Gastric atrophy (GA) is the first premalignant condition described in the path towards gastric adenocarcinoma. Gastric biopsies are recommended by current western guidelines to grade histological severity. Patients with severe histologic GA (i.e., OLGA III/IV) are considered to have increased risk of gastric cancer.1
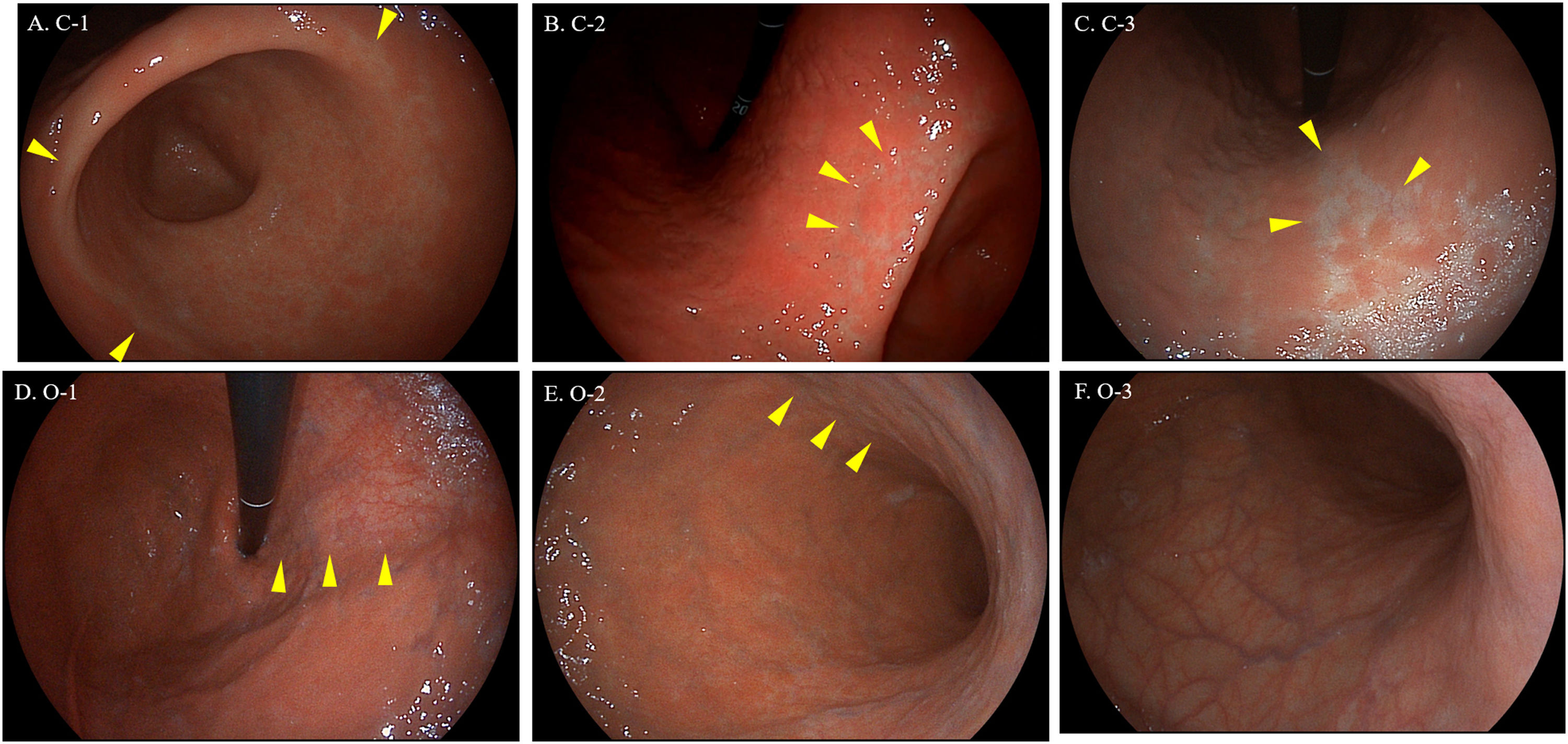
The Kimura-Takemoto classification is a visual scale commonly used in eastern countries to assess GA endoscopically. It classifies GA into two main types based on the location of atrophic border: the closed types (C-1, C-2 and C-3) and the opened types (O-1, O-2 and O-3).2 Despite a moderate agreement among expert endoscopists, the severity of GA according to this classification has shown a correlation with histology,3 and most important, it is also related with gastric cancer risk in eastern populations.4 Although few studies have been carried out in Europe,3 it could be useful to select patients who deserve mapping gastric biopsies in our daily practice.5
High-definition and SFI (Spectral Focused Imaging) technology developed by SonoScape (Medical Corp, Shenzhen, China), improves the atrophic border identification by increasing the contrast between mucosa and vessels: the red becomes redder, and the white becomes whiter. This classification and advantages of SFI are shown in Figs. 1, 2, and video (supplementary material).
Kimura-Takemoto classification with High-Definition White-Light endoscope by SonoScape. (A) C-1: atrophic border is identified in antrum or incisura angularis. (B) C-2: atrophic border reaches to the distal area of lesser curvature of gastric body. (C) C-3: atrophic border reaches to the proximal area of lesser curvature of gastric body. (D) O-1: atrophic border no longer lies on the lesser curvature, but instead between the lesser curvature and anterior wall. (E) O-2: atrophic border affects anterior wall of the gastric body. (F) O-3: atrophic border affects the greater curvature of gastric body (in this case atrophy affects all gastric body). Arrowheads indicate the atrophic border.
SFI virtual chromoendoscopy by SonoScape has an optical phase followed by digital processing. In the optical phase, the blue-violet light is intensified at 415nm where the hemoglobin light absorption peaks. The digital processing then sharpens and de-noises the image, while increasing the brightness and further the contrast. (A) C-1; (B) C-2; (C) C-3; (D) 0–2. Arrowheads indicate the atrophic border.