To describe in detail the epidemiology, diagnosis, clinical management, treatment options, impact on quality of life and unmet needs of patients with advanced liver fibrosis (F3–F4) associated with non-alcoholic steatohepatitis (NASH) in Spain.
MethodologyDelphi study of two rounds of consultation rounds with 41 expert hepatologists from 16 autonomous communities to collect their experience in clinical practice.
ResultsThe estimated prevalence of adult patients diagnosed with F3–F4 fibrosis associated with NASH in Spain is 0.019% (95% confidence interval [CI]: 0.019−0.020%). Approximately 7588 adults with this condition are currently diagnosed and managed in the Digestive System Services of Spanish hospitals, and around 1881 new patients are diagnosed each year. Management is multidisciplinary and includes the specialties of Digestive System, Endocrinology and Internal Medicine, considering the frequently associated metabolic comorbidities (obesity, type 2 dia-betes mellitus or dysmetabolic iron overload). Despite a clear impact on quality of life, this it is not routinely evaluated in clinical practice. The most widely used non-invasive diagnostic tech-niques are transitional elastography and liver fibrosis index 4 (FIB-4). The absence of effective and safe treatments appears as the main unmet need for the management of these patients.
ConclusionsThis study provides a representation of the current situation of patients diagno-sed with F3-F4 fibrosis associated with NASH in Spain, increasing the evidence available and contributing to informed decision-making by professionals and the health system.
Describir de manera detallada la epidemiología, diagnóstico, manejo clínico, opciones de tratamiento, impacto en calidad de vida y necesidades no cubiertas de los pacientes con fibrosis hepática avanzada asociada a esteatohepatitis no alcohólica (NASH) en España.
MetodologíaEstudio Delphi de dos rondas de consulta con 41 hepatólogos expertos de 16 comunidades autónomas para recoger su experiencia en práctica clínica.
ResultadosLa prevalencia estimada de pacientes adultos diagnosticados de fibrosis avanzada asociada a NASH en España es de 0,019% (IC95%: 0,019%-0,020%). Aproximadamente 7.588 pacientes adultos con fibrosis avanzada asociada a NASH están actualmente diagnosticados y son manejados en los Servicios de Aparato Digestivo de los hospitales españoles, y aproximadamente 1.881 nuevos pacientes son diagnosticados cada año. El manejo de los pacientes es multidisciplinar e incluye las especialidades de Digestivo, Endocrinología y Medicina Interna, considerando las frecuentes comorbilidades metabólicas asociadas (obesidad, diabetes mellitus tipo 2 o sobrecarga férrica dismetabólica). A pesar del claro impacto en calidad de vida, éste no se evalúa rutinariamente en práctica clínica. Las técnicas diagnósticas no invasivas más utilizadas son la elastografía de transición y el índice de fibrosis hepática FIB-4. La ausencia de tratamientos eficaces y seguros se presenta como la principal necesidad no cubierta para el manejo de estos pacientes.
ConclusionesEste estudio proporciona una representación de la situación actual de los pacientes diagnosticados con fibrosis avanzada asociada a NASH en España, incrementando la evidencia disponible y contribuyendo a la toma de decisiones informadas por parte de los profesionales y el sistema sanitario.
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of long-term liver damage worldwide, with an estimated prevalence of 25%, and is generally associated with metabolic risk factors such as obesity, diabetes and dyslipidaemia.1–5 Non-alcoholic hepatic steatosis (NASH), with a prevalence of 3–5%,5 is an advanced stage of NAFLD characterised by intrahepatic fat accumulation and a pro-inflammatory state. It is in these NASH patients where we find the most advanced stages of fibrosis (approximately 20% with advanced fibrosis [F3–F4]),6–9 which leads to end-stage liver disease, hepatocellular carcinoma and can even lead to liver transplantation or death.10
Establishing the epidemiological characteristics of NASH is therefore extremely important. However, the results of epidemiological studies published to date are very limited and diverse, probably due to the large variability in the populations studied and in the way the disease is managed.5,11–13 A recent nationwide study provides somewhat more reliable estimates of the epidemiology in the Spanish population, with prevalences of patients with NASH stage F2-F3 and cirrhosis of 1.33% (95% confidence interval [CI]: 0.29–5.98 %) and 0.70% (95% CI: 0.10–4.95 %) respectively.14 It is important to highlight that, although for the management of patients with NASH, the "gold standard" diagnostic technique is liver biopsy, this is associated with major ethical, practical and safety dilemmas in routine clinical practice, and its use therefore tends to be avoided.15 A number of national and international consensus documents have been published on the management of patients with NASH,4,13,16–18 but it is not known to what extent these guidelines are followed in routine clinical practice.
The Delphi technique is a qualitative research method based on obtaining expert opinions on a real clinical practice problem. The technique allows consensus to be reached or the reality of a particular clinical problem to be described through anonymous group interaction based on a series of consultation meetings. Experts can reconsider their views based on the contribution of others, thus allowing for an element of reflection not found in studies based on individual interviews.19,20
In summary, this is a disease with a high incidence and prevalence, in which patient management is not fully optimised. Knowledge of the current situation involving patients with NASH in Spain could therefore help provide healthcare professionals with a better understanding of the disease, while identifying the main needs, improving patient management, and helping healthcare systems to make informed decisions at a time of increasing financial and structural pressure. The aim of this study was to depict the epidemiology of the disease at a national level, describe the clinical management (including diagnosis and follow-up of patients), identify the therapeutic approach to these subjects, assess the impact on quality of life and describe the main unmet needs of NASH-associated F3-F4 fibrosis, based on the opinion and practical experience of and available data from Spanish physicians treating these patients.
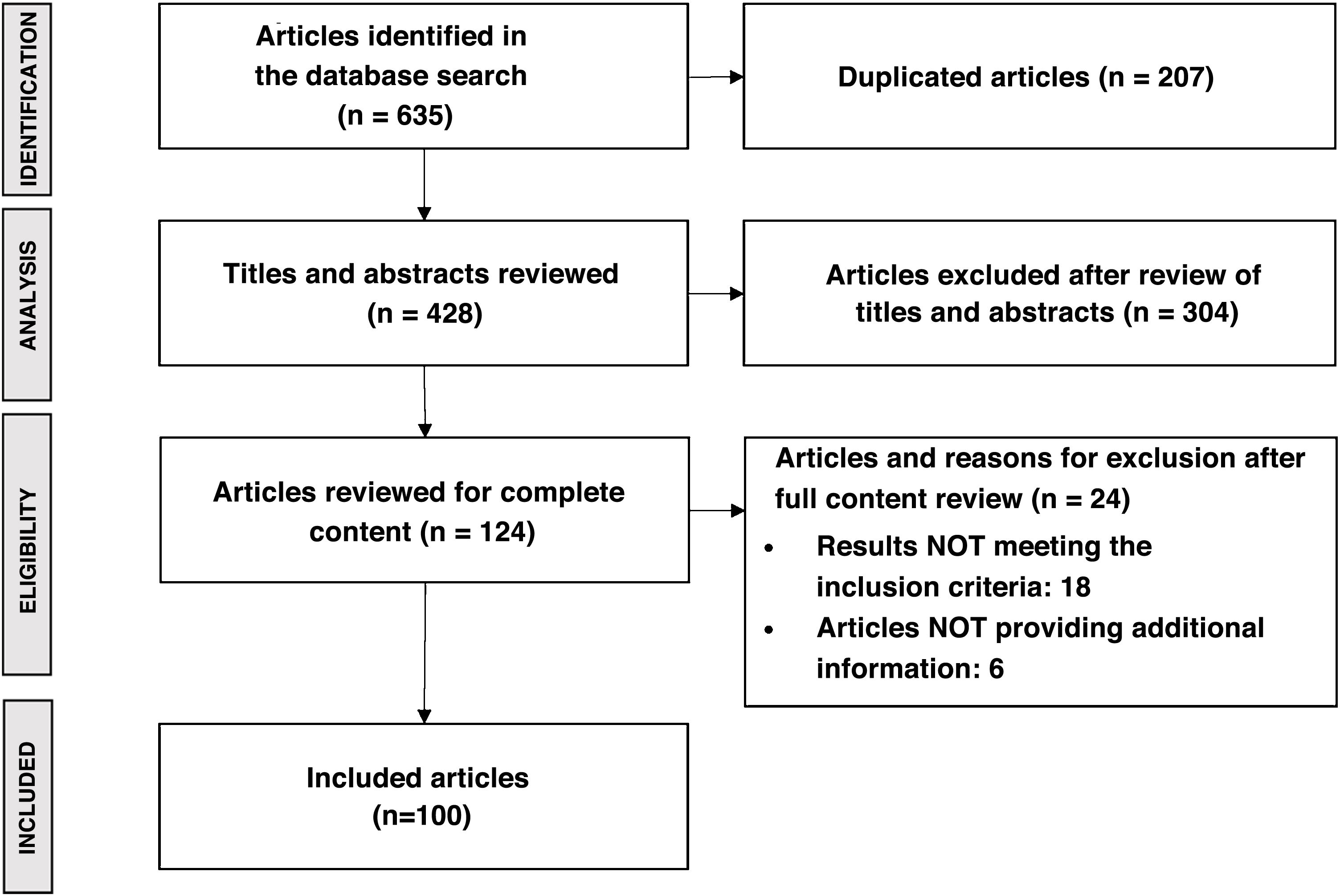
MethodsStudy designThe study was carried out using Delphi methodology, including two rounds of consultation with an expert panel of 41 national hepatologists with experience in the management of patients with NASH, from 41 hospitals in 16 of Spain's 17 autonomous regions (Fig. 1). In addition, five hepatologists were identified as high-level experts and participated in the validation of the study questionnaires and the results obtained. These experts also took part in the consultation rounds of the study, representing Spanish centres of excellence in NASH management.
The questionnaires were developed based on the results of a literature review with a final search date of November 2020. All articles published in national and international journals were included, with priority given to those in the context of the Sistema Nacional de Salud (SNS) [Spanish National Health System]. The main terms of the literature search were: epidemiology; impact on quality of life; clinical management (diagnosis and follow-up); treatment; and main unmet needs. Articles were included if they contained information relevant to describing the management of NASH-associated F3–F4 fibrosis. Exclusion criteria applied to duplicate papers, those written in a language other than English or Spanish, those that mentioned NASH-associated F3–F4 fibrosis but focused on another disease, and those that dealt with animal studies. Search sources included international databases (PubMed and Cochrane) and MEDicina en ESpañol (MEDES), the Spanish national biomedical database. The search for unconventional literature was carried out using Google and on the websites of scientific societies and patient associations related to the disease. The literature search yielded a total of 635 publications, which after review resulted in 100 articles. Fig. 2 shows the PRISMA diagram summarising the results of the literature review.21
The first-round questionnaire was developed based on the results of the literature review and was structured into the sections of epidemiology, impact on quality of life, clinical management (diagnosis and follow-up), treatment and main unmet needs of patients with NASH F3-F4 fibrosis in Spain. The second-round questionnaire was designed on the basis of the analysis of the data obtained in the first round and focused on reaching greater agreement on the questions where there was a larger dispersion of responses during the first round. The study was conducted online from January to July 2022.
Statistical analysisData analysis was performed using descriptive statistics.22 The distribution of responses for quantitative variables was determined by means of the Shapiro-Wilk and Kolmogorov-Smirnov tests of normality.23 In cases with a large dispersion of responses, results were presented including median and interquartile range (IQR).22,23 An outlier analysis was performed to detect and validate results above or below 1.5 times the IQR.24 The qualitative variable analysis was carried out by expressing the percentage of experts who selected each response option. Agreement was reached when at least 80% of the experts agreed with the answer, as indicated in the Delphi methodology.19,20 Dispersion in responses to quantitative questions was defined as a coefficient of variation (CV = standard deviation [SD]/mean) greater than 1, and in qualitative questions when fewer than 80% of respondents agreed in their answer. The first-round questionnaire included 73 questions and the second-round questionnaire 15 questions, which were those where dispersion was identified in the first-round results. Statistical analysis was performed with the IBM® SPSS® Statistics software program, version 28.0.
Epidemiology was estimated from the number of prevalent and incident patients diagnosed with NASH according to the stage of fibrosis currently managed in the Gastroenterology Department of each of the participating hospitals. Fibrosis stages were classified as significant fibrosis (F2-F4), F3-F4 and cirrhosis (F4), according to the latest clinical guidelines.16,25 The diagnosis of NASH-associated fibrosis was made through clinicians' suspicion following non-invasive testing, or a confirmatory liver biopsy. Estimates at a national level were obtained by extrapolating hospital data to the Spanish population, using the most recent data available from the Instituto Nacional de Estadística (INE) [National Statistics Institute].26
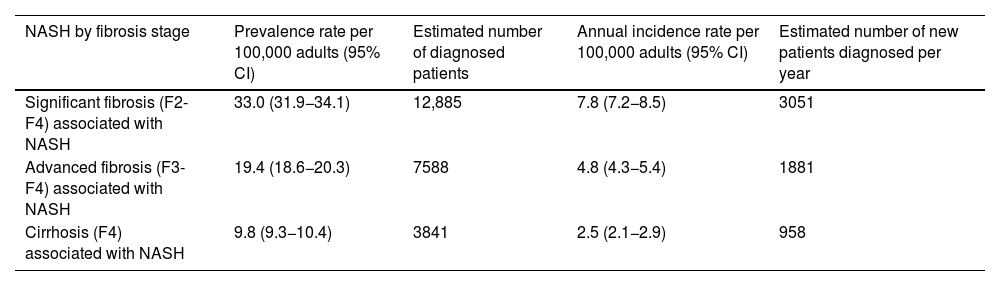
ResultsEpidemiologyApproximately half of the experts (56%) reported having a specific registry to compile cases of patients with a diagnosis of NASH at their hospital. However, the available data is limited; 15% considered they could not estimate the prevalence at their hospital and 46% that they could not estimate the incidence. The epidemiological data provided by the experts are shown in Table 1. The estimated prevalence of patients diagnosed with NASH-associated F2–F4 fibrosis is 0.033% (95% CI 0.032−0.034%) of the Spanish adult population, representing a prevalence rate of 33.0 (95% CI 31.9−34.1) patients per 100,000 adults. The estimated prevalence of NASH-associated F3–F4 fibrosis is 0.019% (95% CI 0.019−0.020%), representing a prevalence rate of 19.4 (95% CI 18.6−20.3) patients per 100,000 adults, and the prevalence of NASH-associated F4 is estimated at 0.010% (95% CI 0.009−0.010%), corresponding to a prevalence rate of 9.8 (95% CI: 9.3−10.4) patients per 100,000 adults. Considering the data available for the Spanish adult population at the time of the study (July 2020: 39,080,614 population aged ≥18),26 this means that, by fibrosis grade, there are approximately 12,885 adult patients diagnosed with F2–F4 fibrosis, 7588 with F3–F4 fibrosis and 3841 with F4 fibrosis associated with NASH currently in the Gastroenterology Departments of Spanish hospitals. About 51% of these patients may have had a confirmatory liver biopsy.
Estimated prevalence and incidence of patients diagnosed with NASH in Spain by fibrosis stage.
| NASH by fibrosis stage | Prevalence rate per 100,000 adults (95% CI) | Estimated number of diagnosed patients | Annual incidence rate per 100,000 adults (95% CI) | Estimated number of new patients diagnosed per year |
|---|---|---|---|---|
| Significant fibrosis (F2-F4) associated with NASH | 33.0 (31.9−34.1) | 12,885 | 7.8 (7.2−8.5) | 3051 |
| Advanced fibrosis (F3-F4) associated with NASH | 19.4 (18.6−20.3) | 7588 | 4.8 (4.3−5.4) | 1881 |
| Cirrhosis (F4) associated with NASH | 9.8 (9.3−10.4) | 3841 | 2.5 (2.1−2.9) | 958 |
CI, confidence interval; NASH, non-alcoholic steatohepatitis.
The incidence of NASH by fibrosis stage was also estimated. Incidence rates per 100,000 adults per year are 7.8 (95% CI 7.2−8.5) for NASH-associated F2-F4 fibrosis, 4.8 (95% CI 4.3−5.4) for NASH-associated F3–F4 fibrosis and 2.5 (95% CI 2.1−2.9) for NASH-associated F4. This means that approximately 3051 new adult patients with F2–F4 fibrosis, 1881 with F3-F4 fibrosis and 958 with F4 associated with NASH are diagnosed each year.
Impact on quality of lifeThere was agreement among experts (98%) that quality of life is affected in patients with NASH F3-F4 fibrosis. Over and above other comorbidities, the stage of fibrosis is the main factor determining the impact on quality of life, and this impact becomes greater as the disease progresses. Asthenia, mobility problems, anxiety and depression are the main factors affecting quality of life in these patients with NASH. Despite the clear impact, most experts (98% agreement) stated that they do not routinely assess the effect of NASH-associated F3-F4 fibrosis on patients' quality of life in any standardised way in their usual clinical practice, mainly due to lack of time (88% agreement).
Clinical managementThe patient's route in the Sistema Nacional de Salud [Spanish National Health System]The study made it possible to describe the patient pathway within the Spanish Health System. Primary care specialists and hospital healthcare professionals who are not hepatologists refer these subjects to the Gastroenterology Department when F3–F4 fibrosis is suspected. There was agreement (83%) that a patient with suspected NASH is referred to the Gastroenterology Department within three months of initial suspicion. The majority of experts (71% agreement) considered that it takes four to 12 weeks from the initial visit to the general Gastroenterology clinic until the NASH diagnosis is confirmed. Some experts (20% agreement) thought this period could be as long as 12–16 weeks. On average, patients make two visits to the hepatologist before NASH is confirmed. There was agreement (95%) that people with NASH F3-F4 fibrosis attend follow-up visits every six months.
The management of subjects with suspected NASH is multidisciplinary due to the coexistence with other metabolic diseases. Endocrinology specialists (63% agreement) and primary care physicians (59% agreement) are the ones most commonly involved in the management of patients with this disease, followed by internal medicine (39% agreement), who are the most appropriate specialists for treating metabolic comorbidities. Other specialities (20% agreement), such as dermatology, rheumatology, surgery (general or bariatric) or psychiatry may also participate in an optimal multidisciplinary approach to manage the disease.
Clinical practice guidelinesThe most widely used clinical practice guidelines for the management of NASH in Spain are those of the European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity (EASL-EASD-EASO 2016),17 used by 93% of experts and consulted as first option by 49%. In fact, Spanish hepatologists follow various guidelines and expert consensus documents in their clinical practice, such as that of Aller et al.13 (83% of experts), the recommendations of Caballeria et al.4 (78%), and the guidelines issued by the American Association for the Study of Liver Diseases (AASLD)18 (73%). Lastly, half (51%) of the specialists use an internal hospital protocol for the management of patients with NASH-associated fibrosis.
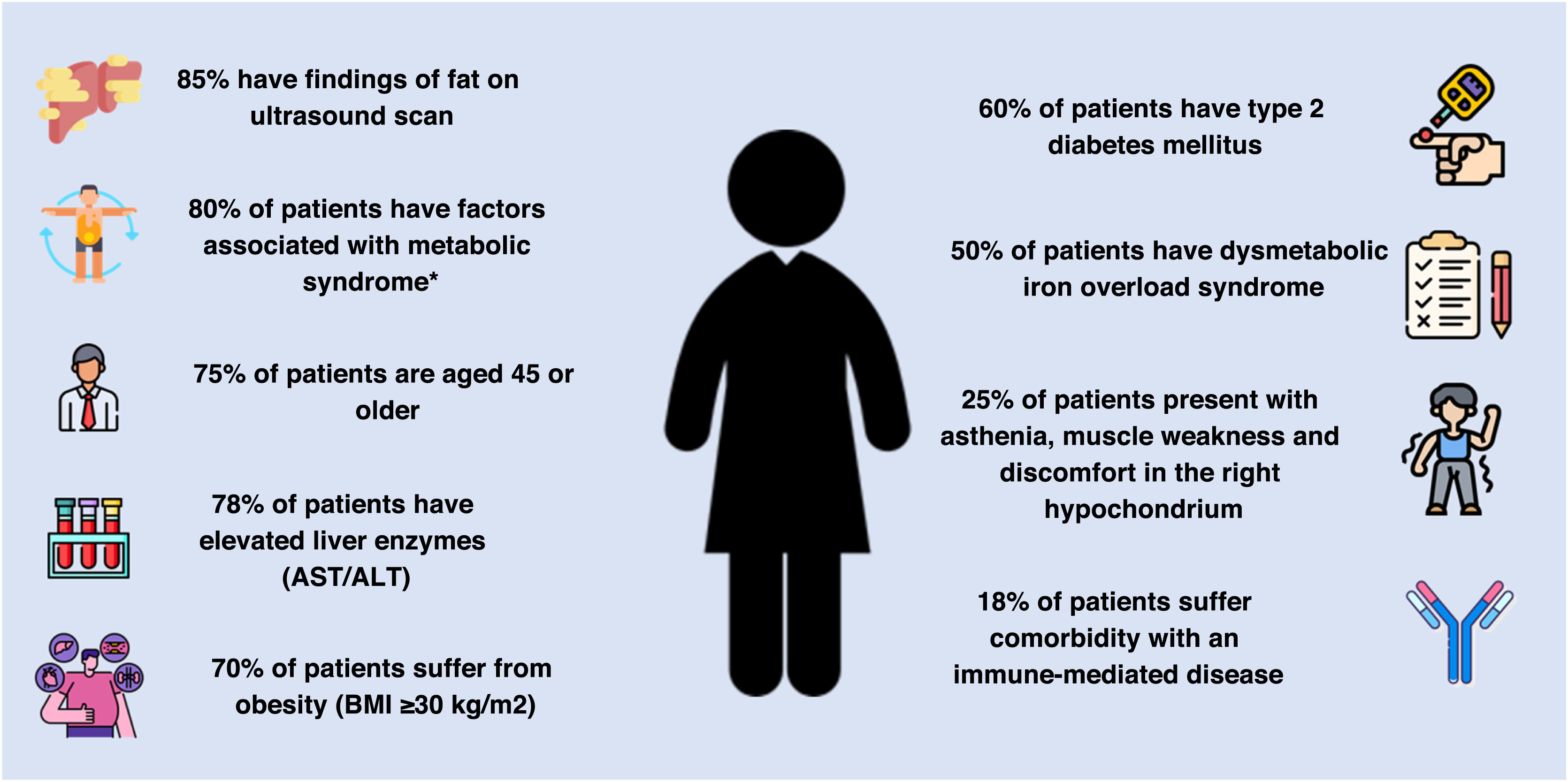
Patient profileThe most common features in patients with suspected NASH F3-F4 fibrosis include ultrasound findings of fat (85%; IQR 66–90%), factors associated with metabolic syndrome (80%; IQR 70–90%), being over the age of 45 (75%; IQR 70–80%), and having elevated liver enzymes (75%; IQR 65–85%). Obesity (70%; IQR 65–80%), type 2 diabetes mellitus (60%; IQR 50–70%) and dysmetabolic iron overload (50%; IQR 30–55%) are also common. Asthenia, muscle weakness and discomfort in the right hypochondrium (25%; IQR: 15–30%), as well as comorbidity with an immune-mediated disease (15%; IQR: 10–21%) occur to a lesser extent (Fig. 3).
Common characteristics of the patient with suspected advanced NASH-associated fibrosis.
AST/ALT: aspartate aminotransferase and alanine aminotransferase; BMI: body mass index; NASH: non-alcoholic steatohepatitis.
*Factors associated with metabolic syndrome include abdominal obesity, decreased high-density lipoprotein (HDL) cholesterol, hypertriglyceridemia, high blood pressure and fasting hyperglycaemia.
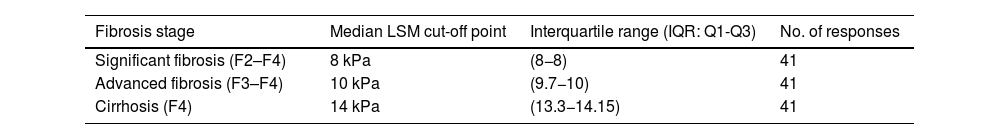
Both imaging and serological non-invasive tests (NIT) are used for the diagnosis of NASH-associated fibrosis. The most commonly used imaging NIT is transient elastography (FibroScan® Echosens, Paris, France) (95% of experts), while the most commonly used biochemical NIT is fibrosis index-4 (FIB-4) (78% of experts). There is broad agreement on the liver stiffness values on elastography considered as cut-off points for diagnosis of fibrosis stage: stiffness ≥8 kPa for F2-F4 fibrosis, stiffness ≥10 kPa for F3-F4 fibrosis; and stiffness ≥14 kPa for F4. (Table 2)
Proposed LSM thresholds as cut-off points for fibrosis stage diagnosis by transition elastography (FibroScan®).
| Fibrosis stage | Median LSM cut-off point | Interquartile range (IQR: Q1-Q3) | No. of responses |
|---|---|---|---|
| Significant fibrosis (F2–F4) | 8 kPa | (8−8) | 41 |
| Advanced fibrosis (F3–F4) | 10 kPa | (9.7−10) | 41 |
| Cirrhosis (F4) | 14 kPa | (13.3−14.15) | 41 |
LSM: liver stiffness measurement.
Determining disease progression between fibrosis stages is a major challenge, particularly from F3 to F4, even when liver biopsies are performed, as sampling error can lead to misdiagnosis. The experts reported their impressions and experience with fibrosis progression in clinical practice. Approximately 10% (IQR: 5–20%) of patients progress annually from fibrosis stages F2–F3 to F3–F4. In addition, the experts estimated that a median of 5% (IQR: 2–7%) of patients with NASH-associated F4 suffer at least one episode of decompensation per year and have to be hospitalised for a median of seven days (IQR: 6–10). Last of all, annually 2% (IQR: 1–5%) of patients with NASH-associated F3–F4 fibrosis develop hepatocellular carcinoma.
TreatmentThe current standard treatment for NASH-associated F3–F4 fibrosis is based on diet and lifestyle modifications, taking into account the metabolic origin of the disease and the absence of pharmacological treatments with an approved indication (100% agreement). However, about half (51%) of the hepatologists prescribe some drug therapy to patients, even if it is not specifically indicated for the treatment of NASH. The most prescribed treatments are statins (37% of experts), semaglutide (24%), fibrates (22%) and vitamin E (20%). These are mainly indicated to treat comorbidities associated with NASH, such as hypertension, type 2 diabetes mellitus, hypercholesterolaemia or metabolic syndrome.
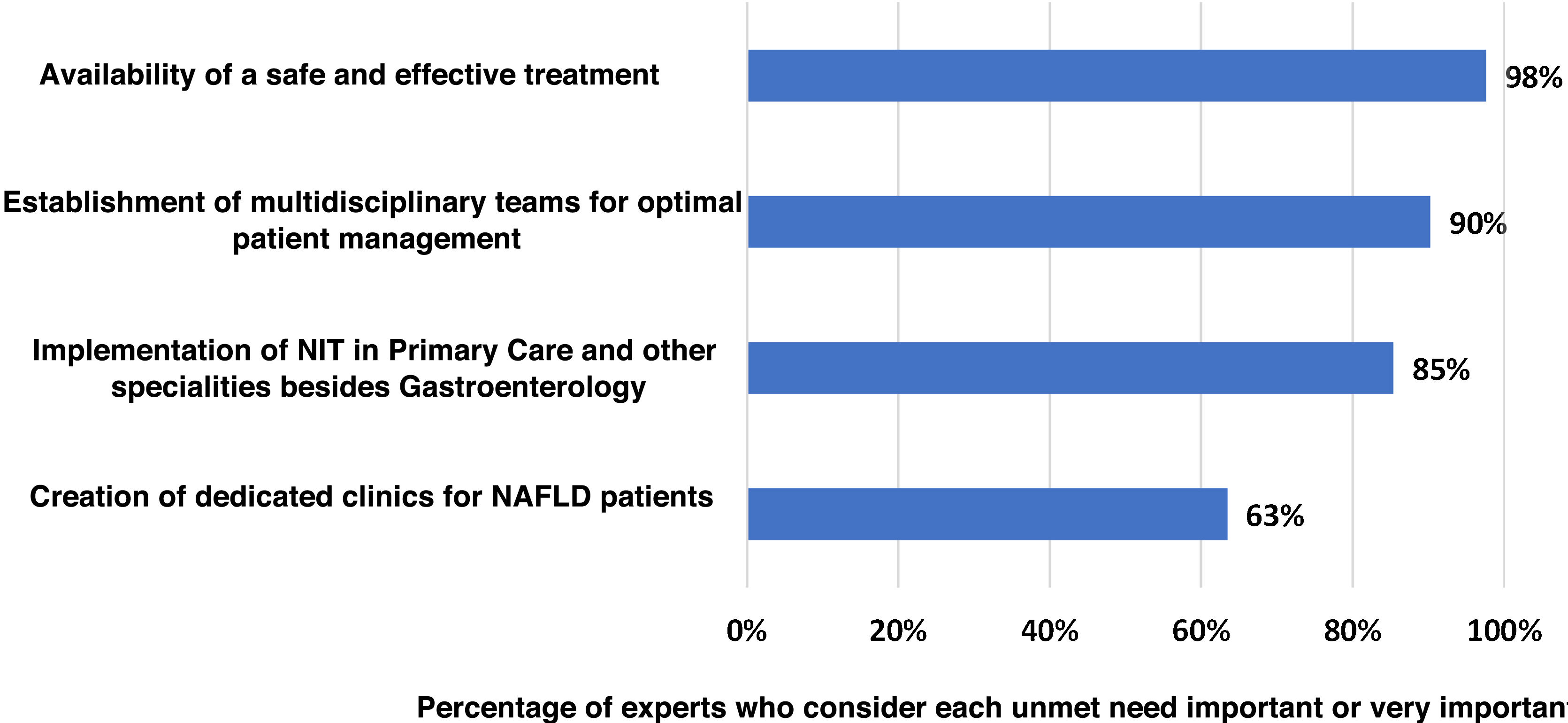
Main unmet needsThe experts agreed (100% agreement) that there are still important unmet needs in the management of NASH-associated F3–F4 fibrosis in Spain (Fig. 4). Lack of effective and safe treatment was identified as the main unmet need (98% agreement). Secondly, the experts considered the establishment of multidisciplinary teams for the management of patients (90%) as an unmet need. Lastly, introduction of the NIT in primary care and in medical specialities other than Gastroenterology (85%) and setting up dedicated clinics for patients with NASH in Gastroenterology Departments (63%) were also considered as important or very important areas for improvement.
DiscussionThis study provides an accurate and detailed overview of the epidemiology, impact on quality of life, clinical management, including diagnosis and follow-up, treatment and the main unmet needs of patients currently diagnosed with NASH-associated F3-F4 fibrosis in Spain. The results, based on the opinion and real clinical practice data of expert hepatologists, the size of the group of participants in the study (n = 41) and the broad representation of all Spain's autonomous regions (16 out of 17), have provided insight into the current approach to patients with NASH-associated F3–F4 fibrosis in Spain, helping offset the lack of evidence found in the literature.
Estimating the epidemiology of NASH is a major challenge due to the limited availability of patient registries.27 More specifically, approximately half of the experts who participated in our study do not have adequate registers to collect data on subjects with NASH, making it difficult to estimate the epidemiology in Spain accurately. In addition, the variability in the techniques and criteria used to establish a diagnosis in different Spanish hospitals, as well as the lack of validated non-invasive tests to confirm the diagnosis, are major barriers to accurately estimating the incidence and prevalence of the disease. The total number of patients with NASH (including those undiagnosed) in the Spanish population was recently estimated according to fibrosis stage.14 The results of this Delphi study contribute accurate information reported by clinical experts on subjects diagnosed with F3-F4 fibrosis due to NASH currently in the Gastroenterology Departments of Spanish hospitals. The results on the prevalence of diagnosed patients obtained in this study are in line with those reported in European studies.15,28 The difference we found between the estimated prevalence of people with NASH-associated F3–F4 fibrosis diagnosed in Spanish hospitals (0.033%) and the estimate of the total prevalence of the disease in the Spanish population (1.45%)14 is significant and reveals high levels of underdiagnosis. One study published in recent years states that only 1.3% of patients with NASH-associated F3-F4 fibrosis in Spain may have been diagnosed.15 The increasing incidence of NAFLD and its potential economic impact on healthcare systems have been widely described in the literature, including the resources needed to manage fibrosis, cirrhosis, carcinoma and liver transplantation, which are more prevalent in individuals suffering from NASH.5,29 The clinical and financial burden of the disease is significantly higher in cases which remain undiagnosed and/or untreated until the disease progresses to fibrosis stage F3–F4. Therefore, in view of the large number of undiagnosed patients, it is extremely important to improve early identification. An early diagnosis of NASH and the establishment of an appropriate course of management may contribute not only to improving the patient’s prognosis but also to significantly reducing the potential cost of managing the disease and its complications.5,29,30
The expert advisory panel agreed that early detection and diagnosis of patients with NASH-associated F3–F4 fibrosis is essential in preventing, or at least slowing, the progression of fibrosis, thus improving the long-term health outcomes for these subjects. Although liver biopsy remains the technique of choice for definitive diagnosis, its current use in clinical practice is limited. In our study, only half of the patients with suspected NASH had their diagnosis confirmed by liver biopsy. The use of this technique is limited mainly due to ethical issues, as in many cases it exposes the patient to unnecessary risk.16,17 In contrast, NIT are increasingly used because of their predictive diagnostic potential and recent clinical practice guidelines have recognised that these techniques may be able to reduce the need for liver biopsy.17,31,32 Recently published literature indicates that the implementation of NIT in primary care could be a key factor in diagnosis and referral to the gastroenterology specialist, as primary care is the first point of contact for most patients with NAFLD.33 However, access to NIT is currently very diverse and variable depending on the hospital level and region, and this should be a focus for future work to optimise the management of the disease.
Defining the patient profile is also a challenge, as the disease is very heterogeneous and associated with a range of different metabolic comorbidities. Increasing the use of NIT in clinical practice, as well as the awareness and involvement of primary care and other medical specialities, such as endocrinology and internal medicine, are key factors for early diagnosis and appropriate management of patients with NASH. This is especially important because experts agree on the significant reduction in quality of life experienced by these patients. However, they agreed that there is no standardised assessment of quality of life in clinical practice. The main reason, common with other disorders, is the lack of time to carry out these assessments. Another worrying finding provided by the study is the need to improve diagnostic criteria, which is resulting in lower rates of diagnosis or referral to the gastroenterology specialist for subjects with F3–F4 fibrosis caused by NASH. The expert panel of this Delphi study considers it important that more work is carried out on the identification of patients at primary care level and on referral to specialists in gastrointestinal medicine, in order to optimise detection and management. Several recently published studies have demonstrated benefits in liver function, cardiovascular risk and weight loss in patients treated by a multidisciplinary NAFLD unit, compared to those treated by the gastroenterology specialist alone.33–35 The lack of detailed inter-speciality referral protocols is likely caused by the absence of effective and safe treatment for patients with this disease, which has been agreed by experts as the main unmet need in the management algorithm for these patients. Currently, in the absence of approved treatment options for NASH, experts focus therapeutic management on the treatment of the associated comorbidities. In the coming years, new treatment options are expected to be approved, and this could lead to a significant change in the current therapeutic approach to patients. Lastly, mention should be made of other unmet needs of relevance in the management of people with NAFLD, such as addressing the cardiovascular risk associated with the disease, with this being one of the priorities for optimising management in the coming years.36
We should point out that the main limitations of this study are the inclusion of hospitals from different regions and levels of specialisation, as well as the diverse range of experience of the experts consulted in the management of the disease. It also has to be taken into account that our results are based on the opinion and experience of the clinical experts treating these patients, and are therefore susceptible to variability between centres and specialists. This may have resulted in a higher degree of variability in responses than if the study had only included reference centres. However, it also helps make the results obtained more relevant and representative, as they describe the real situation of clinical practice at national level and provide key information about the current situation of these patients, in the absence of other commonly used sources of information such as patient registries. Finally, it should be noted that the study participants were gastroenterology specialists, although as identified in the results, other experts are involved in the management of these patients. This study is intended as a first step with the specialists who most frequently see subjects with NASH-associated F3–F4 fibrosis, but would benefit from being repeated in a second phase involving healthcare professionals from other medical areas who are also involved.
In conclusion, this study represents the first accurate and detailed estimate of the current situation of patients diagnosed with NASH-associated F3–F4 fibrosis in Spain, based on real clinical practice data. Our results complement the available evidence and may increase knowledge and awareness among healthcare professionals from the different medical specialities involved in the management of these patients, as well as help healthcare systems to make informed decisions on the resources allocated to managing these subjects. At the time of writing, no published data related to the objectives of this study are available for Spain, making the results presented all the more relevant.
FundingThis study was funded by Advanz Pharma Specialty Medicine Spain SLU.
Conflicts of interestR. Aller, J.L. Calleja, J. Crespo, M. Romero-Gómez and J. Turnes received remuneration from Advanz Pharma Specialty Medicine Spain SLU for their participation in the study. O.
Benmarzouk-Hidalgo is an employee of Advanz Pharma Specialty Medicine Spain SLU. R. Subirán and A. Gil are employees of Omakase Consulting S.L., which received funding from Advanz Pharma Specialty Medicine Spain SLU for the conduct of the study.
The authors would like to thank the clinical experts who participated in this study for their contribution: Drs. Agustín Albillos, Raúl Andrade, Ana Arencibia, Pablo Bellot, Salvador Benlloch, Marina Berenguer, Lucía Bonet, Mª Teresa Broquetas, Marta Casado, Laura Castillo, Moisés Diago, Mª Desamparados Escudero, Pamela Estévez, Javier Fuentes, Luisa García-Buey, Isabel Graupera, Diana Horta-Sangenís, Luis Ibáñez, Nahikari Irazábal, Miguel Jiménez, Francisco Jorquera, Manuel Macías, Jose Antonio Martínez Otón, Esther Molina, José Luis Montero, Isidoro Narváez, Antonio Olveira, Roberto Patón, Mercedes Pérez-Carreras, Manuel Rodríguez, Isabel Serra, Germán Soriano, Francisco Suárez, Juan Uriz, Víctor Vargas and Mercedes Vergara.
Thanks also to Paula Iruzubieta for her contribution to the revision of the manuscript.