Inflammatory bowel disease comprises two conditions: ulcerative colitis and Crohn's disease. Inflammatory Bowel Disease Questionnaire 32 (IBDQ-32) is a specific questionnaire which has been translated from English into Spanish and validated. In the Spanish-speaking countries of America it has not been validated. The aim was to determine the psychometric properties, validity and reliability of the Mexican version of the IBDQ-32 questionnaire.
MethodsA total of 316 patients with inflammatory bowel disease and 100 healthy controls participated in the study. The questionnaires IBDQ-32 and SF-36 were issued on two occasions (separated by 15 days). The psychometric properties of the Mexican version of the IBDQ-32 questionnaire were determined.
ResultsPatients with inflammatory bowel disease had an impaired quality of life compared to healthy controls. There were no differences between ulcerative colitis and Crohn's disease in the total scores of IBDQ-32 and its domains. The internal consistency reliability was good. The intraclass coefficient showed good reliability (repeated measurement) for total scale and all four subscales. Factor analysis explained variance is higher than 50% therefore is considered adequate/acceptable. The correlation between IBDQ-32 and SF-36 showed a satisfactory association. The social domain is the only one that presented a ceiling effect.
ConclusionsThe Mexican version of the IBDQ-32 quality of life questionnaire is valid and reliable. This sample included the entire spectrum of inflammatory disease (remission and activity) and was comparable when assessing quality of life with the SF-36 generic questionnaire.
La enfermedad inflamatoria intestinal comprende dos afecciones: colitis ulcerosa y enfermedad de Crohn. El Cuestionario de enfermedad inflamatoria intestinal 32 (IBDQ-32) es un cuestionario específico que ha sido traducido del inglés al español y validado. En los países de habla hispana de América no ha sido validado. El objetivo fue determinar las propiedades psicométricas, validez y confiabilidad de la versión mexicana del cuestionario IBDQ-32.
MétodosParticiparon en el estudio un total de 316 pacientes con enfermedad inflamatoria intestinal y 100 controles sanos. Los cuestionarios IBDQ-32 y SF-36 se aplicaron en dos ocasiones (separadas por 15 días). Se determinaron las propiedades psicométricas de la versión mexicana del cuestionario IBDQ-32.
ResultadosLos pacientes con enfermedad inflamatoria intestinal tenían una calidad de vida deteriorada, en comparación con los controles sanos. No hubo diferencias en las puntuaciones totales de IBDQ-32 y sus dominios entre la colitis ulcerosa y la enfermedad de Crohn. La confiabilidad de la consistencia interna fue buena. El coeficiente intraclase mostró buena confiabilidad (medición repetida) para la escala total y las cuatro subescalas. La varianza explicada del análisis factorial es superior al 50%, por lo que se considera adecuada/aceptable. La correlación entre IBDQ-32 y SF-36 mostró una asociación satisfactoria. El dominio social es el único que presentó un efecto techo.
ConclusionesLa versión mexicana del cuestionario de calidad de vida IBDQ-32 es válida y confiable. Esta muestra incluyó todo el espectro de la enfermedad inflamatoria (remisión y actividad) y fue comparable al evaluar la calidad de vida con el cuestionario genérico SF-36.
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the intestine that comprises two conditions: ulcerative colitis (UC) and Crohn's disease (CD). The incidence and prevalence of IBD in Mexico is uncertain. Yamamoto reported in 2009, 847 cases diagnosed with UC between 1987 and 2006.1 However, a recent nation-wide cohort study in Mexico reported calculated incidence rates of 0.21, 0.16 and 0.04 cases per 100,000-person year for IBD, UC and CD respectively. The prevalence rates of IBD, UC, and CD, were 1.83, 1.45, and 0.34 cases per 100,000-person-year respectively in a period of 15 years.2
Traditionally, treatment and follow-up of patients with IBD is based on clinical symptoms, laboratory tests, endoscopic and histological findings, however, these findings fail to express the experience of how the patients perceive their health status in their daily life.3,4
Several activity indexes have been used for assessing the grade of disease activity based on physical and biological aspects, but do not evaluate the impact of the disease on the perception of patients’ life, especially their emotional and social well-being.5 The clinical course of disease and different type of therapies impact different activities within for example, professional, emotional, social and educational domains.6
Health status is related to the quality of life that is also known as health-related quality of life (HRQOL) in patients with IBD.5 Based on the definition of Health by the WHO, both UC and CD are chronic conditions that influence the physical, family and social dimensions of life.7
The IBDQ-32 questionnaire is a specific questionnaire for IBD, which has been translated from English into Spanish and validated.7 In Spanish-speaking countries of America it has not been validated.
The aim of this study was to evaluate and determine the validity and reliability of the Mexican version of the IBDQ-32 questionnaire.
MethodsSubjectsPatients at the outpatient IBD clinic at the National Institute of Medical Sciences and Nutrition were invited to participate in the current study. According to Terwee et al.8 the required sample size (for performing factor analysis) should be at least a 100 subjects, but is also dependent on the number of items of the questionnaire (seven patients for each item of the questionnaire). Following this rule of sample size calculation, at least 224 patients with ulcerative colitis (UC) should be included. Crohn's disease (CD), however, is a rare condition in Mexico. As such, a convenience, consecutive sample would be recruited of at least 60 patients. In addition, 100 healthy controls would be included. The questionnaires were applied in a control group, defined as subjects without gastrointestinal conditions or chronic conditions, they were individuals (accompanying relatives) of the waiting room of the consultation area.
The inclusion criteria were: (1) patients who agreed to participate in the study; (2) patients older than 18 years old and of Mexican nationality; and (3) patients with confirmed diagnosis of UC or CD for at least 6 months. The exclusion criteria were: (1) psychiatric disorders previously diagnosed or cognitive impairment. The elimination criteria included those patients who did not respond to the questionnaires on 2 different times (separated each one by 15 days).
In order to assess the clinical activity of the disease several indexes were used: the full Mayo score (0 remission, 1 mild activity, 2 moderate activity, 3 severe activity) (the integral disease activity index for UC patients) and the Harvey Bradshaw score (<5 clinical remission, 5–7 mild activity, 8–16 moderate activity, >16 severe activity) for patients with CD.9–11
QuestionnairesThe Inflammatory Bowel Disease Questionnaire (IBDQ) developed by Guyatt and colleagues12 and the extended version of the IBDQ by Love et al.13 have been translated and validated into Spanish in Spain.4,7 Currently, the most frequently used questionnaire is the one by Guyatt et al., named IBDQ-32 (to differentiate from the extended version, IBDQ-36). The IBDQ-32 questionnaire is a specific questionnaire for bowel disease, which has been translated from English into Spanish. In countries of Latin America the IBDQ-32 has not been validated. The questionnaire consists of 32 items with four domains (8 items per domain): gastrointestinal symptoms, systemic symptoms, emotional and social impact. The response to each item is based on a 7-point scale, where 7 is the best and 1 is the worst quality of life (QOL) perceived. The final score ranges from 32 to 224; higher scores indicate a better QOL. A group of Mexican Spanish speaking content experts (KYF, MZS) reviewed the original Spanish questionnaire to identify cultural differences in interpretation between Spanish from Spain and Mexican Spanish.
The modifications in the questionnaire were made in “words” that are not used in the colloquial language in Mexico and are:
Question 1, 22, 24: “evacuar”, instead of “ir de vientre”
Question 11 and 16: “baño”, instead of “lavabo”
Question 18: “usted”, instead of “vosotros”
Question 27: “enojado” instead of “enfadado”
The Short Form-36 Health Survey (SF-36) is one of the most widely used and broadly evaluated generic HRQOL questionnaires. The 36 items of the instrument cover the following scales: Physical function, Physical role, Body pain, General health, Vitality, Social function, Emotional role and Mental health. For each dimension, items are coded, aggregated and transformed into a scale that ranges from 0 (the worst state of health for that dimension) to 100 (the best state of health).14 SF-36 was completed and weighted (www.dgplades.salud.gob.mx/Contenidos/Documentos/CuestionarioSalud.pdf).
ProcedureThe questionnaires were completed by patients with UC and patients with CD as well as by the control group. The questionnaires were completed on two different occasions (separated by 15 days). The first occasion was during the medical consultation after which both questionnaires were sent by post to all patients and controls. All participants received a phone call as reminder to hand in the completed questionnaires when coming to the clinic for their next consultation.
The psychometric properties of the Mexican version of the IBDQ-32 questionnaire were determined based on the quality criteria defined by Terwee et al.8 and the Consensus-based Standards for the selection of Measurement INstruments (COSMIN) taxonomy of psychometric properties and definitions for health-related outcomes.15
Statistical analysisDifferences between median values between UC and CD of clinical variables and IBDQ-32 scores were made with Mann–Whitney test, as well as the comparison between IBD group with the control group.
The comparison between the medians of the total score and of each domain was performed with the U-Mann–Whitney test between patients in clinical remission and those with disease activity.
To assess the reliability and validity of the IBDQ-32 questionnaire, the following was performed and the following statistical terms were considered8,15,16:
Reliability- -
Internal consistency: measure to objectively evaluate that the items of a domain or subscale in a questionnaire are related (measuring the same concept). Cronbach's alpha was calculated for the total score and for each domain separately. Between 0.70 and 0.90 was considered a measure of good internal measure.
- -
Repeated measurement: the degree to which repeated measurements provide similar answers. Intraclass coefficient was performed and 0.70 was considered as the minimum standard for reliability.
- -
Structural validity: Factor analysis was performed with confirmatory maximum likelihood (ML) using all items of the IBDQ-32 to test whether four factors could be distinguished (four subscales).
- -
Hypothesis testing: the main hypothesis of this study was that the IBDQ-32 questionnaire adequately measures quality of life compared to the generic SF-36 quality of life questionnaire, considered as “gold standard”.
- -
Criterion validity: values the extent in a score is related with a gold standard, in this case the SF-36 questionnaire. A positive rating for criterion validity was considered >0.70. Spearman's correlation was performed.
The interpretability by floor and ceiling effects was evaluated too by considering the effect to be present if more than 15% of the surveyed answered the lowest or highest score.
A p value <0.05 was considered as significant. The data were analyzed with the statistical package SPSS v.16.
Ethical considerationsThis work was performed according to the principles expressed in the Declaration of Helsinki. The study was approved by the ethical committee in our institution and a written informed consent was obtained from all patients.
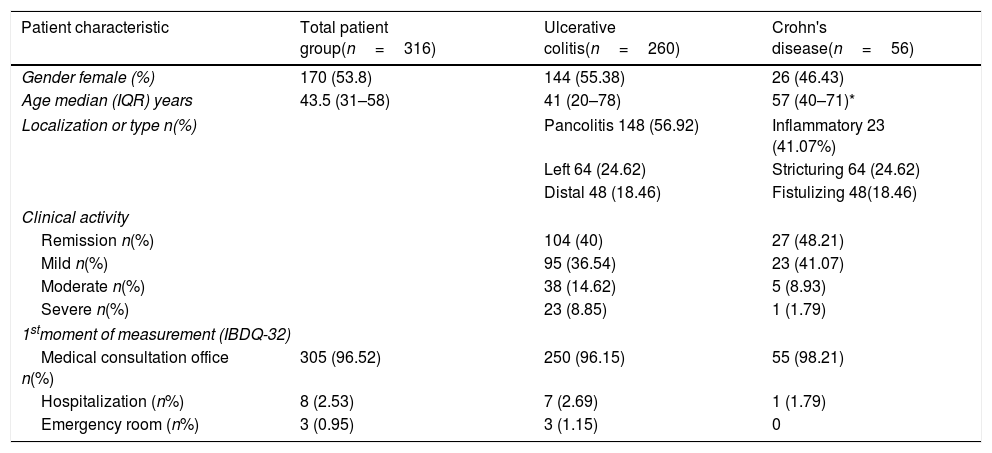
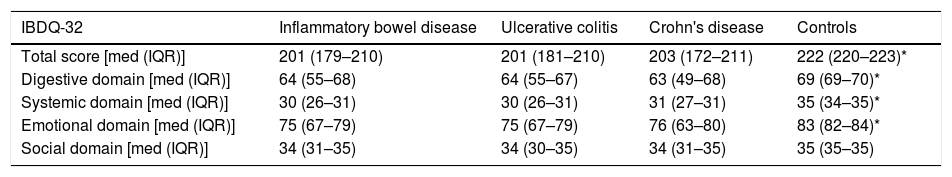
ResultsGroup differencesA total of 416 subjects were included: 260 (62.5%) patients with UC, 56 (13.5%) patients with CD and 100 healthy subjects (24%). Table 1 shows the clinical characteristics of patients with IBD. Table 2 shows the scores obtained for the total IBDQ-32 questionnaire and its four domains for all patients and healthy subjects. Due to non-normal distributions of data, group differences were tested for significance using Mann–Whitney tests. There were no significant differences in scores between the UC and CD groups. Healthy subjects presented significantly higher scores on the total score and the digestive, systemic and emotional domains, than patients in general with IBD and each subtype (p<0.000). No significant differences were identified for the Social domain.
Demographic and clinical characteristics of patients with IBD.
| Patient characteristic | Total patient group(n=316) | Ulcerative colitis(n=260) | Crohn's disease(n=56) |
|---|---|---|---|
| Gender female (%) | 170 (53.8) | 144 (55.38) | 26 (46.43) |
| Age median (IQR) years | 43.5 (31–58) | 41 (20–78) | 57 (40–71)* |
| Localization or type n(%) | Pancolitis 148 (56.92) | Inflammatory 23 (41.07%) | |
| Left 64 (24.62) | Stricturing 64 (24.62) | ||
| Distal 48 (18.46) | Fistulizing 48(18.46) | ||
| Clinical activity | |||
| Remission n(%) | 104 (40) | 27 (48.21) | |
| Mild n(%) | 95 (36.54) | 23 (41.07) | |
| Moderate n(%) | 38 (14.62) | 5 (8.93) | |
| Severe n(%) | 23 (8.85) | 1 (1.79) | |
| 1stmoment of measurement (IBDQ-32) | |||
| Medical consultation office n(%) | 305 (96.52) | 250 (96.15) | 55 (98.21) |
| Hospitalization (n%) | 8 (2.53) | 7 (2.69) | 1 (1.79) |
| Emergency room (n%) | 3 (0.95) | 3 (1.15) | 0 |
The comparison between ulcerative colitis and Crohn's disease was performed with the U-Mann–Whitney (age) and chi-square test (except localization or type).
IBDQ-32 total score and domain for patient groups and control group.
| IBDQ-32 | Inflammatory bowel disease | Ulcerative colitis | Crohn's disease | Controls |
|---|---|---|---|---|
| Total score [med (IQR)] | 201 (179–210) | 201 (181–210) | 203 (172–211) | 222 (220–223)* |
| Digestive domain [med (IQR)] | 64 (55–68) | 64 (55–67) | 63 (49–68) | 69 (69–70)* |
| Systemic domain [med (IQR)] | 30 (26–31) | 30 (26–31) | 31 (27–31) | 35 (34–35)* |
| Emotional domain [med (IQR)] | 75 (67–79) | 75 (67–79) | 76 (63–80) | 83 (82–84)* |
| Social domain [med (IQR)] | 34 (31–35) | 34 (30–35) | 34 (31–35) | 35 (35–35) |
[med (IQR)] median/interquartile range, differences between median values between UC and CD of IBDQ-32 score and subscale were made with Mann–Whitney test, as well as the comparison between inflammatory bowel disease group with the control group.
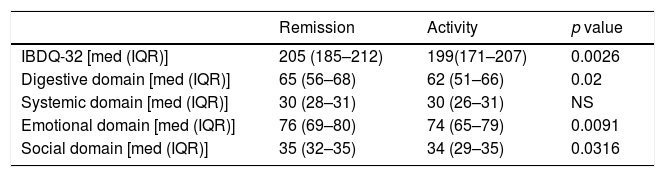
When separating IBD patients between remission (131 patients) and disease activity (mild, moderate, and severe) (185 patients), the IBDQ-32 total score and its digestive, emotional, and social domains showed higher scores than the patients who were in remission (Table 3).
IBDQ-32 total score and for domain by remission vs activity of the disease.
| Remission | Activity | p value | |
|---|---|---|---|
| IBDQ-32 [med (IQR)] | 205 (185–212) | 199(171–207) | 0.0026 |
| Digestive domain [med (IQR)] | 65 (56–68) | 62 (51–66) | 0.02 |
| Systemic domain [med (IQR)] | 30 (28–31) | 30 (26–31) | NS |
| Emotional domain [med (IQR)] | 76 (69–80) | 74 (65–79) | 0.0091 |
| Social domain [med (IQR)] | 35 (32–35) | 34 (29–35) | 0.0316 |
[med (IQR)] median (interquartile) range. Comparison between the medians of the score of the total score and of each domain using the Mann–Whitney U test between patients in clinical remission and those with disease activity.
Cronbach's alpha was considered an adequate measure of internal-consistency reliability. All Cronbach's alpha values lie between 0.76 and 0.95, thus indicating good internal consistency.
Repeated measurement reliabilityRepeated measurement reliability was determined by calculating intraclass coefficients (ICC) between the first moment of measurement and the repeated measurement two weeks apart. Table 4 depicts ICCs for the total score and each domain. All ICCs were above 0.70 demonstrating good reliability (repeated measurement) for total scale and all four domains.
Structural validityA confirmatory maximum likelihood (ML) factor analysis was performed using all items of the IBDQ-32 to test whether four factors could be distinguished (four domains). This four-factor model explained 53.18% of the total variance and was accepted (goodness-of-fit test, p=0.868).
Hypothesis testing for construct validity (convergent validity)As detailed by factor analysis above, the four factors explain 53.18% of the variance. This amount of explained variance is higher than 50% therefore is considered adequate/acceptable.17
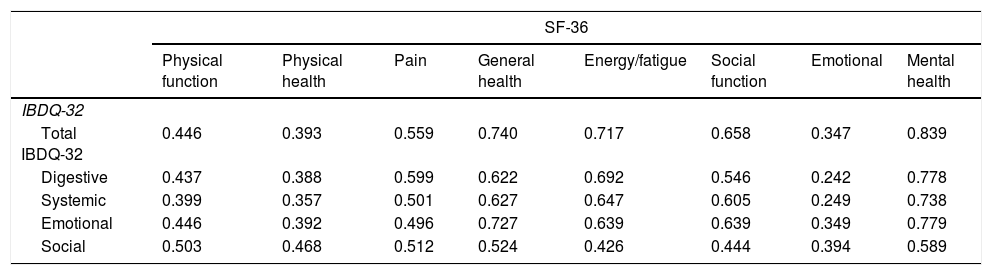
Convergent validity of the IBDQ-32 was determined by comparison with the generic quality of life SF-36 questionnaire. Convergent validity could be determined by comparing the domains from the IBDQ-32 and the SF-36 that were supposed to measure the same concept. Associations were calculated by Spearman's correlation coefficients for non-parametric data.4,5,16 The correlation coefficients for each domain in the IBDQ-32 and SF-36 are depicted in Table 5. A strong correlation was observed between the SF-36 Mental health domain and the total score of IBDQ-32 and its four domains. A substantial correlation was found between the rest of the domains except for the SF-36 emotional domain that had a weak correlation with the IBDQ-32 digestive and systemic domains.
Associations among IBDQ-32 and SF-36 (nonparametric Spearman's correlation coefficients).
| SF-36 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Physical function | Physical health | Pain | General health | Energy/fatigue | Social function | Emotional | Mental health | |
| IBDQ-32 | ||||||||
| Total IBDQ-32 | 0.446 | 0.393 | 0.559 | 0.740 | 0.717 | 0.658 | 0.347 | 0.839 |
| Digestive | 0.437 | 0.388 | 0.599 | 0.622 | 0.692 | 0.546 | 0.242 | 0.778 |
| Systemic | 0.399 | 0.357 | 0.501 | 0.627 | 0.647 | 0.605 | 0.249 | 0.738 |
| Emotional | 0.446 | 0.392 | 0.496 | 0.727 | 0.639 | 0.639 | 0.349 | 0.779 |
| Social | 0.503 | 0.468 | 0.512 | 0.524 | 0.426 | 0.444 | 0.394 | 0.589 |
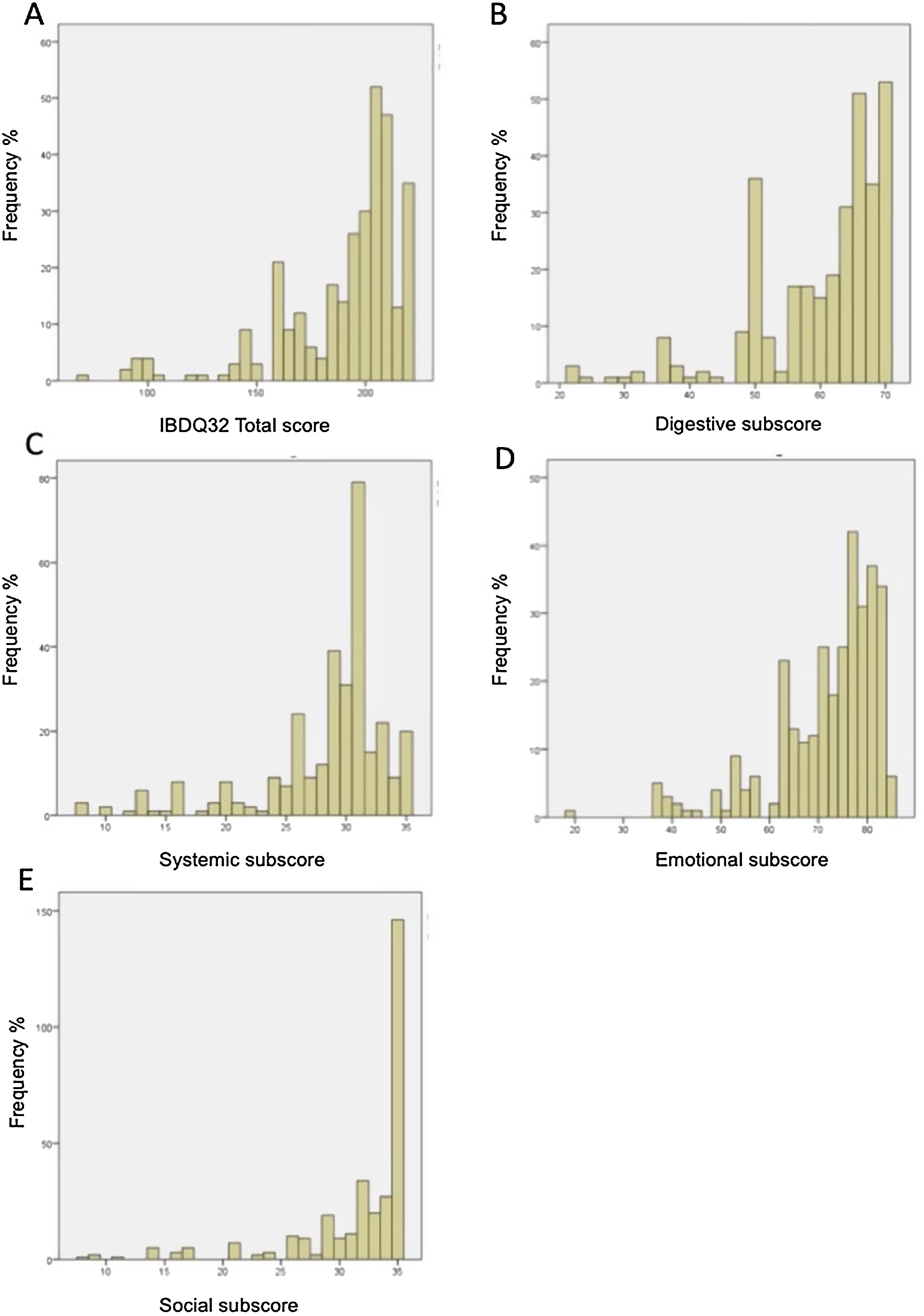
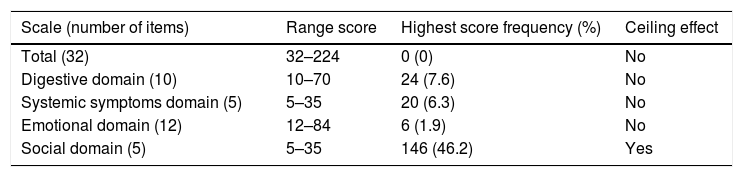
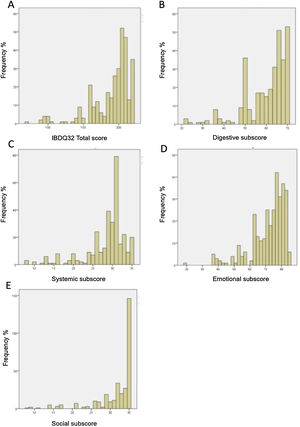
Floor and ceiling effects were based on patient data only. Table 6 shows the ranges and the number of patients achieving the highest and the lowest scores for the IBDQ-32 total score and its domains. Fig. 1A–E shows the corresponding histograms of the data distribution. Only the social domain presented ceiling effects. No floor effects were identified.
Floor and ceiling effects for the IBDQ-32 in inflammatory bowel disease patients (n=316).
| Scale (number of items) | Range score | Highest score frequency (%) | Ceiling effect |
|---|---|---|---|
| Total (32) | 32–224 | 0 (0) | No |
| Digestive domain (10) | 10–70 | 24 (7.6) | No |
| Systemic symptoms domain (5) | 5–35 | 20 (6.3) | No |
| Emotional domain (12) | 12–84 | 6 (1.9) | No |
| Social domain (5) | 5–35 | 146 (46.2) | Yes |
Histogram of data distribution on the IBDQ-32. (A) Histogram of data distribution on the IBDQ-32. The frequency of patients is displayed as a function of the total score on the IBDQ-32. The area under the curve equals the total frequency of patients. (B) Histogram of data distribution on the Digestive subscore. The frequency of patients is displayed as a function of the Digestive subscore. The area under the curve equals the total frequency of patients. (C) Histogram of data distribution on Systemic subscore. The frequency of patients is displayed as a function of the Systemic subscore. The area under the curve equals the total frequency of patients. (D) Histogram of data distribution on Emotional subscore. The frequency of patients is displayed as a function of the Emotional subscore. The area under the curve equals the total frequency of patients. (E) Histogram of data distribution on Social subscore. The frequency of patients is displayed as a function of the Social subscore. The area under the curve equals the total frequency of patients.
This is the first validation of the IBDQ-32 questionnaire in Spanish for Latin America. All modifications were validated for a specific language and culture, and translation into another language Spanish from Spain may decrease its validity as well as its application in our population. Any questionnaire still translated must be validated in the population that is applied to prove that it is equivalent to its original version. Therefore, cross-cultural adaptation requires re-evaluation of psychometric properties, in the linguistic and cultural context to be validated.
In this study, we assessed the validity and reliability of the IBDQ-32 questionnaire in Spanish from Mexico. The IBD population in which it was applied was for CD and UC patients as well as a control group of asymptomatic healthy volunteers. Clearly lower questionnaire scores were obtained in the IBD group compared to asymptomatic healthy volunteers suggesting worse quality of life in IBD patients. The scores between UC and CD patients were similar, so we inferred that their quality of life assessed by this instrument did not show differences between CD and UC.
When disease activity was evaluated, patients in remission had higher scores in the total score and in each domain (except for the systemic domain). This study was not focused on evaluating sensitivity to change; responsiveness was limited because changes were not measured. A further study is required for assessing the change in clinical activity in hospitalized patients.
All patients still attending to the outpatient IBD clinic specially when they have mild to moderate activity evaluated with activity scores that includes clinical and endoscopic aspects like Mayo or a novel integral disease activity score that includes clinical, biochemical, endoscopic and histological findings for UC patients, it is frequent that histological and endoscopic activity is worse than the clinical and biochemical parameters. Generally, patients assessed in consultation are those who perceive themselves to be more clinically stable in their disease. The clinical activity focuses mainly on physical and biological aspects and the general quality of life questionnaires on the feeling of well-being.
In particular, the social scale is similar to the score between IBD patients and asymptomatic volunteers. This could be one reason why ceiling effects (best HR-QoL) for Social subscale was observed. It seems that the patients who mostly come to the consultation do not perceive a limitation in the social domain in their life. We corroborated that this domain was measuring what it should measure by a substantial correlation with the social function of the SF-36.
To date, there are only 4 studies for validation of the IBDQ-32 questionnaire and to our best knowledge, this is the first validation in Spanish for Latin America. Masachs et al.7 concluded that the global score of the IBDQ-32 and its domains were very similar to the IBDQ-36 applied in their sample and the main analysis approach was to assess the sensitivity to change according to the clinical activity in UC and CD. In this study we decided to focus on the validity and reliability of the IBDQ-32 and in a second stage consider its discriminate the power or sensitivity to change according to Terwee et al.8
Leong et al.5 in a Chinese validation of IBDQ-32, they found a strong correlation between systemic domain and SF-36. They did the correlation with the entire score of SF-36 (not recommended) and each IBDQ-32 domains. They acquired a correlation between SF-36 total score and systemic domain in Crohn's disease of r=0.8 (p<0.001) as the highest correlation obtained and the lowest for the Bowel domain of 0.549 (p<0.001). In our study, we did the correlation with each domain of IBDQ-32 and each domain of SF-36. The systemic domain had a weak correlation with the Emotional/Fatigue domain but a strong correlation with the Mental Health domain of SF-36. It is enriching in validating a questionnaire to know all the values that may be interacting in a score. The ICC in both versions were above 0.70 for the total score and for each domain.
The limitations in our study is that the sample of patients with CD is small due to low incidence and prevalence in our country, however, the results of the analysis in this subgroup are similar to those in UC, therefore, we can infer that this questionnaire could be considered valid and reliable in CD patients.
Our questionnaire is valid and reliable to assess HRQOL in patients with IBD, both UC and CD in Mexico and we can assume that in Latin America due to the cultural similarity with the modifications made to the Spanish version. Further studies are required to assess patients in hospitalized patients with severe disease activity and their changes in quality of life. Longitudinal studies are required to assess changes in quality of life over time and according to disease activity and remission.
In conclusion, our Mexican version of the IBDQ-32 quality of life questionnaire is valid and reliable. This study included the full spectrum of inflammatory bowel disease (remission and activity conditions) and was comparable for assessing quality of life with the SF-36 generic questionnaire.
FundsThis research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Authors’ contributionsMónica Rocio Zavala Solares, Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Lucero Salazar, Data acquisition and data curation, Investigation, Writing – review & editing.
Jesús K. Yamamoto-Furusho, Conceptualization, Data curation, Resources, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Conflict of interestsThe authors declare that they have no conflict of interest.
Fabiola Maely González-Ortiz and Azucena Casanova-Lara who contributed to the pilot study in the application of questionnaires.
Renee Speyer who contributed to the analyses for the presentation in a medical venue.