The aim of the study is to evaluate the clinical and biochemical response of inflammatory bowel disease (IBD) patients treated with vedolizumab, 16 weeks after transitioning from intravenous (iv) to subcutaneous (sc).
MethodsAn observational, prospective, single-center cohort study was performed. Patients with IBD and maintenance treatment with vedolizumab, stable for at least 4 mo, were offered to switch to sc formulation. At the same time of treatment administration a blood test was performed, with vedolizumab levels and fecal calprotectin.
ResultsForty-three patients were included, 12 of them (27.9%) chose to transition to sc formulation. All included patients remained in remission during follow-up. At week 16 (w16), no significant differences were found in terms of calprotectin levels in patients on iv treatment (mean 146.6 ± SD 45.9) vs. sc (159.26 ± 53.9) (p 0.9). Vedolizumab serum levels at w16 were higher in the sc group (22364.3 ± 5141.6) vs. iv (11425.9 ± 1514.2) (p 0.009). At w16, 9 (75%) of the patients in the SC group were highly satisfied with the medication and 11 (91.7%) considered it easy to administer. 4 patients (12.9%) in the iv group and 2 (16.6%) in the sc group presented mild adverse effects. The 2 cases (100%) of the sc group the adverse event was local inflammation at the injection site.
ConclusionIn our experience, vedolizumab sc is a convenient alternative to iv administration. Vedolizumab serum levels in patients who transitioned to sc were higher than iv formulation.
El objetivo del estudio es determinar la evolución clínica y bioquímica de pacientes con enfermedad inflamatoria intestinal (EII) tratados con vedolizumab, 16 semanas después de cambiar de la vía intravenosa (iv) a subcutánea (sc).
MétodosSe llevó a cabo un estudio de cohortes prospectivo, observacional y unicéntrico. Se ofreció a pacientes con EII y tratamiento de mantenimiento con vedolizumab, estable durante al menos 4 meses, cambiar a sc. Al mismo tiempo de la administración del tratamiento, se realizó un análisis de sangre, midiendo los niveles de vedolizumab y calprotectina fecal.
ResultadosSe incluyeron 43 pacientes, de los cuales 12 (27.9%) optaron por cambiar a sc. Todos los pacientes incluidos permanecieron en remisión durante el seguimiento. En la semana 16 (s16), no se encontraron diferencias significativas en los niveles de calprotectina entre el grupo iv (media 146.6 ± DE 45.9) y sc (159.26 ± 53.9) (p 0.9). Los niveles séricos de vedolizumab en la s16 fueron más altos en el grupo sc (22364.3 ± 5141.6) vs. iv (11425.9 ± 1514.2) (p 0.009). En la s16, 9 (75%) de los pacientes en el grupo sc estaban muy satisfechos con el medicamento y 11 (91.7%) lo consideraron fácil de administrar. 4 pacientes (12.9%) en el grupo iv y 2 (16.6%) en el grupo sc presentaron efectos adversos leves. En los 2 casos (100%) del grupo sc, el evento adverso fue inflamación local en el sitio de inyección.
ConclusiónEn nuestra experiencia, vedolizumab sc es una alternativa eficaz. Los niveles séricos valle de vedolizumab en el grupo sc fueron mayores que en el grupo iv.
Vedolizumab is a humanized monoclonal antibody directed against integrin α4β7 that acts selectively on the gastrointestinal wall.1 The efficacy and safety of vedolizumab in subjects with inflammatory bowel disease (IBD) has been studied in different clinical trials, in particular the GEMINI trials.2,3 The usual dosage of vedolizumab is 300 mg intravenously (IV) administered at weeks 0, 2 and 6 for induction, and then every 8 weeks for maintenance.4,5
In 2020, the subcutaneous (SC) administration route of vedolizumab 108 mg every 2 weeks in maintenance was approved, after the VISIBLE clinical trials6,7 were completed. In VISIBLE I, 216 subjects with moderate to severe UC [ulcerative colitis] were included; vedolizumab 300 mg IV was initially administered at week 0 and week 2 and subsequently at week 6, randomising responders to 108 mg of vedolizumab SC every 2 weeks, 300 mg of vedolizumab IV every 8 weeks, or placebo, to assess response at week 52.6 In the VISIBLE II trial, subjects with moderate and severe CD [Crohn’s Disease] were included; after induction with vedolizumab IV, the 410 responding subjects were randomised at week 6 to 108 mg of SC vedolizumab every 2 weeks or placebo, and response was assessed at week 52.7 In both clinical trials, vedolizumab SC was shown to be superior to placebo in clinical and endoscopic remission (VISIBLE I 46.2 vs. 14.3% and VISIBLE II 48.0 vs. 34.3%).6,7
The SC route of administration can provide more independence for the patient, avoiding transfer to the day hospital and possible inconveniences associated with it (absence from work/studies, exposure to hospital multi-resistant microorganisms or risk of SARS-CoV-2 infection).8,9 The main drawback of this route of administration is the possible loss of adherence to treatment. No serious adverse events associated with SC treatment have been described and the most frequently reported event is local skin reactions at the injection site.10
The present study aims to evaluate the transition from vedolizumab IV to SC in subjects with IBD on maintenance treatment. The objective is to determine both the clinical and analytical evolution as well as the drug levels in the blood, and to learn patients’ opinion after the change in the usual administration route.
Material and methodsStudy designA single-site, prospective, observational cohort study was conducted between November 2022 and August 2023. Subjects over 18 years of age with an established diagnosis of CD or UC, being followed up in the IBD unit of La Paz University Hospital (Madrid, Spain), were selected. To be included in the study, subjects had to be on stable maintenance treatment with vedolizumab, considered stable for a minimum of 4 mo without a change in dose or administration interval.
Only subjects in whom the indication for treatment was the control of luminal disease were included. Subjects with different indications (prevention of postoperative recurrence without inflammatory activity, complex perianal disease or pouchitis) were not included in the study.
Subjects meeting the inclusion criteria were offered a switch to SC treatment. All subjects who met the criteria and agreed to participate in the study were included, whether they wished to switch to SC formulation or preferred to continue with IV.
Participants who continued IV treatment maintained the regimen they were on prior to inclusion in the study (300 mg IV every 4, 6, or 8 weeks). Subjects who switched routes of administration began receiving 108 mg SC every 2 weeks, regardless of the IV regimen they were previously receiving.
Study proceduresAll subjects included in the study underwent analytical determination before each administration of the drug, including acute phase reactants to monitor inflammatory activity (leukocytes, C-reactive protein [CRP], haemoglobin, platelets, albumin and fibrinogen), trough levels of vedolizumab, antivedolizumab antibodies and measurement of faecal calprotectin. Subjects who agreed to switch to SC were analysed at weeks 0, 4, 8 and 16. In group IV, at weeks 0, 8 and 16.
Vedolizumab levels were measured using the enzyme-linked immunosorbent assay (ELISA) methodology in the hospital’s immunology laboratory. In the SC group, levels at week 0 correspond to the trough levels of the final dose of IV vedolizumab received.
For subjects who switched to SC treatment, a visit was scheduled in the IBD nursing clinic for the administration of the first dose of SC vedolizumab, and to answer possible questions about the dose change.
The presence of side effects directly or indirectly related to treatment with vedolizumab and the route of administration were collected to determine the safety of the treatment.
At the first visit, all subject received a questionnaire about the reasons why they had decided to change or maintain the route of administration of the drug. To objectively determine clinical activity and measure drug response, where appropriate, validated clinical indices were used, such as the Harvey-Bradshaw Index (HBI) for CD and the Simple Clinical Colitis Activity Index (SCCAI) for UC. Subject satisfaction was assessed using the TSQM questionnaire, and quality of life was assessed using the IBDQ-9 questionnaire.
Clinical response was considered a decrease of ≥ 3 points in the HBI compared to baseline and a decrease of ≥2 points in the SCCAI score. The definition of clinical remission was an HBI < 5 and a SCCAI ≤ 2 points, and biological remission for faecal calprotectin results <250 µg/g (<100 µg/g in subjects with ileal CD) and CRP < 5 g/dl.
Statistical analysisA descriptive analysis of the subjects’ baseline characteristics and those related to IBD was performed. For continuous variables, the mean and standard deviation were calculated; for categorical variables, the percentages and 95% confidence intervals were assessed. Provided that the variables had a normal distribution (verified using the Shapiro-Wilk test), the categorical variables were compared using the χ2 test, and the quantitative variables using the Student’s t test. Otherwise, the corresponding non-parametric test was applied. A p-value <0.05 was considered statistically significant. Statistical analyses were performed with Stata version 16.1.
Ethical considerationsThe study was conducted in accordance with the principles laid down in the 1975 Declaration of Helsinki and European Union directives. The study was approved by the Ethics Committee of the Hospital Universitario La Paz (HULP ID PI-5367). All subjects included signed an informed consent form.
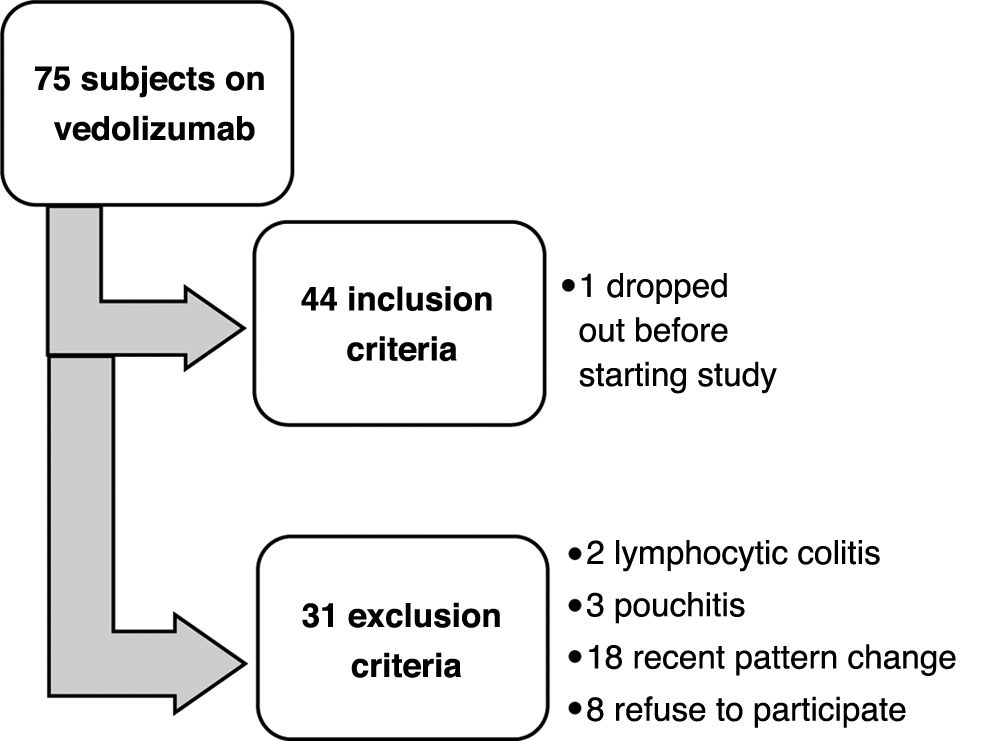
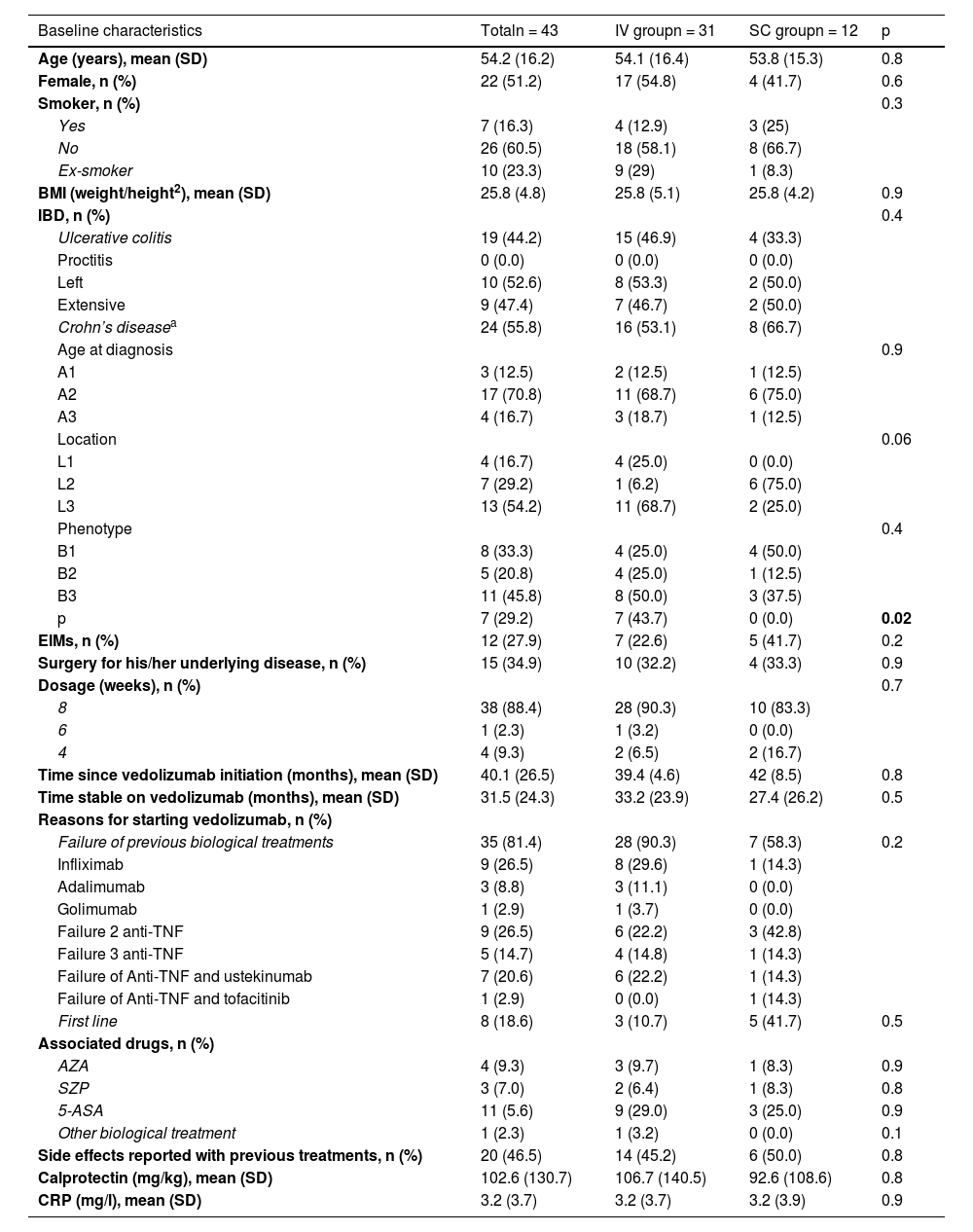
ResultsForty-three subjects were included; Fig. 1 shows the flow chart of subject selection for the study. Thirty-one subjects remained on IV vedolizumab and 12 decided to switch to SC. The baseline characteristics of the sample are found in Table 1.
Baseline characteristics from the sample.
| Baseline characteristics | Totaln = 43 | IV groupn = 31 | SC groupn = 12 | p |
|---|---|---|---|---|
| Age (years), mean (SD) | 54.2 (16.2) | 54.1 (16.4) | 53.8 (15.3) | 0.8 |
| Female, n (%) | 22 (51.2) | 17 (54.8) | 4 (41.7) | 0.6 |
| Smoker, n (%) | 0.3 | |||
| Yes | 7 (16.3) | 4 (12.9) | 3 (25) | |
| No | 26 (60.5) | 18 (58.1) | 8 (66.7) | |
| Ex-smoker | 10 (23.3) | 9 (29) | 1 (8.3) | |
| BMI (weight/height2), mean (SD) | 25.8 (4.8) | 25.8 (5.1) | 25.8 (4.2) | 0.9 |
| IBD, n (%) | 0.4 | |||
| Ulcerative colitis | 19 (44.2) | 15 (46.9) | 4 (33.3) | |
| Proctitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Left | 10 (52.6) | 8 (53.3) | 2 (50.0) | |
| Extensive | 9 (47.4) | 7 (46.7) | 2 (50.0) | |
| Crohn’s diseasea | 24 (55.8) | 16 (53.1) | 8 (66.7) | |
| Age at diagnosis | 0.9 | |||
| A1 | 3 (12.5) | 2 (12.5) | 1 (12.5) | |
| A2 | 17 (70.8) | 11 (68.7) | 6 (75.0) | |
| A3 | 4 (16.7) | 3 (18.7) | 1 (12.5) | |
| Location | 0.06 | |||
| L1 | 4 (16.7) | 4 (25.0) | 0 (0.0) | |
| L2 | 7 (29.2) | 1 (6.2) | 6 (75.0) | |
| L3 | 13 (54.2) | 11 (68.7) | 2 (25.0) | |
| Phenotype | 0.4 | |||
| B1 | 8 (33.3) | 4 (25.0) | 4 (50.0) | |
| B2 | 5 (20.8) | 4 (25.0) | 1 (12.5) | |
| B3 | 11 (45.8) | 8 (50.0) | 3 (37.5) | |
| p | 7 (29.2) | 7 (43.7) | 0 (0.0) | 0.02 |
| EIMs, n (%) | 12 (27.9) | 7 (22.6) | 5 (41.7) | 0.2 |
| Surgery for his/her underlying disease, n (%) | 15 (34.9) | 10 (32.2) | 4 (33.3) | 0.9 |
| Dosage (weeks), n (%) | 0.7 | |||
| 8 | 38 (88.4) | 28 (90.3) | 10 (83.3) | |
| 6 | 1 (2.3) | 1 (3.2) | 0 (0.0) | |
| 4 | 4 (9.3) | 2 (6.5) | 2 (16.7) | |
| Time since vedolizumab initiation (months), mean (SD) | 40.1 (26.5) | 39.4 (4.6) | 42 (8.5) | 0.8 |
| Time stable on vedolizumab (months), mean (SD) | 31.5 (24.3) | 33.2 (23.9) | 27.4 (26.2) | 0.5 |
| Reasons for starting vedolizumab, n (%) | ||||
| Failure of previous biological treatments | 35 (81.4) | 28 (90.3) | 7 (58.3) | 0.2 |
| Infliximab | 9 (26.5) | 8 (29.6) | 1 (14.3) | |
| Adalimumab | 3 (8.8) | 3 (11.1) | 0 (0.0) | |
| Golimumab | 1 (2.9) | 1 (3.7) | 0 (0.0) | |
| Failure 2 anti-TNF | 9 (26.5) | 6 (22.2) | 3 (42.8) | |
| Failure 3 anti-TNF | 5 (14.7) | 4 (14.8) | 1 (14.3) | |
| Failure of Anti-TNF and ustekinumab | 7 (20.6) | 6 (22.2) | 1 (14.3) | |
| Failure of Anti-TNF and tofacitinib | 1 (2.9) | 0 (0.0) | 1 (14.3) | |
| First line | 8 (18.6) | 3 (10.7) | 5 (41.7) | 0.5 |
| Associated drugs, n (%) | ||||
| AZA | 4 (9.3) | 3 (9.7) | 1 (8.3) | 0.9 |
| SZP | 3 (7.0) | 2 (6.4) | 1 (8.3) | 0.8 |
| 5-ASA | 11 (5.6) | 9 (29.0) | 3 (25.0) | 0.9 |
| Other biological treatment | 1 (2.3) | 1 (3.2) | 0 (0.0) | 0.1 |
| Side effects reported with previous treatments, n (%) | 20 (46.5) | 14 (45.2) | 6 (50.0) | 0.8 |
| Calprotectin (mg/kg), mean (SD) | 102.6 (130.7) | 106.7 (140.5) | 92.6 (108.6) | 0.8 |
| CRP (mg/l), mean (SD) | 3.2 (3.7) | 3.2 (3.7) | 3.2 (3.9) | 0.9 |
AZA: azathioprine; SD: standard deviation; IBD: inflammatory bowel disease; BMI: body mass index; EIM: extraintestinal manifestations; CRP: C-reactive protein; SZP: salazopyrines; TNF: tumor necrosis factor; 5-ASA: mesalazines.
In bold p < 0.05, statistically significant differences.
Montreal Classification of Crohn’s disease: A1: age at diagnosis ≤16 years; A2: age at diagnosis between 17–40 years; A3: age at diagnosis >40 years; L1: ileal involvement; L2: colon involvement; L3: ileocolic involvement; B1: inflammatory type; B2: stenosing type; B3: fistulising type; p: perianal involvement.
Among the 31 subjects (72.1%) who decided not to change to SC, the main reason was the fear of needles (17/31; 54.8%). Twelve patients (38.7%) opted not to change to SC for fear that it would not be as effective, and the next most common reason was the feeling of security in receiving it in the day hospital. (7/31; 22.6%). However, subjects in the SC group preferred this route of administration to save trips to the day hospital (5/12; 41.7%) and for the convenience of self-administration (3/12; 25.0%). From the SC group, 10 subjects (83.3%) had previously been administered SC medication, compared to 21 (67.7%) of the IV group (p = 0.14). Fourteen subjects (45,2%) in the IV group and 6 (50%) in the SC group had previously had to miss their studies or work to receive the medication.
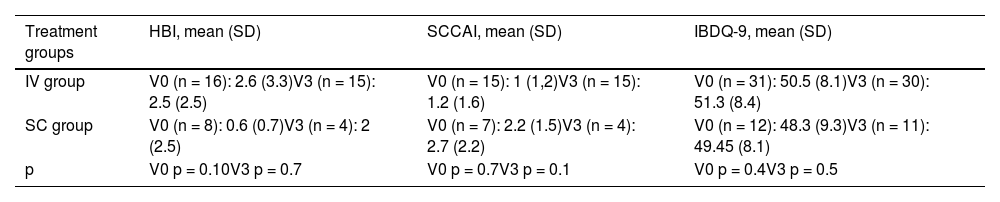
At the beginning of the study, subjects in both groups were in clinical remission and remained in remission until week 16; Table 2 shows the clinical indices (HBI and SCCAI) of both groups at the beginning and end of the study. No significant differences were found between the two groups (p > 0.05). Regarding the IBDQ-9 questionnaire, the results remained stable throughout the study (Table 2) and no significant differences were found between groups (p > 0.05).
Clinical indices (HBI/SCCAI) and IBDQ-9 at baseline (V0) and at week 16 (V3).
| Treatment groups | HBI, mean (SD) | SCCAI, mean (SD) | IBDQ-9, mean (SD) |
|---|---|---|---|
| IV group | V0 (n = 16): 2.6 (3.3)V3 (n = 15): 2.5 (2.5) | V0 (n = 15): 1 (1,2)V3 (n = 15): 1.2 (1.6) | V0 (n = 31): 50.5 (8.1)V3 (n = 30): 51.3 (8.4) |
| SC group | V0 (n = 8): 0.6 (0.7)V3 (n = 4): 2 (2.5) | V0 (n = 7): 2.2 (1.5)V3 (n = 4): 2.7 (2.2) | V0 (n = 12): 48.3 (9.3)V3 (n = 11): 49.45 (8.1) |
| p | V0 p = 0.10V3 p = 0.7 | V0 p = 0.7V3 p = 0.1 | V0 p = 0.4V3 p = 0.5 |
SD: standard deviation; HBI: Harvey-Bradshaw index; IBDQ-9: Inflammatory Bowel Disease Quality of Life Questionnaire, short version; SCCAI: Simple Clinical Colitis Activity Index.
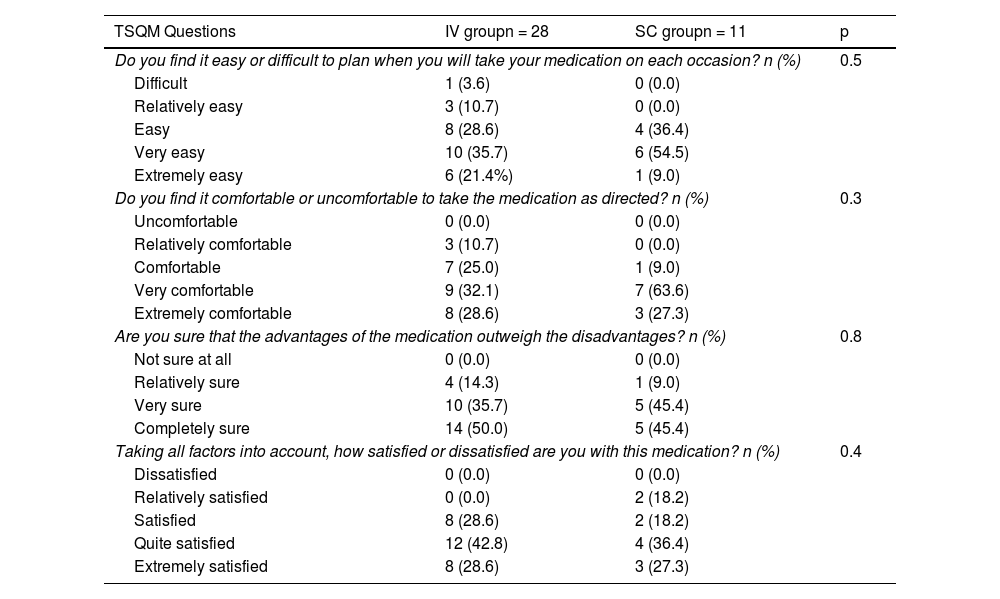
At week 16, 9 (75%) of the subjects in the SC group were satisfied with the medication and 11 (91.7%) considered their administration easy and comfortable. Table 3 shows the most relevant results of the TSQM questionnaire in week 16. During follow-up, one subject (8.3%) of the SC group opted to return to IV treatment. Regarding side effects, 4 subject (12.9%) in the IV group and 2 (16.6%) in the SC group presented mild adverse effects. No serious adverse events were recorded during follow-up. In the SC group, in both cases (100%) the adverse event was local inflammation at the injection site.
Results extracted from the TSQM at week 16.
| TSQM Questions | IV groupn = 28 | SC groupn = 11 | p |
|---|---|---|---|
| Do you find it easy or difficult to plan when you will take your medication on each occasion? n (%) | 0.5 | ||
| Difficult | 1 (3.6) | 0 (0.0) | |
| Relatively easy | 3 (10.7) | 0 (0.0) | |
| Easy | 8 (28.6) | 4 (36.4) | |
| Very easy | 10 (35.7) | 6 (54.5) | |
| Extremely easy | 6 (21.4%) | 1 (9.0) | |
| Do you find it comfortable or uncomfortable to take the medication as directed? n (%) | 0.3 | ||
| Uncomfortable | 0 (0.0) | 0 (0.0) | |
| Relatively comfortable | 3 (10.7) | 0 (0.0) | |
| Comfortable | 7 (25.0) | 1 (9.0) | |
| Very comfortable | 9 (32.1) | 7 (63.6) | |
| Extremely comfortable | 8 (28.6) | 3 (27.3) | |
| Are you sure that the advantages of the medication outweigh the disadvantages? n (%) | 0.8 | ||
| Not sure at all | 0 (0.0) | 0 (0.0) | |
| Relatively sure | 4 (14.3) | 1 (9.0) | |
| Very sure | 10 (35.7) | 5 (45.4) | |
| Completely sure | 14 (50.0) | 5 (45.4) | |
| Taking all factors into account, how satisfied or dissatisfied are you with this medication? n (%) | 0.4 | ||
| Dissatisfied | 0 (0.0) | 0 (0.0) | |
| Relatively satisfied | 0 (0.0) | 2 (18.2) | |
| Satisfied | 8 (28.6) | 2 (18.2) | |
| Quite satisfied | 12 (42.8) | 4 (36.4) | |
| Extremely satisfied | 8 (28.6) | 3 (27.3) | |
TSMQ: Treatment Satisfaction Questionnaire with Medications.
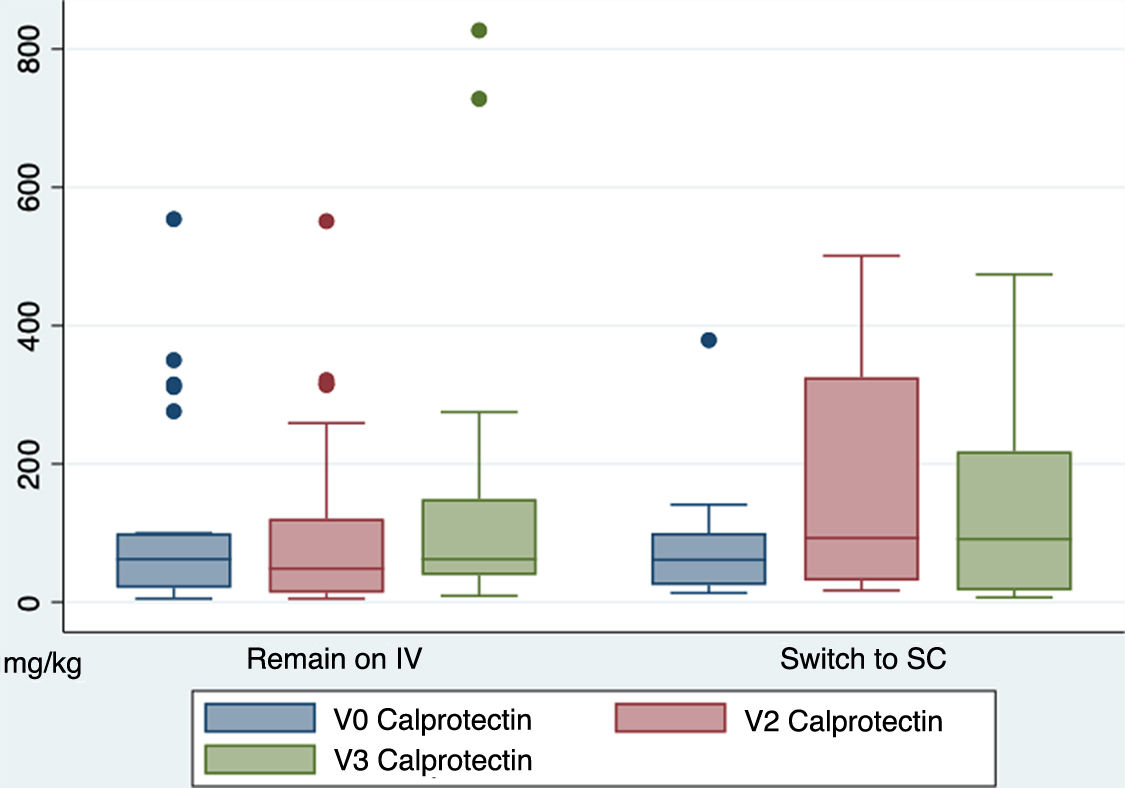
Regarding the biochemical response, faecal calprotectin levels (mg/kg) remained stable between both groups throughout the study, with no significant differences. The mean faecal calprotectin levels of the IV group at V0 were 106.7 (SD 140.5) and those of the SC group were 92.58 (SD 108.6) (p = 0.77). At V3, mean calprotectin levels in the IV group were 146.6 (SD 215.6) and in the SC group 159.3 (SD 170.5) (p = 0.87). Fig. 2 shows the differences in faecal calprotectin levels between both groups throughout the study.
CPR levels (mg/l) also remained stable between both groups, with no statistically significant differences. At V0 the IV group had a mean PCR of 3.2 (SD 3.7) and the SC group 3.2 (SD 3.9) (p = 0.99); At V3, the IV group had 2.9 (SD 3.0) and SC 3.8 (SD 4.6) (p = 0.44).
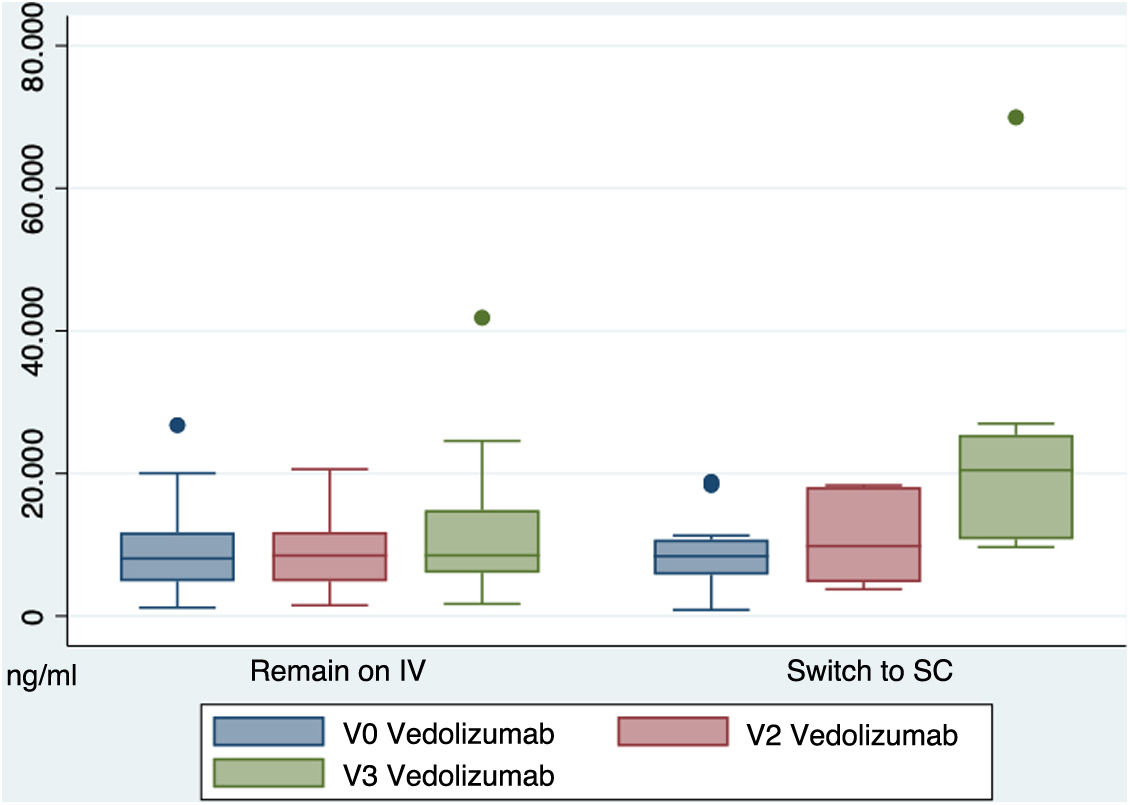
Regarding the trough levels of vedolizumab in the blood (µg/ml), at the beginning of the study in the IV group, these were 8.6 (SD 5.5) and in the SC group 9.1 (SD 5.2) (p = 0.80). At week 16, the SC group had higher blood levels of vedolizumab than the IV group, with statistically significant differences (IV 11.4 ± 8.15 vs. SC 22.4 ± 17.1). (p = 0.009). Fig. 3 shows the differences in blood levels of vedolizumab between the two groups.
During follow-up, no subject presented anti-vedolizumab antibodies.
DiscussionThis is a prospective cohort study that included 43 subjects who were on stable maintenance treatment with vedolizumab, 12 of whom agreed to change to SC and 31 remained on their usual IV regimen. After 16 weeks of follow-up, no differences were found between the two groups in terms of clinical remission or rate of adverse events. Furthermore, most subjects in the SC group were satisfied with the change, highlighting the ease and convenience of administration.
Bergqvist et al. included in their study 85 subjects on vedolizumab maintenance treatment who switched to SC therapy; at 6 mo both clinical indices and biochemical parameters (calprotectin and CRP) remained stable and most subjects were satisfied with the change.11 The study “Transitioning from intRavenous to subcutAneous VEdolizumab in patients with infLammatory bowEl diSeaSe –TRAVELESS” included 124 subjects receiving IV vedolizumab, 81 of whom agreed to switch to SC; after 12 weeks, no significant differences were found regarding clinical activity between the two groups, so it was concluded that both administration routes are similar in terms of effectiveness. In addition, SC treatment resulted in savings in treatment-related costs.9 In the study by Wiken et al., 92.6% of subjects remained on SC treatment for 6 mo after transition, with no changes in clinical or biochemical activity or quality of life.12 In the post-hoc analysis of the VISIBLE studies of subject who transitioned to SC at week 48, 64% had clinical remission and 72% had clinical response.13
In the VISIBLE I trial, vedolizumab SC levels were higher than those in the IV group.6 Furthermore, in both the IV and SC groups, higher levels correlated with a higher proportion of clinical and endoscopic remission.6 Also in the TRAVELESS study, a statistically significant increase in vedolizumab levels was observed in subjects with SC treatment versus IV at week 12, with a greater proportion of subjects in remission with higher concentrations of the drug in the blood.9 In the study by Bergqvist et al., mean trough levels of vedolizumab were 2.3 times higher after switching to SC compared with the previous levels with IV treatment.11 In our study, a significant increase in serum vedolizumab trough levels is also observed at week 16 in the SC treatment arm.
Treatment with vedolizumab has demonstrated long-term endoscopic and histological cure in subjects with IBD14; Higher serum levels of vedolizumab could correlate with a higher and earlier mucosal healing rate.15 In a post-hoc analysis of data from the GEMINI 1 trial, it was observed that during vedolizumab induction, subjects who achieved levels greater than 37.1 μg/l in week 6 achieved clinical remission in week 14, with significant differences compared to those with lower levels.16 In addition, this study proposes a theoretical objective for maintenance treatment with vedolizumab IV to have serum trough levels above 12.7 μg/l.16 The theory for higher trough levels of vedolizumab in patients on SC treatment is that fewer fluctuations in the drug in the blood might occur when receiving 108 mg SC every 2 weeks instead of 300 mg via IV every 8 weeks. However, the mean serum vedolizumab concentration would be similar in both cases.9,17
The SC route has notable administration advantages over the IV route, such as avoiding visits to the hospital and offering flexibility to the patient.18 It is worth noting that in this study, 72.1% of subjects refused to change to the SC route, mainly due to their rejection of self-administration and the sense of security of it being administered in the hospital. In the TRAVELESS study, approximately 30% of patients refused transition for reasons similar to the above.9 In this study no patient with perianal disease (p = 0.02) nor CD with ileal location (p = 0.06) opted to change to SC, which could reflect that more refractory subjects chose to remain in the IV group, although further studies would be necessary to confirm these data. On the other hand, no statistically significant differences were found between subgroups regarding the type of IBD, failure of previous biologic treatments or side effects of other treatments.
Among the main predictive factors when it comes to preferring the SC route are having received SC treatment previously and being older.19 Another predictive factor in the acceptance of the transition to the SC route is a shorter duration of IV treatment.18 However, in our study, no significant differences were found between both groups regarding age, previous SC treatment or time since the start of vedolizumab. Considering the above reasons, it is important to adequately select patients who are candidates for the SC route, individualise the administration route according to the patient's preferences, and take into account their history of adherence to treatment, since one of the disadvantages of this route is the difficulty in monitoring the correct administration of the drug.20,21
In our study, one of the subjects in the SC group discontinued treatment during follow-up because he preferred the IV route. In the study by Bergqvist et al., a treatment discontinuation rate due to local adverse events was reported, and in the study by Wilken et al., there was a 7% 6-mo discontinuation rate.11,12 Further studies are needed to determine adherence to vedolizumab SC.
Regarding adverse events of treatment with vedolizumab SC, in the VISIBLE trials, the most frequently reported adverse event in the SC group was mild local reaction at the administration site (10.4 vs. 1.9% in the IV route vs. 0% in placebo).6,7 Similar results are shown in our study, in which only 2 subjects (16.6%) presented adverse effects and in both cases (100%) it was a local reaction at the injection site. The rate of serious adverse events in the SC group in the VISIBLE trials was 9.4 and 8.4%.6,7 No serious adverse events were reported in the aforementioned studies by Ventress et al. and Bergqvist et al., nor in our study.9,11
Local reaction in SC treatment is a frequent adverse event, dependent on multiple factors of both the drug and the patient, and is a determining factor in the quality of life and adherence to treatment.22 The presence of citrate in the composition has been proposed as an aggravating factor for pain, although there is insufficient evidence to support this.23
In patients treated with vedolizumab SC who present local reactions and return to the IV route, isolated cases of allergic reactions have been described during the first IV infusion (urticaria, erythema at the previous SC injection site, angioedema and hypotension), which in some cases have led to discontinuation of the drug.24–26 Further studies are needed to determine the pathophysiology of this association.
Our study is prospective and compares clinical, biochemical variables and serum levels in patients who maintain vedolizumab IV versus those who transition to SC. The main limitations are the sample size, the follow-up time limited to 16 weeks, not having endoscopic evaluation available, and not having been able to calculate the cost-effectiveness differences between both groups.
In conclusion, this study is consistent with current evidence for Vedolizumab SC in real life. It shows that switching from vedolizumab IV to SC in maintenance therapy for stable patients is possible and there are no differences in efficacy. Furthermore, patients on SC treatment are satisfied with the administration route, and serum trough levels of vedolizumab are significantly higher than in the IV group. Further prospective studies are needed to compare treatment with vedolizumab SC and IV and demonstrate that higher serum drug levels imply greater mucosal healing.
Ethical considerationsThe study was conducted in accordance with the principles laid down in the 1975 Declaration of Helsinki and European Union directives. The study was approved by the Ethics Committee of the Hospital Universitario La Paz (HULP ID PI-5367). All subjects signed an informed consent form in order to take part in the study.
FundingThis study has received no specific funding from public, private or non-profit organisations.