It has been reported that professional cyclists had an accelerated solid gastric emptying which decreased by increasing the exercise intensity. That could be explained by a predominance of stress-dependent motility inhibitors such gastrointestinal hormones, neurotransmitters and or the predominance of the gastric inhibitory vagal motor circuit. The aim of this preliminary study was to evaluate the role of β-endorphins, inhibitors of gastric motility, in these findings.
MethodsGastric emptying of solids marked with Tc99 while resting and plasmatic levels of β-endorphins were evaluated in 27 healthy controls and 19 professional cyclists (day 1). Besides, gastric emptying of solids was also assessed in cyclists when they reached 50% (day 1) and 75% (day 2) of the maximum oxygen consumption (low and high, respectively), during exercise on the cycle-ergometer. The third day, naloxone was administered in cyclists in order to block the β-endorphins receptors and gastric emptying was measured when they reached 75% of the maximum oxygen consumption.
ResultsBasal β-endorphin levels were lower in cyclists vs controls (p<0.05) and they increased with the exercise intensity (p<0.001). There were no significant differences in gastric emptying of solids with or without naloxone when 75% of the maximum oxygen consumption was reached.
ConclusionsThe inhibitory effect of the exercise in the gastric emptying of solids does not seem to be secondary to the action of β-endorphins, that leaves the gastric inhibitory vagal motor circuit a more likely predominant role.
Se ha informado de que los ciclistas profesionales tienen un vaciado gástrico sólido acelerado que disminuye al aumentar la intensidad del ejercicio. Esto podría explicarse por un predominio de los inhibidores de la motilidad dependientes del estrés, como las hormonas gastrointestinales, los neurotransmisores y o el predominio del circuito motor vagal inhibidor gástrico. El objetivo de este estudio preliminar fue evaluar el papel de las β-endorfinas, inhibidores de la motilidad gástrica, en estos hallazgos.
MétodosSe evaluó el vaciado gástrico de sólidos marcado con Tc99 mientras se evaluaban los niveles en reposo y plasmáticos de β-endorfinas en 27 controles sanos y 19 ciclistas profesionales (día 1). Además, también se evaluó el vaciado gástrico de sólidos en los ciclistas cuando alcanzaron el 50% (día 1) y el 75% (día 2) del consumo máximo de oxígeno (bajo y alto, respectivamente), durante el ejercicio en el cicloergómetro. El tercer día, se administró naloxona en los ciclistas para bloquear los receptores de β-endorfinas y se midió el vaciado gástrico cuando alcanzaron el 75% del consumo máximo de oxígeno.
ResultadosLos niveles basales de β-endorfina fueron menores en los ciclistas frente a los controles (p<0,05) y aumentaron con la intensidad del ejercicio (p<0,001). No hubo diferencias significativas en el vaciado gástrico de sólidos con o sin naloxona cuando se alcanzó el 75% del consumo máximo de oxígeno.
ConclusionesEl efecto inhibidor del ejercicio en el vaciado gástrico de sólidos no parece ser secundario a la acción de las β-endorfinas, lo que deja al circuito motor vagal inhibitorio gástrico un papel más probablemente predominante.
Few studies have evaluated the gastric emptying rate (GE) in professional athletes.1–10 Those studies employed different methodologies and obtained discordant or inconclusive results.1 The basal vagal state in the GES described in professional cyclists disappears as the intensity of the exercise increases, and the GES gradually slows down.11 Two different physiological processes dependent on the grade of exercise could be involved in this response: (1) The existence of increasing concentrations of gastric motility inhibitors such gastrointestinal hormones or neurotransmitters. (2) A progressive control of the sympathetic nervous system in gastric motility, however, different studies observed recently a double and opposite vagal action in gastric motility, one inhibitory by activating the gastric inhibitory vagal motor circuit (G-IVM) and other excitatory through the gastric excitatory vagal motor circuit (G-EVM).12 For more than 30 years, it is well known that opioids induce intense effects in gastrointestinal motility.13,14 They inhibit gastric compliance after ingestion, fundic phasic contractility,15 antrum motility and increase the pylorus tone, which provoke a gastric emptying retardation.16–18 β-Endorphins are opioids and thus can inhibit motility and gastric emptying. Nevertheless, it has not yet been assessed if the opioid blockage could be responsible for the delay in the GES during exercise. The aim of this study was to evaluate the effect of β-endorphins activity blockage using naloxone on gastric emptying in professional athletes.
Materials and methodsParticipantsWe studied 19 professional cyclists, within the age range 15–49 (mean±SD, 22±9.5) and 15±9.5 training hours per week, and 27 healthy male controls, within the age range 20–38 (mean±SD, 27.6±9.8), whose basal values were taken as reference. The exclusion criteria were history of gastrointestinal surgery, gastrointestinal motility disorders and the intake of medication which could modify the gastrointestinal motility or β-endorphins levels.
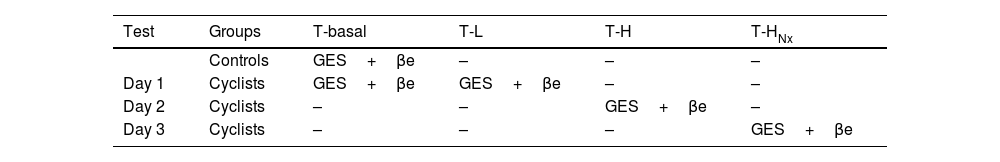
ProcedureGES was evaluated at the resting time (T-basal) at T-L (low intensity exercise) and T-H (high intensity exercise) Values while exercising, correspondent to the time they reached 50% and 75% of the peak oxygen uptake, VO2max. Oxygen uptake was obtained with a Aeromonitor AE-280 gas exchange system (Minato Medical Science, Japan) and the heart rate with a Polar FS-2c monitor (Kemple, Finland). Exercise test was performed on the cycle-ergometer (Lode Excalibur. Gröningen, Netherlands) in three different days: the first day we evaluated the GES with low-intensity exercise (L), the second day the GES with high-intensity exercise (H), and the third day we tested GES with high intensity exercise (H) and an initial opioid receptor blockade with naloxone (HNX) (Table 1). Previously, a progressive resistance exercise test was performed in order to measure VO2max.2 Exercise started with 50W intensity and a pedaling rate of 70rev/min. Then, power was increased 50W every 3min until they manifested signs of fatigue (increased heart rate and subjective sensations). In that moment, power was increased 20W each minute until the voluntary limit was reached. If the cyclists were not able to maintain the cycling load, it was lowered by 5%. The VO2max was obtained 1min before stopping, and 50% (L) and 75% (H) levels were calculated from it. In every session, cyclists completed the programmed exercise (L, H and HNX) in 105min, time required for the GES test. During the test, every subject was able to take water ad libitum to stay hydrated.
Diagram of the study.
| Test | Groups | T-basal | T-L | T-H | T-HNx |
|---|---|---|---|---|---|
| Controls | GES+βe | – | – | – | |
| Day 1 | Cyclists | GES+βe | GES+βe | – | – |
| Day 2 | Cyclists | – | – | GES+βe | – |
| Day 3 | Cyclists | – | – | – | GES+βe |
T-L=time for reaching 50% of the peak oxygen uptake: 50%-VO2max; T-H=time for reaching 75% of the peak oxygen uptake: 75%-VO2max; T-HNx=time for reaching 75% of the peak oxygen uptake: 75%-VO2max, with an initial opioid receptor blockade with naloxone.
The GES was measured in accordance with a previously described procedure in our laboratory,19 after an overnight fast of at least 8h, all subjects ingested two cooked scrambled eggs in front of the gamma camera. Each serving was labeled by injecting 22.3MBq technetium-99m diethylene triamine penta-acetic acid (DTPA). The total number of radioisotope counts in the abdominal cavity was recorded in the gamma camera; this value was 100% of the food retained in the stomach at time=0. Radioactivity emitted by the radioisotopes was measured with an Acticamera-CGR gamma camera connected to an Imag-7300-CGR computer. Acquisition time was 60s at 0, 5, 15, 45, 75 and 105min after ingestion of the test meal, in a 128×128-pixel resolution matrix. The pulse height analyzer was set at the 140-keV photopeak with a 10% window for 99mTc. The counts were measured with the subject prone (posterior projection) and supine (anterior projection) to avoid attenuation error. The computer stored images were analyzed, and the area of interest, comprising the gastric area, was outlined with the digitalizing system's pencil to determine the number of counts within. The geometric mean of the values obtained in the posterior and anterior projections was used as the count value. Due to the long scanning intervals, the fractional solid meal retention values were analyzed using the function y(t)=1−(1−e−kt)β. A power exponential curve was computer-fitted to the proportional gastric emptying data to obtain two representative indices, β and k, and the T1/2 (GE half time). The y(t) factor was the fractional meal retention at time t, k was the GE rate in min–1, t was the time interval in minutes, and b was the y-intercept extrapolated from the terminal portion of the curve. The unknown parameters β and k were determined with a non-linear least square algorithm using the measured fractional meal retention [y(t)] versus time data (t). The initial delay portion of the curve was characterized by a lag phase index, Tlag, which was numerically equal to ln β/k, and was the time in minutes when the second derivative of the function became equal to zero. The k index provided an estimate of the rate of emptying or the later rapid phase of the emptying curve; the β index provided an estimate of the shape of the curve or the earlier and more gradual phase of emptying. GES was evaluated with T1/2 (min).
Measurement of β-endorphinsIn order to determine the β-endorphins plasmatic levels (pg/ml), venous blood was drawn and collected in chilled EDTA-containing glass tubes, they were centrifuged immediately and plasma stored at −80°C. Blood samples were obtained as following: day 1, at T-basal (controls and cyclists) and at T-L (cyclists), day 2, at T-H (cyclists), and day 3, at T-HNX (cyclists) (Table 1). Analysis was done by radioimmunoassay (Euro diagnostica AB. Ideon S-205. 12. Sweden). Naloxone was administered in cyclists at the third day in the beginning of the exercise in order to block the β-endorphins opioid receptors described as following: naloxone hydrochloride 0.4mg IV over 2min (Narcan®; Du Pont Pharma, Germany), followed by an automatic IV infusion of naloxone hydrochloride 40μg/(kgh),16 until the time T-HNX of GE was reached.
Statistical analysisThe obtained results followed a normal distribution. For quantitative variables results were presented as average±standard deviation and qualitative variables as proportions. Comparisons of quantitative variables between groups were calculated using One-way variance analysis ANOVA tests, completely randomized from the ‘t’ Student distribution. The comparison between data pre- and post-naloxone administration was conducted for paired groups with nonparametric test (Wilcoxon test). We compared basal β-endorphins levels of controls and levels in cyclists at different times: T-basal, T-L, TH and T-HNX. Between cyclists, levels of β-endorphins were also compared at times T-basal, T-L, T-H and T-HNX. GES was evaluated with T1/2 (min). GES values were compared at T-H vs T-HNX and T-HNX vs T-basal in controls. The level of significance was established at 5%. The local Ethics Committee (CEIC) approved the study and the informed consent was signed by all subjects before enrollment.
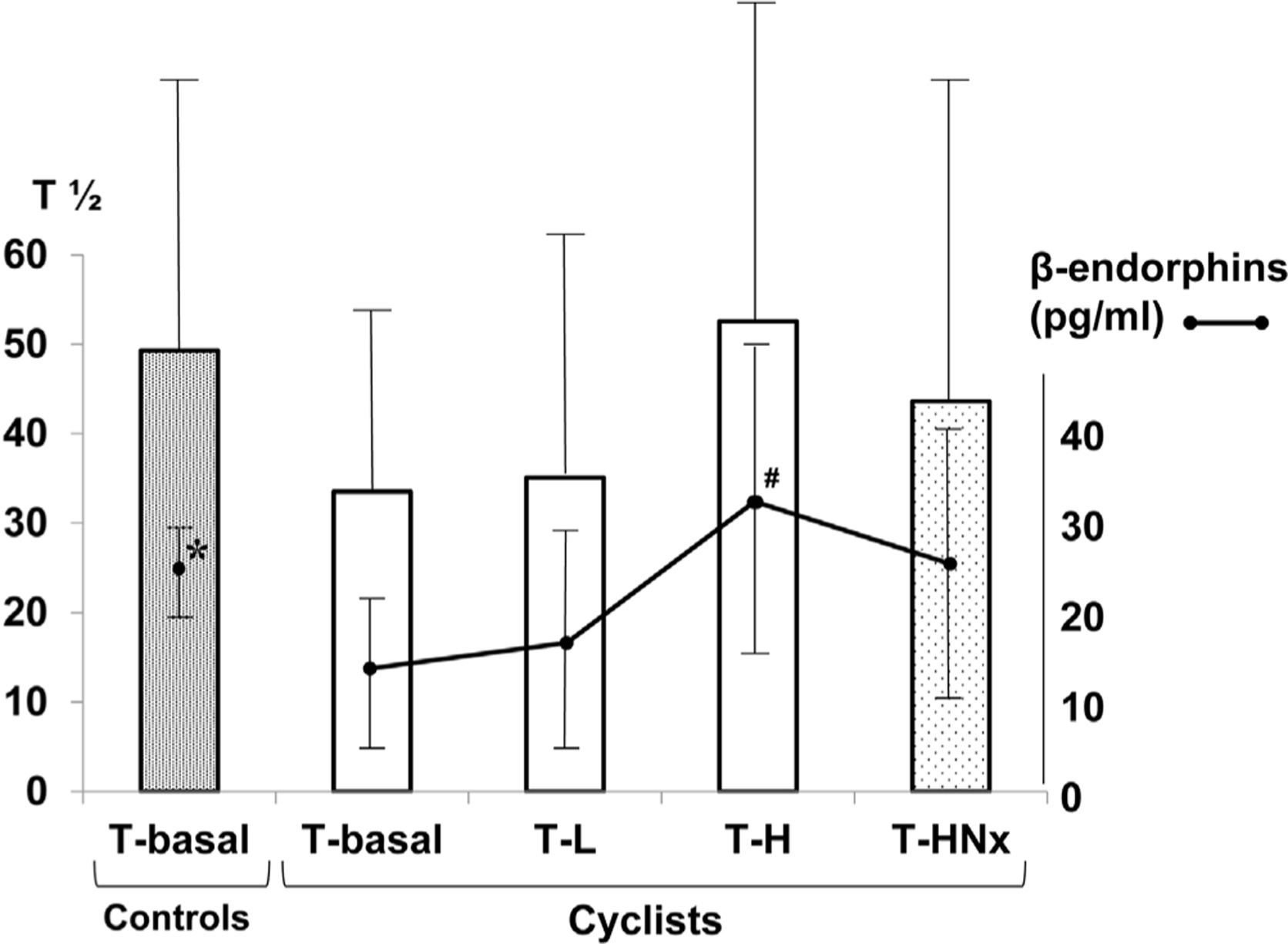
ResultsAt T-basal, plasmatic concentrations of β-endorphins (pg/ml) in cyclists vs controls were significantly lower (14.9±8.5 vs 25.0±4.5; p<0.05) (Fig. 1). β-Endorphin levels raised as the intensity of the exercise increased. At the times T-L, T-H and T-HNX, cyclists showed higher levels of β-endorphins vs T-basal, but similar to those found in controls at T-basal (17.7±12.0, 32.4±17.4, and 27.2±15.1 vs 25.0±4.5; p=ns). However, plasmatic levels of β-endorphins were significantly higher than those at T-basal only at T-H (32.4±17.4 vs 14.9±8.5; p<0.001). After naloxone administration, GES (T1/2) values at T-HNX were similar to that at T-H without naloxone, and T-basal in controls (43.61±19.05 vs 52.55±17.68 and 43.61±19.05 vs 49.30±15.23; p=ns).
Results of the GES (T1/2) relation with exercise intensity and blockade with naloxone, and plasmatic levels of β-endorphins in sedentary controls and professional cyclists. β-Endorphins: *controls vs cyclists (T-basal) (p<0.05); #cyclists (T-basal) vs T-H (p<0.001) (T-L=time for reaching 50% of the peak oxygen uptake: 50%-VO2max; T-H=time for reaching 75% of the peak oxygen uptake: 75%-VO2max; T-HNx=time for reaching 75% of the peak oxygen uptake: 75%-VO2max, with an initial opioid receptor blockade with naloxone). T1/2: GE half time (min).
In a previous communication of partial results from this study, we described the existence of a basal vagal state in professional cyclists11 as a consequence of a predominance of the parasympathetic nervous system, an adaptive response to intense exercise from training programs for athletes.1,3,4 That basal vagal state can be recognized by detecting a basal sinus bradycardia or, as in our case, detecting the presence of a basal accelerated GES (vs controls). We observed that basal GES slows down with the exercise intensity at T-L and T-H. GES can vary depending on not only the intensity of the exercise, but also on the type and duration of the exercise (walking, jogging, cycling, …), and the volume, osmolality or the energetic value of the administered meal.1 Although the high number of variables make the comparison with other similar studies difficult, our findings agree with others previously described and obtained in cyclists with scintigraphic technique.1,6,7 In this preliminary study, we tried to evaluate the effect of β-endorphins in GES in professional cyclists with different levels of exercise intensity, and the motor gastric response after blocking its receptors with naloxone. Some studies found a similar basal levels of β-endorphins in controls and athletes; however, other studies obtained significant low basal plasmatic levels of β-endorphins in trained athletes,20 as we did. This finding could be secondary to methodological differences, but could also be explained by the effect of adaptation to training and or a lower perceived stress level, with a higher comfort during the exercise test. Besides, this finding would explain, in part, the basal vagal status observed in athletes: low levels only would produce a discreet blocking of gastric motility and pyloric phasic and tonic activity, promoting an accelerated GES.17 However, the accelerated basal GES progressively slows down with exercise intensity, while at the same time plasmatic levels of β-endorphins increase. Thus, the highest β-endorphin levels coincide with T-H and the slowest GES rate. In other words, intense exercise in professional cyclists normalizes β-endorphin levels and GES, being both parameters at the time of maximal exercise intensity similar to those in resting controls. The parallelism between these two parameters and exercise intensity has not been previously described in the literature. We tried to evaluate the role of β-endorphins in the observed GES slowdown at T-H, by blocking opioid receptors with naloxone. The result was intriguing: the GES rates were similar at T-HNX and T-H, (with and without naloxone); that is to say, there was no inhibitory effect of β-endorphins on gastric motility. According to Geeraerts et al.,15 blockade by naloxone did not affect the gastric sensibility, tone, and compliance or GES. However, the postprandial accommodation was significantly inhibited. Similar results concerning GES were also obtained by Reber et al.21 Other researchers, with similar approaches and different methodologies, found that blockade of opioid receptors by naloxone produced an accelerated,13 retarded14 or normal GES.21–24
It could be argued that the opioid receptors blockade was not enough, but it has been proved that the doses used were adequate for that purpose.16 It can also be suggested the interference of different gastrointestinal hormones in these processes. Some of them vary their concentrations with exercise: GLP-1, pancreatic polypeptide, cholecystokinin and YY peptide increase and ghrelin decrease. It has been described that YY peptide, GLP-1 and pancreatic polypeptide induce a delayed GES (braking hormones) and ghrelin accelerates it (accelerating hormones).1,21,25,26 The proposed physiological mechanism of these findings explains that intense exercise (≥70% of VO2max) produces a decline of 80% in the splanchnic flow, the subsequent hypoxia in the gastrointestinal tract would induce the release of different hormones with influence in the GES.27 However, Mattin et al.2 could not find significant changes either in GES of a semi-solid meal, nor in different appetite regulating hormones (YY peptide, pancreatic polypeptide, insulin, GLP-1 and ghrelin) during exercise. After evaluating studies with similar methodologies, it seems to be proved that exercise increase plasmatic concentrations gastrointestinal hormones with inhibitory effect on GES.1,16,25,26 Nevertheless, in accordance with these and other studies,12 this effect seems to be short and/or scarce. This would be explained if the action of some gastrointestinal hormones was mainly paracrine, with a short local effect and a rapid plasma clearance.28 However, the design of this study could not answer these questions. In this respect, Reber et al.18 found that IV naloxone infusion did not change GES, but it increased significantly the concentrations of YY peptide, an inhibitor of GES. That is to say, two stimuli (intense exercise and naloxone) can induce the release of high levels of YY peptide, but its inhibitory effect was not enough to slow down the GES.25,26 These findings might seem contradictory, but they could be explained by the activity of the G-IVM on the gastric motility vs the effect of the G-EVM regulating the acid gastric secretion and the hormone release.12,29 Thus, GES in trained cyclists could be divided in two phases: a first basal phase where the G-EVM activity would predominate, manifested by an accelerated GES and the release of paracrine hormones with a fast degradation. As the exercise intensifies, a second phase would start, with an increasing activity of the G-IVM and a GES slowdown. The inhibitory final action of the G-IVM on the gastric smooth muscle is exercised through the release of ATP, VIP and mainly NO from the non-adrenergic non-cholinergic neurons in the myenteric plexus via muscarinic and nicotinic receptors.1,30 The design of this study does not allow to confirm this hypothesis or answer these two questions: Why is the G-EVM activated in trained athletes during their basal phase? and what mechanisms drive the change from G-EVM to G-IVM when increasing the physical activity, mainly during the intense exercise?
In conclusion, naloxone-sensitive endogenous opioids like β-endorphins have little or no effect in regulation of the GES in male professional cyclists during exercise. This finding has not been previously described and opens new avenues for research. According to recent studies, the role of the sympathetic nervous system and its inhibitory catecholamines in the GES slowdown during intense exercise does not seem predominant. On the other hand, the activation of the G-IVM seems essential. Although this is a preliminary study with a modest sample size and further studies with a higher number may be required, our findings help to understand the gastrointestinal physiology during exercise and its influence in the GES, which could be helpful in nutrition plans for athletes during exercise.
Ethical approvalThe local Ethics Committee (CEIC) approved the study and the informed consent was signed by all subjects before enrollment.
FundingThe authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that they have no conflicts of interest.