifungal therapy, we will see drug A and drug A plus drug B, but rarely drug B alone. So already we are at some disadvantage. The waters are muddied further in that, when we do salvage studies, many patients have previously received courses of AMB, an agent with very slow terminal clearance. We have generally ignored this potential contribution of at least low levels of a “third” drug. Other salvage studies include clinical failures and toxicity failures. As one example of the differences, caspofungin (CSP) salvage shows much more benefit in toxicity failures than clinical failures. The following comments present a view that we have not yet reached a point of adopting combined antifungal as standard for either Candida or mold infections. I will present some reasoning as to why this may be inevitable, given the nature of our mold IFI, and I will suggest another line of investigation which is at our doorstep, and may prove more beneficial than our selection of antifungal drug combinations. This is not a unique view, and has recently been published in an excellent review by Dr Luis Ostrosky-Zeichner5. Not so much has changed between 2008 and 2012.
The role of preclinical data (which I am not going to detail here)In vitroThere have been many publications presenting in vitro data on combination versus single drug effect on fungal pathogens. Many of these present the fractional inhibitory concentration of individual and combination regimens. When the activity of the combination exceeds the activity of single drugs this is defined as an additive or a synergistic effect. By contrast, when the effect of combinations is less than single drugs this is defined as an antagonistic effect. The advantage of this system is the ability to change the conditions of the test in many ways, including: drug concentrations, the time they are added to the cultures, incubation conditions, measurement of overall inhibitory effect (inhibiting growth, killing organisms, or changing the morphology of organisms [echinocandins and molds]), or by interference with metabolic processes of pathogens. The same advantages, of multiple conditions for tests, may also be a disadvantage when we try to extrapolate from the test tube to the patient. Through these in vitro tests we reach a consensus, usually rather empirically, on what may be clinically significant… but without a great deal of certainty in many cases.
I find in vitro data on antifungal agents, alone, very unconvincing. As examples I would use 1) AMB, which kills Aspergillus, Mucor, and a host of other organisms very rapidly in vitro, but is very often associated with clinical failure and 2) echinocandins, which were initially, on the basis of MIC, considered less effective against Candida parapsilosis6. Recently, and very quietly, the recommendations against echinocandins for C. parapsilosis group were downgraded. The reason is that echinocandins were clinically effective per response and survival against C. parapsilosis7. So what role do in vitro data play in development of new regimens? Essentially, they give us a series of “effective” concentrations to take to the next level.
Animal studies: The next levelPreclinical animal studies add several critical criteria to the evaluation of novel antifungal regimens. These include:
- 1)
Drug kinetics: This includes whether the drug is absorbed orally or must/can be given intravenously (for the latter it must be solubilized in vehicles, which are themselves soluble in an aqueous environment). Amphotericin B and echinocandins are used clinically only intravenously, while azoles, in general, can be given orally or intravenously (excepting posaconazole at this time). Animal studies help determine whether the drug achieves serum levels effective against fungus “X” as determined by previous in vitro studies. They also answer whether the drug penetrates to and remains in a target site of infection long enough to have efficacy (pharmacodynamics). Finally, they give us the rate of clearance and how a drug is metabolized? However, laboratory animal drug clearance is not analogous to human clearance, and in mice is often much more rapid.
- 2)
Drug efficacy: animal studies help us determine the dosing route, amount, and frequency conditions for maximum efficacy (measured as survival and/or morbidity and/or reduction of fungal burden) and minimal or “acceptable” toxicity. Acceptable is determined, in part, by the nature of the mycosis being treated. Kinetics of drug disposition are very different in animal models than in humans (for example, in mice oral AMB is absorbed and effective, but not in humans). For chronic diseases one must perform chronic studies of drug treatment to check long term toxicity. One must also use at least two different animal models to determine an effective regimen. And finally, if one is to treat meningitis, which may restrict drug access across the blood brain barrier, one must use appropriate animal models… of meningitis.
- 3)
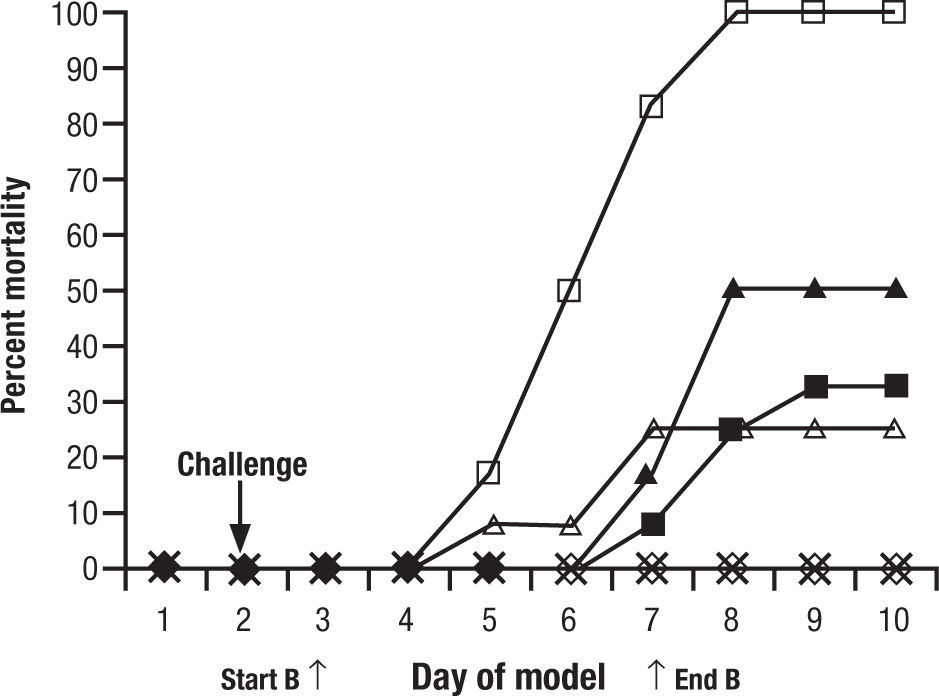
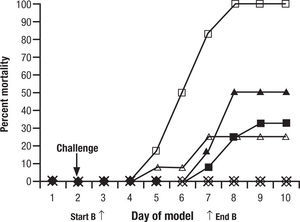
Animal models are a critical antecedent to clinical studies. In 15 years of studies with new antifungals provided through the National Institutes of Health, we have found many effective in vitro, but much less than 5% effective and tolerated in animals. This is for single drugs. When one considers combinations, the process may become even more complex. To evaluate a single regimen one must use at least 8–10 animals in each treatment group. That is, for drug A, drug B, and drug A+B, 30 animals are used… just to look at one dose for one duration of each drug. It is very difficult to do a full dose ranging study with two drugs and combinations. A common recourse is to use a dose just at or below minimal efficacy for each individual drug, and then the combination, hoping for a dramatic improvement seen only with the combination. One must interpret these studies carefully. For example, one frequently quoted study of combined CSP and VOR for aspergillosis in guinea pigs showed reduced “positive lung cultures” of the combination over VOR alone and controls, but no increase in survival benefit8. This was considered a confirmation of the combination over single drug therapy (Fig. 1). However, if one looks carefully, there is no significant difference between the VOR alone and the combination. All animals survived. In other words, the dose of VOR was too high to have the desired “sub-threshold effect”. These studies are difficult to conduct, and readers often see conclusions in them that are beyond the strength of the data. Yet, despite inconclusive results, this and other such studies were used to launch the concept of combined antifungal therapy as superior to one drug.
Figure 1.Cumulative mortality of 12 guinea pigs per treatment group treated with caspofungin, voriconazole, caspofungin plus voriconazole, or amphotericin B. Guinea pigs were challenged on the second day. Controls (□) received no antifungal therapy. Treatment of the guinea pigs with amphotericin B at 1.25mg/kg/day (Δ), caspofungin at 1mg/kg/day (¿) or 2.5mg/kg/day (¿), voriconazole at 5mg/kg/day (◊), caspofungin at 1mg/kg/day plus voriconazole at 5mg/kg/day (*), or caspofungin at 2.5mg/kg/day plus voriconazole at 5mg/kg/day (•) was initiated 24h after challenge and was given daily for 5 day. Reused with permission.
Original source: Antimicrob Agents Chemother. 2002;46:2564–8.8
Even in animal models the results in one laboratory do not always confirm those of the other. In one example, van de Sande and colleagues examined VOR and anidulafungin (ANID) alone and combined in a rat model of advanced IA9. In this study equivalent doses were used for the area under the curve… total drug exposure (AUC)… in humans for both drugs. Rats treated early in the course all survived, whereas rats treated late in the course had 56% survival at day 23. ANID given in late IA showed 18% survival, and combined with VOR added no benefit. In essence ANID was poorly active and gave no benefit in combination therapy.
Preclinical studies: SummaryIn my view the in vitro data are useful to select a drug which has potential in the test tube and may then be taken to the much more costly animal studies. The animal studies incorporate pharmacokinetics, pharmacodynamics, and give both efficacy and toxicity data in various regimens. However, they are very cost-limiting (as well as in ethics of using the masses of animals required) to study full ranges in combination studies. The few attempts (by ourselves and others) have severe limitations in exploration of dose size and duration, and whether treatment is initiated early or late in the course of disease. What these data do give us are some cues as to how to approach human studies…which unfortunately. Preclinical studies do not give us any assurances regarding benefit, antagonism, or toxicity. For that, we must go directly to the patient, and my personal view is that for combination therapy all rises or falls on the clinical data alone.
Clinical evidence on combination antifungal therapyIt has been made abundantly clear for cryptococcosis, candidiasis, and aspergillosis that treatment very early in disease is much more effective than delayed therapy. Yet in clinical practice our innovative changes in drug regimens are usually made for patients in the last stages of a desperate course of “nothing is working”. We ask a great deal.
It is worth beginning with a brief review of the most clear-cut benefit of combination antifungal therapy. This is, of course, cryptococcal meningitis, and it is for this benefit that we strive with the treatment of other mycoses.
Cryptococcal meningitisThe first studies of combination antifungal therapy were conducted in non-HIV patients by Bennet et al., and published in 19791. In that study combined flucytosine (5FC, 150mg/kg/day) and AMB were found more effective than AMB alone, and also less nephrotoxic than the latter. Four weeks of the combination was as effective as 6 weeks10. Flucytosine was not considered an effective single agent due to toxicity of the dose at 37.5mg/kg/6h, and to the emergence of resistance (to both C neoformans and to Candida as well)11. This combination regimen became the standard course of therapy for cryptococcal meningitis until the sharp rise in cryptococcal disease associated with HIV/ AIDS. The major problem then was that combination therapy arrested meningitis, but with the continued immune suppression associated with HIV, the disease recurred after treatment was stopped. This and the cumulative toxicities of both AMB and flucytosine led to two major changes.
The first of these changes was the reduction in dose of 5FC to 25mg/kg/6h12. This abrogated most of the myelotoxicity of flucytosine. A lower dose of Amphotericin B also reduced the nephrotoxicity and indirectly, the flucytosine toxicity, as flucytosine is excreted renally, and levels of its toxic metabolite, 5-fluoruracil, also rise in renal failure.
The second change was the development of fluconazole (FLU), a fungistatic drug for C. neoformans. While fluconazole had some benefit on its own, it was incorporated into studies of combined therapy by the MSG. The MSG found that an initial 2 weeks regimen of AMB and 5FC was marginally (P=.06) more effective in converting cerebro-spinal fluid (CSF) cultures negative at 2 weeks than was AMB alone13 (Table 1). The actual difference favoring the addition of 5FC was only 9%, which does not convince me that 5FC really added much. At the end of 2 weeks, this induction regimen was followed by fluconazole at 400mg per day for 8 more weeks, then 200mg per day for 10 months (non-HIV) or until the CD4 count rose (above 100 in HIV patients, with non-detectable HIV RNA for 3 months allows one to terminate therapy). Many patients clinically responded, and an important part of these studies was the adoption of a surrogate for clinical response…the CSF culture conversion after 2 weeks of induction therapy. Patients with persistent positive CSF cultures were often continued longer on the induction regimen. Because 5FC is not available in many parts of the world, and because the results only marginally favored it, many clinicians use combined induction therapy, while others use a single drug with AMB (or liposomal AMB) alone. The brevity of AMB, and the 25mg/kg/6h 5FC dose, makes for a regimen with less toxicity and a lower need for monitoring hematology or renal function.
Outcomes in prospective randomized trial of fluconazole versus fluconazole and amphotericin B in systemic candidiasis. Reused with permission.
| FLU 800mg/day | FLU + AMB | P | |
| Number | 107 | 112 | - |
| Success | 57% | 69% | 0.043 |
| Persist. Pos. Blood Culture | 17% | 6% | 0.02 |
| Death 90 days | 39% | 40% | NS |
A prospective randomized open label trial in Thailand and the US compared AMB (0.7mg/kg for 14 days, followed by fluconazole 400mg per day to day 56) versus AMB plus FLU at 400mg per day for 56 days or AMB plus FLU 800mg per day for 56 days14. The results were difficult to interpret, in part due to comparing several time points for response with patients in 2 different countries, with some on or off antiretroviral treatment, and using a overall clinical response which included both clinical improvement and negative CSF cultures at 2 weeks. At day 14, 41% of those on standard AMB followed by FLU, 27% of those on the FLU 400mg combination, and 54% of those on the 800mg FLU combination had successful responses. The authors claimed a “trend” to benefit of the combinations. In my view, this study is flawed by not using enough FLU (400 and 800mg doses are fungistatic while 1200mg is fungicidal) and that the major difference was FLU in just the first 2 weeks of therapy… i.e. not a very bold study design and containing patients grouped in too many cells.
Use of the CSF culture conversion after 2 weeks of therapy was eventually considered an insensitive surrogate for microbiological response. Bicanic et al. correlated outcome with initial fungal burden and with early fungicidal activity (EFA), measured as daily log reduction counts in the CSF, determined through multiple lumbar punctures in the first 2 weeks of therapy15. High fungal burden was associated with poor outcome, and high EFA was associated with improved outcome.
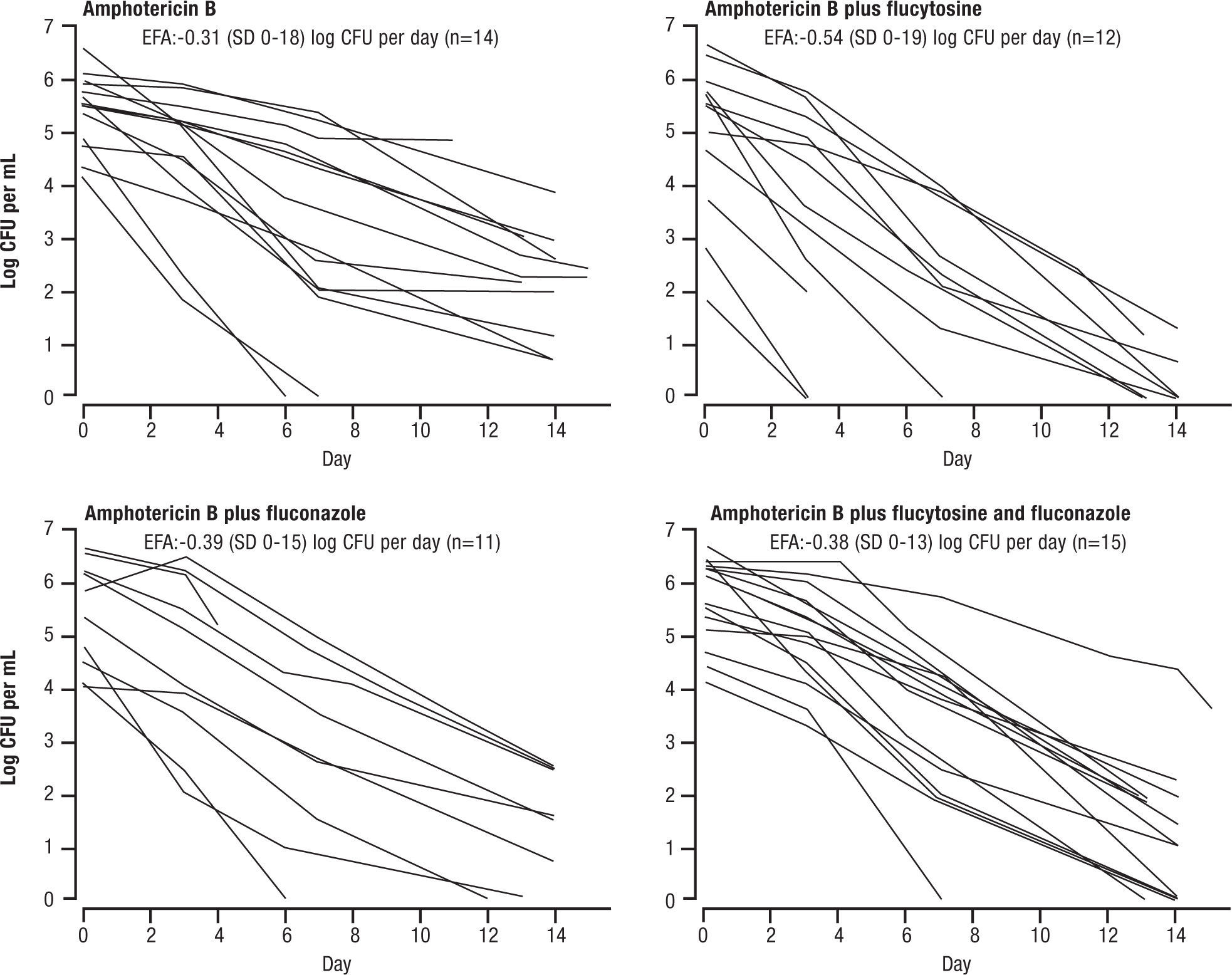
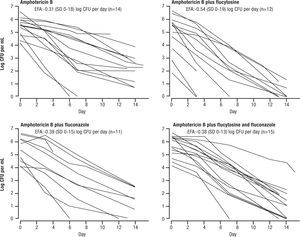
Several, more recent, studies adopted the EFA as the surrogate for microbiological response, replacing the 2 week culture sterilization. This was initially used in animal models, and when advanced to clinical application, was found to be very sensitive in a study by Brouwar et al.16. In their study, groups of 15 patients were randomized to AMB+5FC, AMB alone, AMB+FLU, or all 3 drugs. The investigators found that a high fungal burden (i.e. late stage disease) correlated with poor outcome. Just as important, the investigators changed their surrogate for response to the early reduction in cryptococcal counts in cerebrospinal fluid (ERC, same as EFA). This necessitated more lumbar punctures, but gave a more sensitive index of response than the 2 week culture conversion. In most of the more recent studies this criterion has been used. The EFA, as per Figure 2, showed superiority of AMB and 5FC (EFA = -.54±0.19 log CFU/ml CSF/day) over the other regimens.
Amphotericin B and flucytosine (5FC had a higher early fungicidal activity compared with Amphotericin B alone (p=0.006), Amphotericin B + fluconazole (p=0.02) or triple drugs therapy (p=0.02). Reused with permission.
However, results were not always reproducible. A follow-up study by Loyse et al., compared regimens with combinations of AMB and other drugs17, Their responses showed that AMB (0.7–1mg/kg/day) + 5FC had an EFA of −0.41±0.22 log CFU/ml CSF/day, AMB + FLU 800mg per day (EFA – 0.38±0.18 log CFU/ml CSF/day), AMB + FLU 600 twice daily (EFA −0.41±0.35 log CFU/mL CSF/day), and AMB + VOR 300mg twice daily (EFA-0.44±0.20 log CFU/mL CSF/day), all had similar EFA. These results could not select out any superior regimen, and had the disadvantage that all regimens include AMB. Further, none were as good as AMB+5FC results by Brouwer et al.16. There were only 13–23 patients per group.
Immune augmentation has been examined only once. The Mycoses Study Group supported a Phase II comparison of a traditional regimen of AMB + 5FC for 2 weeks, followed by FLU 400mg per day for 8 weeks, with this regimen plus interferon gamma administered 3 times per week for the full 10 weeks18. At 2 weeks the negative CSF cultures were 13%, 36%, and 32% of placebo, IFN-γ 100μg, and 200 μg recipients, respectively. In this study of 70 patients, there was a “trend” (P=.22, for the 100mcg dose of IFNG versus 0.078 for both 100 and 200mcg doses combined). I would also consider this study inconclusive rather than clear evidence of superior efficacy for the IFNG / antifungal drug combination.
A more exciting era in chemotherapy came with combinations of 5FC and FLU, in an effort to eliminate AMB altogether. A randomized study of FLU versus FLU + 5FC in Uganda showed no significant differences in 6 month survival, but the FLU dose was only 200mg per day19. An open study by Larsen et al. suggested that an all oral regimen of fluconazole with 5FC was effective in cryptococcal meningitis20. This was found effective in open studies, with higher doses of FLU (1200mg) giving better results. Indeed, high doses of FLU given alone were found to reduce C. neoformans counts in CSF more effectively than lower doses… so the “usual” dose may not be optimal21,22. FLU at 1200–2000mg/day was recommended in the 2010 IDSA Guidelines as an alternative therapy, based on results at high doses12.
These studies describe a progression from early therapy with combined AMB and 5FC to more recent studies with combined AMB and FLU and FLU and 5FC. These regimens are being further examined in countries where cryptococcosis is a greater clinical issue than in the United States (e.g. South Africa, Thailand).
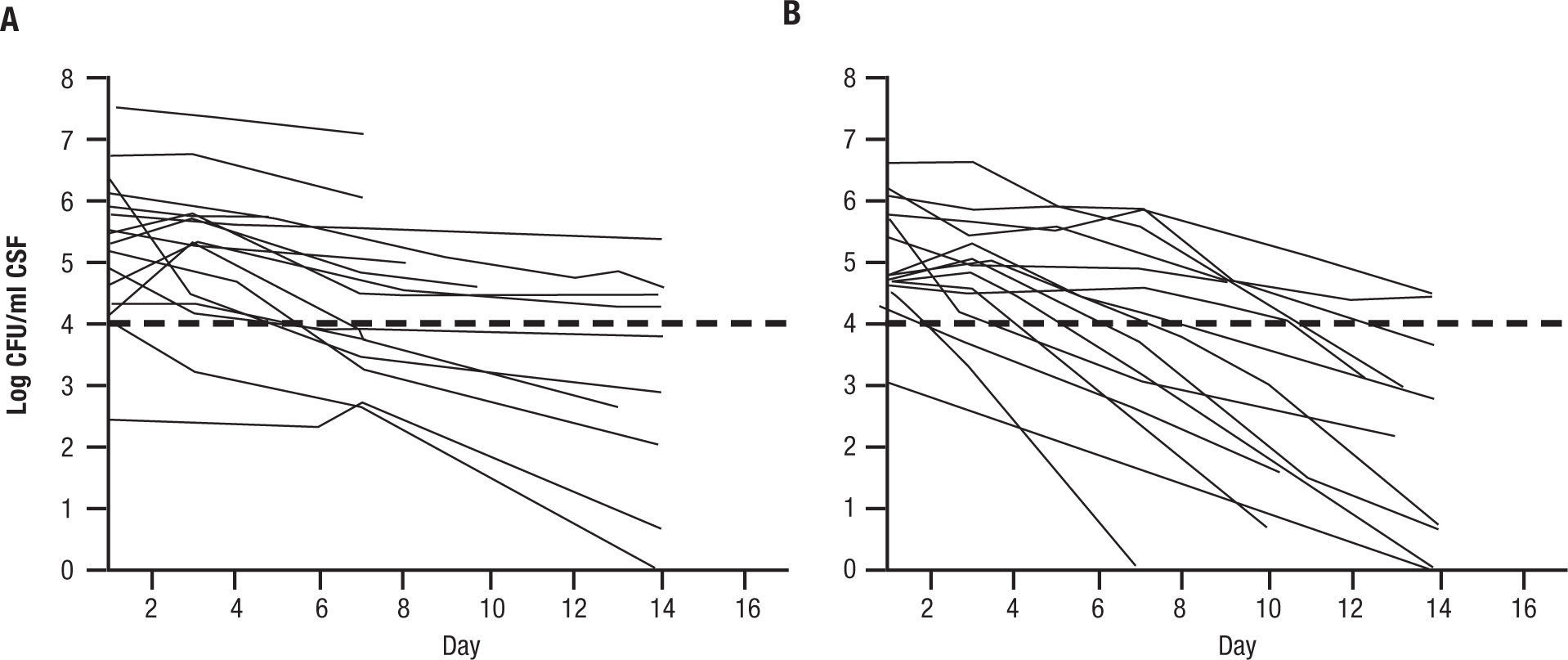
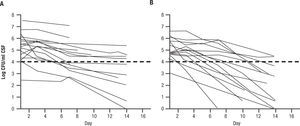
One randomized prospective trial was conducted in Malawi with FLU1200mg per day alone or combined with 5FC at 100mg/kg/day on HIV positive, non HAART treated patients with their first episode of cryptococcal meningitis23. EFA was the primary end point, and was determined as the slope of reduction of cryptococcal counts from lumbar punctures done on day 1,3,7, and 14. EFA of the combination was -.28±0.17 log CFU per day for the combination versus -0.11±0.09 log CFU per day for FLU alone (P<.001) (Fig. 3). This was remarkable in that only 41 patients were analyzed. The conclusion of this study was that an all oral regimens with high dose fluconazole and oral flucytosine can be constructed in Africa, where it is very difficult to administer AMB. This small study also showed a reduction in mortality at 2 weeks for the combination (10% of 21) versus fluconazole alone (37% of 19) [P=.05].
Early fungicidal activity in patients treated with fluconazole (1200mg per day) versus fluconazole and flucytosine (100mg/kg/day). A: fluconazole 1200mg per day. B: fluconazole and 5FC 100mg/kg/day. Reused with permission.
In summary, there is strong support for combined 5FC and AMB for induction therapy of 2 weeks, followed by FLU for long term therapy, which continues until the CD4 counts rise in HIV patients, or 10 weeks in non-HIV patients. However, the more important discoveries, also based on multiple studies, have been the increased efficacy of high dose FLU, and the ability to use an all oral regimens (FLU and 5FC) for induction therapy. These major advances depended in part on the adoption of EFA as a surrogate for response, a practice unfortunately not done universally. They make a strong case for distribution of 5FC worldwide, and not to penalize patients in countries where this component of an all oral regimen is not available. Cryptococcus gattii is managed in just the same way as C. neoformans for meningitis.
For the rare patient unable to tolerate fluconazole, both VOR and posaconazole have some efficacy in cryptococcal meningitis. But they have not been extensively studied in primary therapy or with combinations.
Finally, the immune reconstitution syndrome is manifested by headache and increased inflammation in the CSF. It is not associated with antifungal therapy failure, which should not be changed. If needed, brief courses of corticosteroids may reduce the manifestations of immune reconstitution syndrome24.
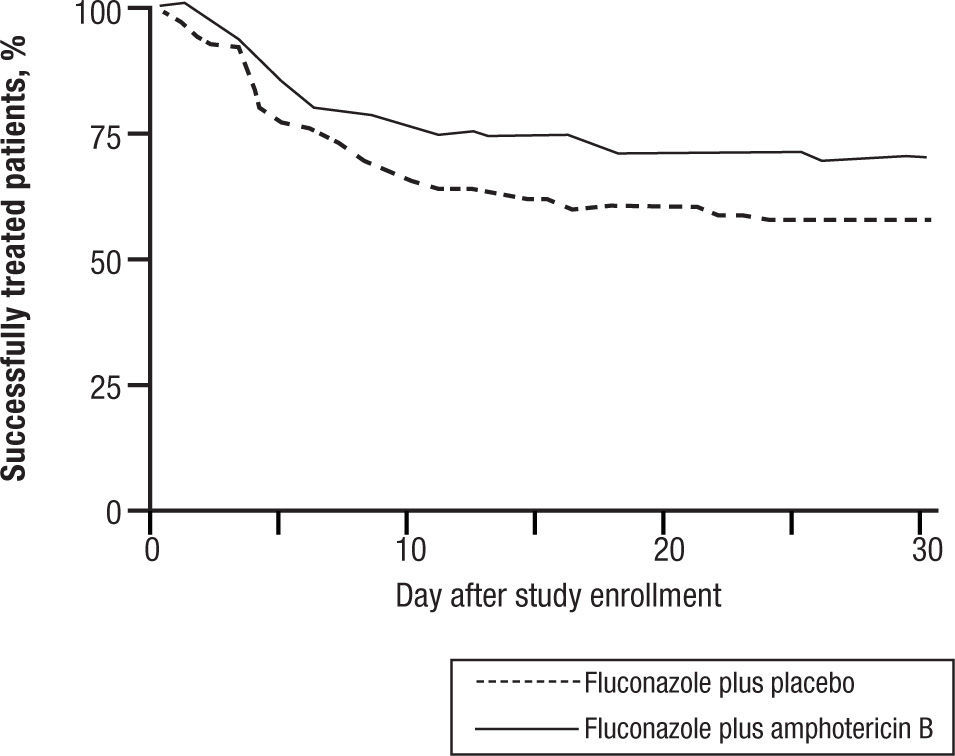
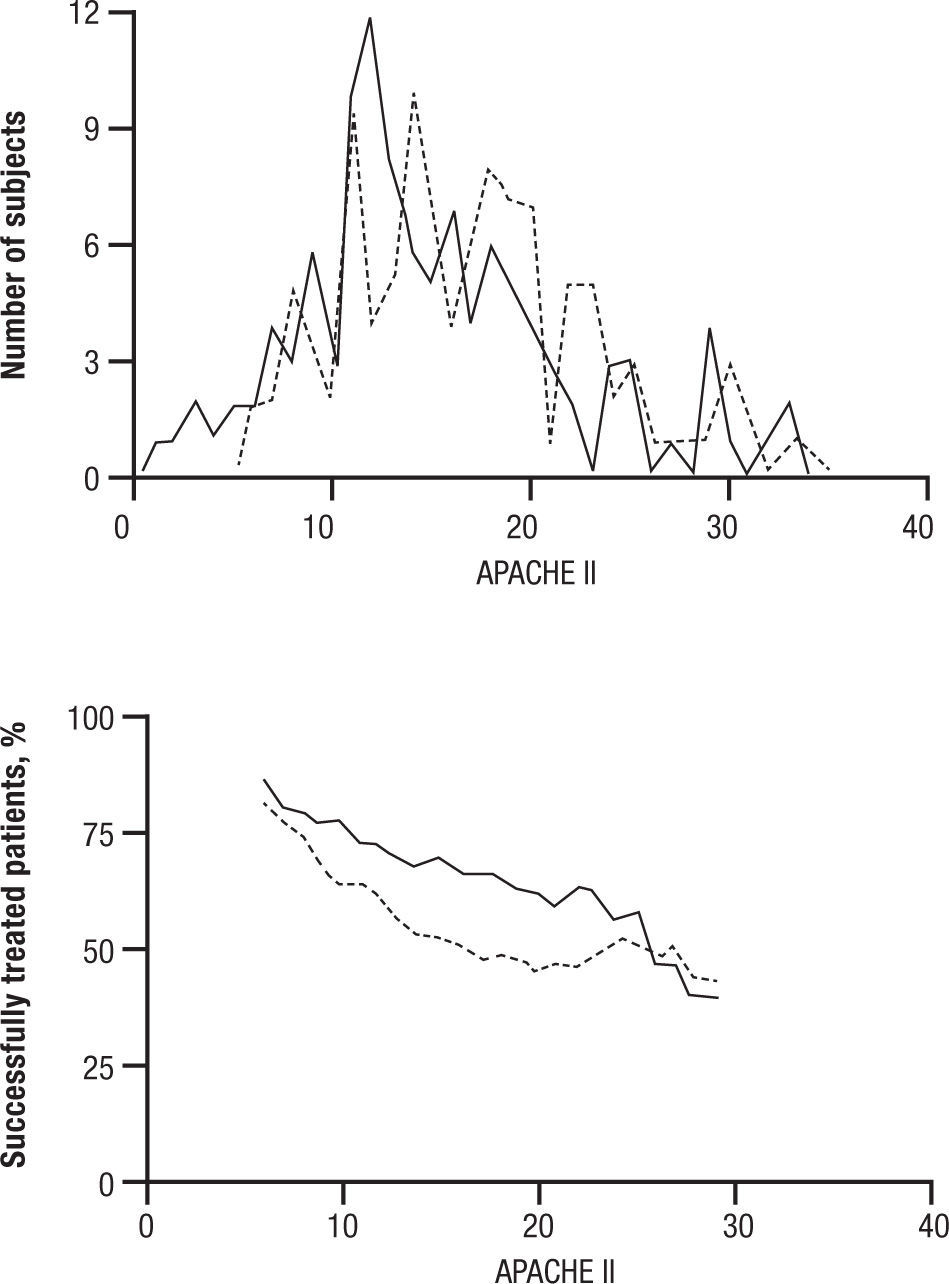
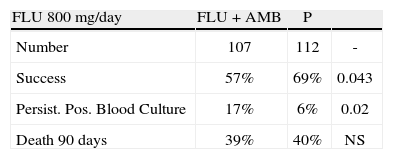
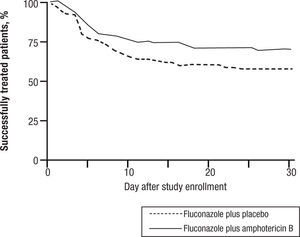
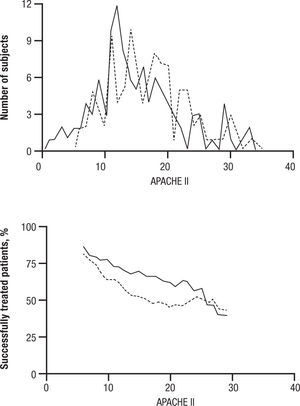
CandidemiaInitial studies of AMB combined with 5FC were scattered cases. The use of 5FC in neutropenic patients was a detriment, and no clear benefits were shown. The appearance of FLU, a relatively non-toxic but fungistatic azole, led to studies of combined AMB and FLU in candidemia. While FLU had activity of its own in candidemia, there was not enough push to set up a 3 arm study. Hence FLU was compared with FLU and AMB. The clinical data related to combination therapy essentially hang on the prospective randomized study by Rex and the MSG25. In this large prospective randomized trial, patients were randomized to 800mg per day FLU versus FLU and AMB 0.7mg/kg/day. Success was defined as resolution of symptoms and negative blood cultures (Table 1). The combination was favorable, P=.048, and candidemia persisted longer with FLU therapy. However, this is presented a little more clearly than it really is. The pre-defined criteria for success included time to failure, where the P was 0,08 (Fig. 4). Ninety day mortality also showed no differences. If overall success rate is used, (a secondary analysis) the P required for significance, was .045, and 0.043 was achieved. The clearest benefit for the combination was found in another secondary analysis, when the authors broke down their patients according to APACHE II (fig. 5). Herein, the combination performed better than single drug than was noted for patients with midrange APACHE II scores (approximately between 10 and 22). In other words, when the APACHE II score was low, patient immune defenses were sufficient to cause low mortality, with a relatively minor contribution of either single or combined antifungal therapy. In patients with APACHE II scores in the 20's, mortality was high no matter what regimen was used. Host immune defenses clearly played a critical role, and when these were abrogated severely, antifungal regimens were less critical. This shows, ever so clearly, the effect of the severity of underlying disease on treatment outcome, and the limitations of antifungal therapy. As most of us do not use APACHE II scoring to determine our therapy, using these data in clinical decision making is not easily done.
Time to failure of patients with candidemia treated with fluconazole or fluconazole and amphotericin B. P=0.0925.
APACHE II distribution and success rate, by APACHE II score. A. Distribution of APACHE II scores for the fluconazole plus amphotericin B deoxycholate (solid line) and fluconazole plus placebo (stippled line) groups. B. Outcome by group (solid line, fluconazole plus amphotericin B deoxycholate; stippled line, fluconazole plus placebo) for subjects with the same APACHE II score ± 5 points (e.g., the leftmost point of each line is the success rate for subjects having an APACHE II of 6 ± 5, or 1–11). The cell width of ± 5 for this rolling stratified analysis was chosen because it placed ≥ 10 subjects into each cell. Qualitatively similar results were obtained for cell widths ± 3 and ± 4. Reused with permission.
The 2009 IDSA Guidelines had only this curious and somewhat dismissive comment about the Rex study: “In large clinical trials, fluconazole demonstrated efficacy comparable to that of AmB-d for the treatment of candidemia…”6 Indeed, the IDSA Guidelines do not recommend combined AMB and FLU for candidemia. The final icing on this cake was provided by the echinocandins, which had equal or higher efficacy than other antifungals. As one example, in a randomized comparison ANID (200mg day 1 then 100mg per day) was more effective at end of therapy than FLU alone (800mg day 1 then 400mg/day), (P=.02)26. At present, echinocandins are recommended as primary therapy in patients severely ill with candidemia and FLU or AMB are recommended as alternatives for some patients6.
In summary, the Rex study is the definitive study on antifungal drug combinations in candidemia, at least for azoles and AMB. By days to response there was no significant difference. By number of responders there was a barely significant difference. By clearance of candidemia there was a very significant difference. By mortality there was no significant difference. AMB is toxic. One cannot conclude that there is much value for this combination.
A second series of studies involved immune defense as one of the arms of antifungal therapy. In this study, with supporting murine data, patients were randomized to treatment with AMB, with or without the addition of a monoclonal heat shock protein Candida antibody27. Results for this randomized placebo controlled trial were very impressive, strongly favoring the combination. Of 119 patients analyzed by modified intent-to-treat, complete response (improvement and negative blood cultures) was seen in 48% of those who received (liposomal AMB) LAMB versus 84% of those who received LAMB plus a Candida heat shock protein antibody (P=.001). More rapid sterilization of blood cultures was also seen. However, there have been some challenges to the reported efficacy of Mycograb (also named Efungumab), and a cytokine release syndrome has been associated with unstable blood pressure and cardiac output. Mycograb has not been approved in Europe, pending further controlled studies, and is an orphan drug per the US Food and Drug Administration28. This is particularly unfortunate, as a number of other studies with antibodies and cytokines against Cryptococcus neoformans and against inflammatory mediators in other diseases, such as coccidioidomycosis, have been tried only in a few cases, with no conclusive results.
Invasive aspergillosisThis disease is the current leading problem of IFI. Invasive aspergillosis is presently associated with a variety of predisposing factors, including high dose steroid usage, hematological malignancy neutropenia, stem cell transplantation, especially with graft versus host disease, solid organ transplantation. Pathogenesis is almost always by infection following inhalation of conidia, germination in alveoli and small airways, penetration of hyphae into blood vessels, and ultimately widespread hematological dissemination. IA is associated with mats of hyphae obstructing blood vessels, initially in the lungs, but also in other target organs. Distal tissue infarction is a hallmark of this disease. Initial manifestations of IA are early onset of pneumonia following an immunologic suppression…. and efforts were made to prevent this disease by using HEPA filtration in the hospital rooms of vulnerable patients. However, in more recent years IA has appeared in both early and later phases of leukemia, commonly associated with graft versus host disease, treatment with steroids, and other immune suppressive medications. Furthermore, the rise of heart/lung transplantation has generated a whole new series of manifestations of IA, including local necrotizing tracheobronchitis, focal pulmonary invasion,
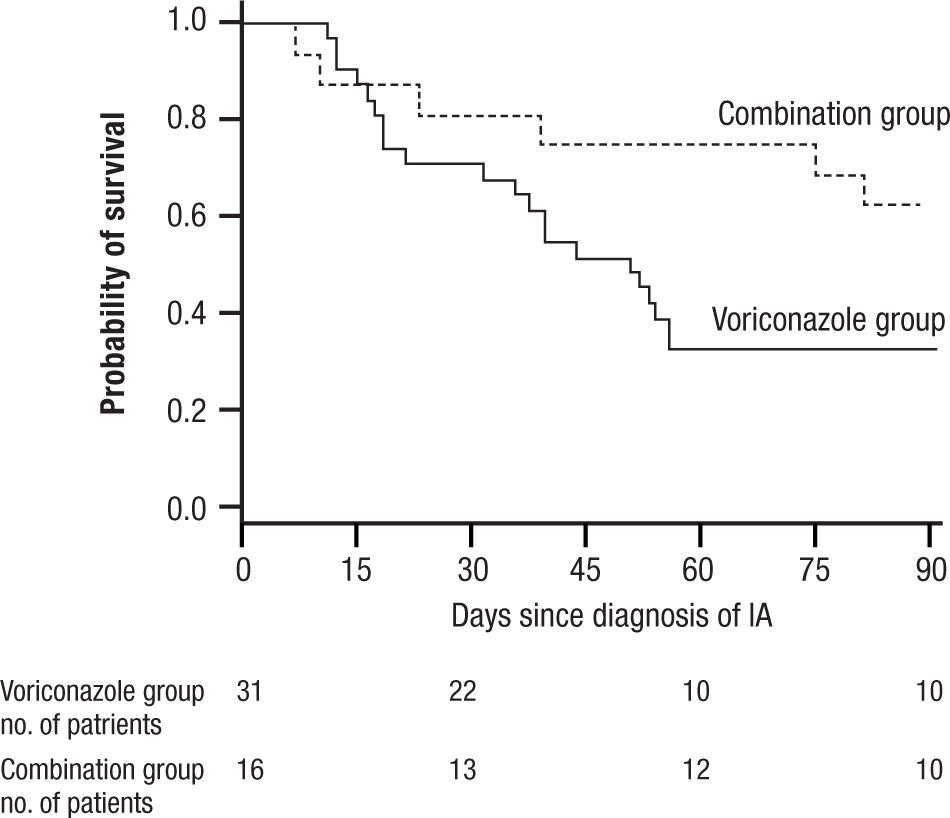
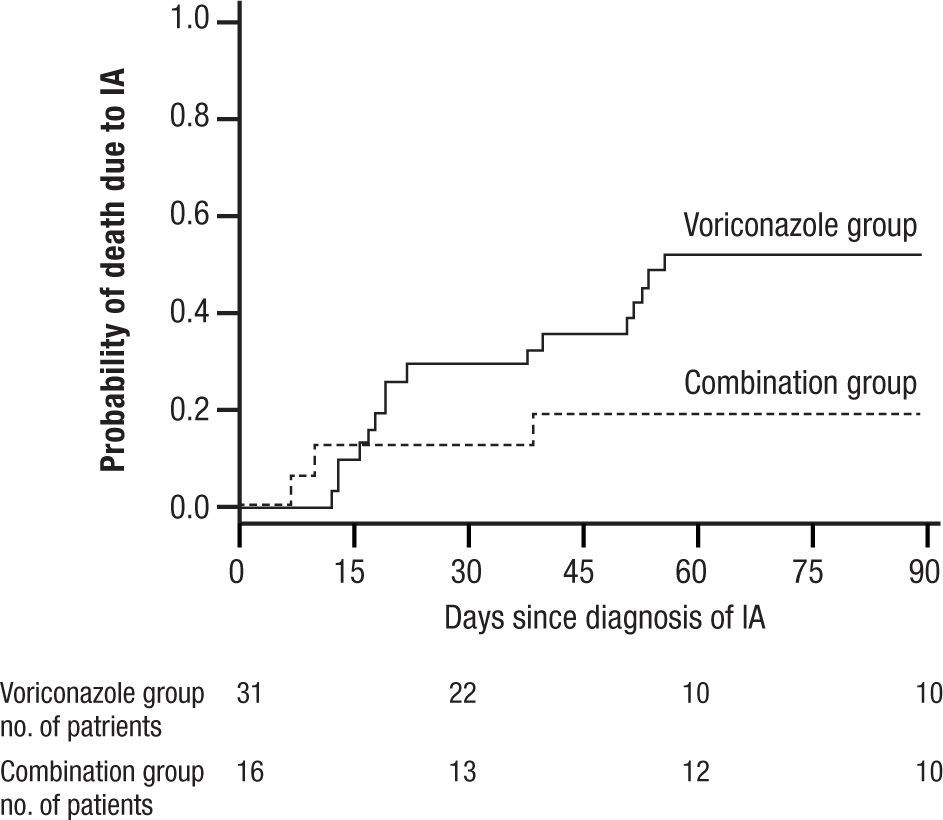
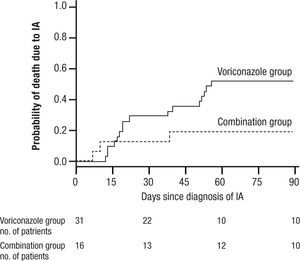
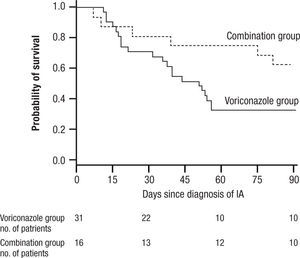
and widespread pneumonia. All of these have given IA a much broader range of disease and virulence than in earlier years. Treatment initially was acute, associated with high mortality often exceeding 80% for AMB, and then was dramatically improved with the introduction of VOR. More recently, a switch to LAMB has substantially reduced the toxicity of AMB. VOR associated mortality was still over 29% at 12 weeks, and interest soon turned to the possibility of combination therapy29.In 2004 Marr et al. published a very provocative paper, a study of salvage VOR response to IA associated with HSCT, compared with combined VOR and CSP30. There were 47 patients treated between 1997 and 2001. Forty one of these patients were HSCT recipients. Standard practice at that time was to use AMB ≥ 1mg/kg for primary therapy and VOR for rescue therapy. In February 2001 salvage therapy was altered to include VOR plus CSP 70mg day 1 then 50mg/day. Survival at 3 months after diagnosis was higher for the combination (P=.08) and when measured after initiation of salvage therapy it had a P=.045, favoring the combination (Fig. 6). With univariate Cox regression, the combination gave a hazard ratio of 0.42 and P=.048, thus favoring the combination. However, non-myeloablative therapy had a hazard ratio of 0.30 with a P=.052. Is there much difference between P=.048 and P=.052? With multivariate Cox regression analysis, the combination reduced the risk of death associated with VOR alone, HR 0.27, P=.008, with receipt of transplant giving a P=.06, and non-myeloablative therapy dropping out.
Kaplan Meier survival plot of patients receiving VOR or VOR and CSP for salvage therapy of IA. P is 0.048 from the likelihood ratio test using Cox regression. Note small number of patients at 90 days. Reused with permission.
A main strength of this study included using all cause mortality (no fudging as to who died of IA). The responses to combined therapy were clearly better than to VOR alone, or were they? The initial appearance of a positive effect needs to be examined more closely. First, this was not a randomized trial, but a prospective study of a combination compared with a retrospective analysis of VOR alone. This was, in essence a historic comparison, and the numbers were very small, 31 for VOR, and only 16 for combined therapy. Despite the similarity of groups in the demographics presented, such studies are prone to unappreciated differences in the demographics of treatment groups. The small numbers, and some differences between the treatment groups (HSCT as one example) exaggerated this weakness. Furthermore, when the comparisons of outcome were extended beyond the initial published observations, the overall mortality of both groups came together. The very small numbers at the end of observation (10 per group) seriously limit comparisons.
Another unappreciated problem is the generally limited database for echinocandins in IA. Why did the authors choose CSP? An open study of rescue therapy with CSP in very well defined patients showed efficacy in a very small number of CSP recipients31. Even in this carefully conducted study of 83 patients, there were two distinct groups. One was 71 patients failing conventional therapy, and the other was 12 patients treated with CSP for drug toxicity of the primary therapy (AMB). This latter group had a 75% response while that for failing patients was 39%… only half as good. The combined response to CSP was 45%. Combinations of CSP with VOR and other agents have been reported to be in the same range, 55%32. Other studies had similar results, using a variety of combinations. Overall, echinocandin efficacy in IA is based on a variable data base, often retrospectively collected, and not readily comparable with other studies. And the results are not especially encouraging in patients with late stage disease. The newer echinocandins, micafungin and ANID, have a much more poorly constructed data base than CSP. Most of the data with micafungin are based on combination with AMB or VOR, so it is impossible to determine MF result alone33. Response rates of 194 patients given micafungin in combination with other drugs was collectively only 34%, so this is not much to get excited about. So while we have very good data on VOR and AMB response in IA, it is much more sketchy for the echinocandins in IA. Nevertheless, based on the Marr study, there was considerable popular support for using echinocandins with VOR, at least in rescue therapy for IA. Merck did acquire data on toxicity of combined CSP and VOR (no added toxicity), but did not support a primary randomized trial of single vs combined therapy.
Yet another study, a “prospective observational study”, was reported by Singh et al., on patients with liver transplantation and primary treatment of IA34. This really was a historical study in which 47 patients treated in 1999 to 2002 received LAMB, versus 40 patients from 2001–2005, who were given combined VOR and CSP (n=40). Ninety day survival was 67% for the combination recipients versus 51% for the LAMB recipients (P=.117). However, in secondary analyses the authors found a significant benefit in those with renal failure and those with Aspergillus fumigatus. The problems with this study include a) its historical nature b) small size and, c) the secondary analyses, done with small groups of patients, poorly powered, and reaching for something, anything, which was “good”. The authors concluded that combination therapy “might be considered” preferable for special groups. That is a very qualified endorsement.
These types of studies in themselves are extremely rarely the foundations for major changes in medical practice. The best that they can properly do is to raise the question of whether combination therapy is superior to a single agent. The question should be answered by a sufficiently powered randomized prospective comparison. However, as time passed, many physicians did not wait, and began to use combinations in primary or rescue therapy of IA.
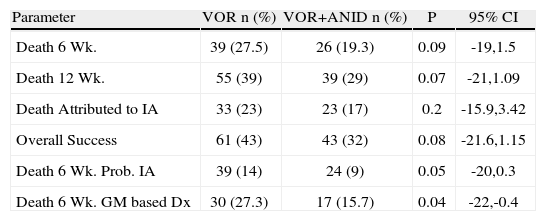
After a considerable delay, a multicenter prospective study was developed by the MSG for HSCT transplant recipients with probable or documented IA. The sponsors and the investigators are to be complimented for diligently pursuing this study. The regimens were VOR (6mg/kg/12h IV decreasing to 4mg/kg/12h) versus ANID (200mg V day 1 decreasing to 100mg/day thereafter) and VOR randomized 1:1. After 1 week the VOR could be switched to oral drug at 300mg/day. Combination therapy was continued for 2–4 weeks. The endpoint was death at 6 weeks in patients with confirmed or probable IA diagnosed at day 7. Secondary endpoints were global response at 6 weeks, all cause mortality at 12 weeks, and safety/tolerability. The study recently closed, and preliminary data were presented at the 2012 ECCMID meeting35. Of 454 patients entered, 277 were adjudicated to have documented or probable IA). Six week mortality in the 135 patients who received combination therapy was 19%, versus 28% of those who received VOR alone (P=0.09), which just makes the cut for a “trend,” favoring the combination. In other words the responses were close to good enough, but not quite good enough for a “conclusive” answer. If we want to use the combined regimen, this may be “good enough” for some. The authors reinforced this by presenting the data for patients in whom the microbiological basis for probable IA was only the galactomannan assay, the results significantly favored the combination therapy group, with a pjust a bit below .05. Details are also given in Table 2. The authors appropriately concluded that their results showed a trend to improved survival, but then threw in the “probable IA diagnosed with GM only” as an analysis. Is this playing by the rules of analysis according to success criteria defined before the study commenced, or is this post hoc analysis? This is a preliminary presentation, which I did not attend, and I cannot easily judge.
Outcome of invasive aspergillosis in patients randomized to voriconazole or voriconazole and anidulafungin35
| Parameter | VOR n (%) | VOR+ANID n (%) | P | 95% CI |
| Death 6 Wk. | 39 (27.5) | 26 (19.3) | 0.09 | -19,1.5 |
| Death 12 Wk. | 55 (39) | 39 (29) | 0.07 | -21,1.09 |
| Death Attributed to IA | 33 (23) | 23 (17) | 0.2 | -15.9,3.42 |
| Overall Success | 61 (43) | 43 (32) | 0.08 | -21.6,1.15 |
| Death 6 Wk. Prob. IA | 39 (14) | 24 (9) | 0.05 | -20,0.3 |
| Death 6 Wk. GM based Dx | 30 (27.3) | 17 (15.7) | 0.04 | -22,-0.4 |
My point is that we are not neutral, but want to use what we hope will be more effective. A suggestion (P=.09) is better than nothing at all… or is it? If we cannot quite prove a benefit, does “almost proof” become good enough? What if the P is .06, or .08? Fortunately, there were no additional toxicities, which is good, but this is of much less importance compared with efficacy. But is the trend to benefit good enough to adopt combination therapy routinely? I think not. Let us return to cryptococcosis, where multiple studies using different regimens have clearly confirmed the value of combined therapy. It should not be that hard to show a real benefit in IA if there is a real benefit… at least with these regimens given for primary therapy, i.e. early disease. Recall that this is not salvage therapy, with patients treated late in the course of disease. This should be more like the VOR vs AMB study, or the patients given CSP for “toxicity” salvage (70% response), not clinical salvage (35% response).
With a large study such as this, with patients carefully selected for high probability of IA, if the initial response was not quite clear-cut, the authors had to work to show a benefit, which was barely significant when other predefined criteria for success were not achieved? This type of secondary analysis, is often used, and may have significant contributions. But is it objective, or turning over the ground looking for something, anything? This is surely where Dr Kibbler and I disagree.
ZygomycosisThis has been a fertile area of preclinical investigation. Because zygomycetes have an avid iron capturing system, and because one predisposing clinical factor is iron overload disease, investigations were conducted in mice using combined AMB and an iron chelating compound. The combination appeared superior to AMB alone, decreasing tissue fungal burden presumably by starving the fungus of iron36. Unfortunately, zygomycosis is so uncommon that randomized prospective trials are almost impossible to contemplate in humans. A few cases gave a mixed report. In one of them a patient was treated with AMB and CSP for zygomycotic liver abscess, and then posaconazole was added. There appeared to be some initial stabilization. Then deferasirox was added and the disease became much more aggressive locally and metastasized, with a lethal outcome37. An initial case series suggested a worse outcome with the combinations, and this was dropped. Another area of interest is echinocandins. These have no demonstrable activity alone in zygomycosis in vitro, but in animal studies they added AMB38. Again, positive animal studies should initiate clinical investigation, but these on their own rarely indicate definitive change in therapy. A few case studies have suggested that combined therapy might be superior to AMB alone39. The largest of these is a series of 41 cases collected from 1994 to 200640. Of these, 83% were diabetics of whom 41% were given corticosteroids. Only 12% were neutropenic. Success, (being alive and not on hospice care 30 days after hospital discharge) was achieved by 54%. Five patients received CSP and ABLC, while 2 received CSP and LAMB as initial therapy. Four patients (one combination and 3 monotherapy with polyene) were lost to follow-up within 30 days of discharge, and were considered non-evaluable. Of the evaluable patients, 100% of 6 combination therapy recipients (including 4 with CNS disease) versus 45% of 31 patients given polyene alone (and 25% of 16 with CNS disease) were considered successes (p=.01). The authors noted that from 1992–1997 AMB was the primary mode of therapy (60% of 5 cases successes); from 1997–2002 lipid forms of AMB were used as monotherapy (44% of 18 patients successes), and from 2004–1006 combination therapy was used (57% of 14 patients successes). There appeared to be no differences in outcome between these periods by multivariate analysis, and they claimed that only combination therapy remained significant. However, there are 2 major problems with this study. The first is that it is historic… an uncontrolled case series. There was no clear indication as to why some got the combinations versus monotherapy. Although the surgeons were unchanged, there was no straightforward way to evaluate the impact of surgery on these patients. Clearly the time of intervention is critical. The interval from onset of symptoms to initiation of therapy was often not given (how many were early versus late in their course). And how much variation was there in underlying immune deficiency among patients receiving monotherapy versus combination therapy? Any of these factors are multiplied by considering that there were only 6 patients receiving combination therapy (during a narrow time frame from between 2005 and 2007) compared with 31 patients treated over a much longer time frame. Six is a very small number. Indeed, if one examines only the 14 patients treated between 2004 and 2006, only 3 of the 8 patients receiving monotherapy responded (37%). This appears to be lower than the patients in earlier years given monotherapy with polyene. This could be consistent with “cherry picking” the patients most likely to respond to any treatment for primary combination therapy. Calls for randomized studies by these authors and those of an accompanying editorial40–42 are not likely to generate much response, with this uncommon group of mycoses. These cases are hard to evaluate, as medical therapy is often combined with surgical debridement to remove infarcted tissue, and early debridement also improves outcome. Issues of funding remain paramount for these uncommon mycoses. The area of zygomycosis will probably remain unresolved as regards combination therapy.
SummaryThe above review suggests that one can clearly show efficacy of antifungal combinations in cryptococcal meningitis, but this has been very difficult to show for other major mycoses. This leads one to consider what other measures are important in determining outcome. For the angioinvasive molds, surgical debridement is a major component of both early and late treatment. Commonly used in zygomycosis, it may also be appropriate in persons with IA and other invasive molds. The reasons are both to prevent hemorrhage of infarcted tissues and to remove tissues where the antimicrobials do not penetrate. The best option is to initiate treatment very early, when there is little infarcted tissue, to allow antifungals penetrate the areas around the focus of infection. Initial studies by Garey et al. showed markedly improved outcome for candidemia (not strongly associated with infarction) if treatment was initiated within a day of the blood culture43. Treatment of patients with the halo sign, indicative of early invasive aspergillosis (strongly associated with infarction), was associated with better outcome than other cavities or solid lesions representing later disease44. Similar results were presented for zygomycosis. Delaying treatment for more than 6 days doubled mortality, from 48 to 84% in 70 consecutive patients (p=.004)45. It is my belief that very early initiation of therapy has more benefit than chasing will o’ the wisp combinations, if for no other reason than antifungal drugs are likely to penetrate already necrotic tissue. This is not a call for abandonment of exploration of combination antifungal therapy. It is recognition that the regimens we have thus far, except for cryptococcosis, have not been solidly effective, and that attention may be better focused on improving standards for very early diagnosis, and launching therapy either pre-emptively or even empirically limited to very high risk groups. This might be combined with early withdrawal if IFI is not quickly confirmed. This takes us into an area beyond the scope of this paper.
Conficts of interestThe author declared no conflicts of interest.