Managing Crohn's disease (CD) requires addressing factors beyond medical treatment alone, including health-related quality of life (HRQoL) and physical activity. This study aimed to understand the relationships between different intensities of physical activity, HRQoL, and inflammatory biomarkers in CD, considering sex as a factor that could influence these associations.
Patients and methodsA cross-sectional, observational study was conducted in 63 CD patients. Sociodemographic and clinical data, including C-reactive protein and fecal calprotectin determinations, were collected. The Inflammatory Bowel Disease Questionnaire (IBDQ-9) and the Global Physical Activity Questionnaire (GPAQ) were used to measure HRQoL and physical activity, respectively.
ResultsAlthough females had similar inflammation levels to males, they engaged in less physical activity and reported lower HRQoL (especially in psychosocial wellbeing). Sedentary behavior and intense physical activity negatively impacted HRQoL in the overall sample and only in females. Higher fecal calprotectin concentrations were associated with poorer HRQoL in the total group and only in males. No correlation was found between inflammation and physical activity.
ConclusionsSex differences influence the relationship between inflammation, physical activity, and HRQoL in CD. Given the greater impact of CD on emotional and social wellbeing in females, irrespective of physiological measures of inflammation, our findings support considering sex differences, which may inform more individualized approaches to improve HRQoL.
El manejo de la enfermedad de Crohn (EC) va más allá del tratamiento médico, abarcando la calidad de vida relacionada con la salud (CVRS) y la actividad física. Este estudio analiza la relación entre distintos niveles de actividad física, CVRS y biomarcadores inflamatorios en la EC, considerando la influencia del sexo.
Pacientes y métodosSe realizó un estudio observacional transversal en 63 pacientes con EC. Se recogieron datos sociodemográficos y clínicos, incluyendo proteína C reactiva y calprotectina fecal. La CVRS y la actividad física se evaluaron mediante el Cuestionario de Enfermedad Inflamatoria Intestinal (IBDQ-9) y el Cuestionario Global de Actividad Física (GPAQ).
ResultadosAunque mujeres y varones presentaban niveles similares de inflamación, las mujeres realizaban menos actividad física y tenían una CVRS más baja (especialmente en el bienestar psicosocial). Tanto el sedentarismo como la actividad física intensa afectaron negativamente la CVRS en la muestra total y solo en mujeres. Mayores niveles de calprotectina fecal se asociaron con peor CVRS en el grupo total y solo en varones. No se encontró correlación entre inflamación y actividad física.
ConclusionesLas diferencias de sexo influyen en la relación entre inflamación, actividad física y CVRS en la EC. Dado el mayor impacto de la EC en el bienestar emocional y social en mujeres, independientemente de las medidas fisiológicas de inflamación, nuestros hallazgos apoyan la consideración de las diferencias entre sexos, lo que puede orientar enfoques más individualizados para mejorar la CVRS.
Inflammatory bowel disease (IBD) encompasses two different disorders, ulcerative colitis and Crohn's disease (CD). CD is a chronic, progressive, inflammatory, immune-mediated inflammatory disease of the gastrointestinal tract.1–3
CD is characterized by transmural, discontinuous and segmental inflammatory bowel lesions, with areas of inflammation between normal-appearing mucosal sections affecting any part of the gastrointestinal tract, although the most common involvement is the terminal ileum and proximal colon. This results in chronic abdominal pain, diarrhea, obstruction and/or perianal lesions. Other symptoms such as fatigue, fever, weight loss, and anemia may also occur.2–4 The disease presents in periods of clinical remission alternating with periods of active inflammatory episodes.5 In addition, they may present extraintestinal systemic manifestations,2,5 which together with the risk of hospitalization and surgery, greatly affect their quality of life and can lead to disability. The chronic and unpredictable nature of the disease and its debilitating effect on all aspects of life are the main concerns of CD patients.3,4
Combined measurements of inflammatory markers, such as C-reactive protein (CRP) and fecal calprotectin (FC), have significant diagnostic and prognostic importance for CD, as they provide a precise measure of the patient's inflammatory activity, correlating with endoscopic and clinical findings of inflammation in IBD.6–8
In clinical practice, patient-reported outcomes (PROs) such as health-related quality of life (HRQoL) and activities related to functional status such as physical activity performance should be prioritized alongside morbidity and disease activity. PROs provide valuable insight into how patients experience their disease and treatment, comprehensively capturing multifaceted aspects that are not always evident through clinical or laboratory measures, which are particularly relevant in CD due to its broad impact on physical, psychological, and social functioning. This way, PROs allow for a more individualized and comprehensive approach to disease management. The World Health Organization (WHO) described HRQoL as “an individual's perception of their position in life in the context of the culture and value systems in which they live, and in relation to their goals, expectations, and standards and concerns”.9 The chronic and progressive nature of CD has a debilitating and disabling effect on activities of daily living, social, educational, professional, and family activities that together with fatigue, abdominal pain, the presence of anorectal symptoms, as well as surgeries and hospitalizations, can lead to a considerable worsening in the HRQoL of these patients.3,10
On the other hand, the benefits of physical exercise are well known, both in healthy people and in some diseases that involve a chronic inflammatory state.11 In fact, the practice of physical activity is an effective strategy for the prevention and treatment of several chronic diseases. Regular physical exercise offers multiple health benefits, such as improving cardiovascular, muscular, and bone function, regulation of body composition, and reducing the risk of disease.12,13
Some studies have demonstrated improvements in the HRQoL of patients with CD through physical activity.14–17 However, this type of complementary therapy for disease management has not been sufficiently investigated and, therefore, is often omitted from current good practice guidelines.8 Indeed, the interactions between physical activity, HRQoL, and inflammatory activity of the disease have not yet been completely elucidated. Besides, although some sex differences in HRQoL have been previously reported in patients with CD, most studies have not examined these differences in relation to physical activity or inflammation. In fact, this study is the first to examine the influence of sex on the complex relationships among different dimensions of HRQoL, physical activity (considering intensity) and inflammatory status in patients with CD. Potential sex-based differences in these aspects among individuals with CD may be influenced by a combination of biological, psychological, and social mechanisms, such as gender roles, caregiving, self-esteem, disease-related stigma, societal expectations or hormonal factors.
According to the updated Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE-II) recommendations for monitoring treatment response, key treatment goals for patients with CD include normalization of serum and fecal markers, absence of disability, restoration of quality of life, and symptomatic relief. Due to the strong correlation of PROs with patient well-being and functional status, these targets should be assessed early and regularly throughout the disease course.18 Moreover, as it becomes increasingly clear that managing CD requires more than medical treatment alone, further research on complementary approaches, such as physical activity, that could contribute to improve HRQoL and disease activity is necessary. These efforts may contribute to enhancing the quality of care and to increasing adherence to the STRIDE-II recommendations.
Taking all the above into account, the objective of this study was to analyze the relationships between different intensities of physical activity, HRQoL, and predictive inflammatory biomarkers of CD, such as CRP and FC, considering sex as a factor that could influence these associations.
MethodStudy design and participantsThis is a cross-sectional, observational study that included patients diagnosed with Crohn's disease. Inclusion criteria required patients to be over 18 years of age, to have been diagnosed with Crohn's disease by the Extremadura Health Service, and to be included in the gastroenterology patient registry of the University Hospital of Cáceres (Spain).
The sample size was calculated using G*Power version 3.1.9.7 (Universität Kiel, Germany) based on a two-tailed test, an effect size (d) of 0.80, α error probability of 0.05, and statistical power of 0.80. The minimum number of subjects was estimated to be 54.
Patients diagnosed with CD were recruited from the gastroenterology nursing outpatient clinic at this hospital. Once eligible patients were identified and provided informed consent, they were enrolled during their scheduled outpatient follow-up visits. Patients who were unwilling to participate, under 18 years of age, or had missing data were excluded prior to enrollment. No patients were excluded after enrollment.
Those who agreed to participate gave their informed consent. Numerical codes were used to identify each volunteer to preserve their anonymity. The study was approved by the Ethics Committee of the University of Extremadura (201/2022) and the Research Ethics Committee of Cáceres (1092022), following the principles and regulations of the European Community Council Directives and the Declaration of Helsinki.
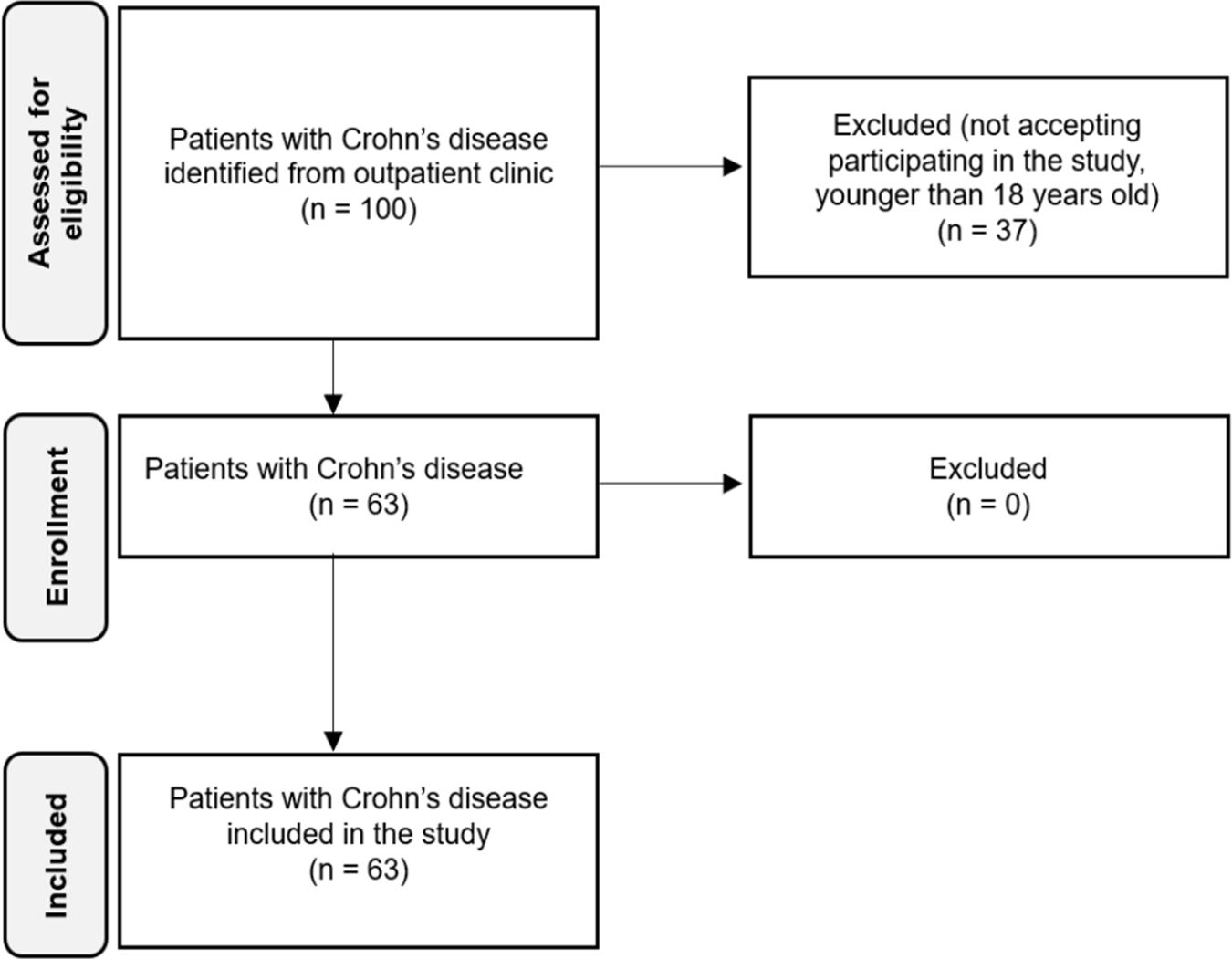
Finally, 63 patients with Crohn's disease were included in the study. Flowchart of patients is represented in Fig. 1.
Data collection: sociodemographic and clinical data, health-related quality of life and physical activityIn addition to the sociodemographic and clinical data obtained from clinical history, information was collected through two questionnaires to measure HRQoL and physical activity levels.
Sociodemographic and clinical dataPatient records were reviewed to collect data on sex, age, height, weight, body mass index (BMI) and CD-related treatments. Information was gathered on prescription of anti-inflammatory drugs, immunomodulators, corticosteroids, and biological therapy (Adalimumab, Ustekimumab, Infliximab, Vedolizumab) at the time of data collection.
In addition, data of inflammatory parameters such as serum CRP and FC in stool samples, assessed by standard techniques, were collected as markers of inflammatory activity. Normal reference ranges were considered as follows: CRP, 0–5mg/L in serum; and FC, 0–50mg/kg in stool.
Measurement of CRP and FC was performed at a single time point within six months prior to the administration of the questionnaires, to capture biomarker levels that most closely reflected the patient's status at the time of the study assessment.
Quality of lifeQuality of life was measured with the short Inflammatory Bowel Disease Questionnaire (IBDQ-9). IBDQ-9 is one of the most commonly used measures to assess IBD patients’ HRQoL. The version used in the study has been validated in Spanish and is reported to have acceptable validity and reliability in people with some type of IBD.19 This questionnaire was administered by hospital nursing staff during scheduled outpatient follow-up visits to previously diagnosed patients. This questionnaire uses 9 items to evaluate HRQoL in four domains: gastrointestinal, systemic, social, and emotional. The gastrointestinal domain evaluates bowel movements, cramps, nausea and vomiting, flatulence and bloating. The systemic domain evaluates energy, fatigue, and malaise, while the social domain assesses the frequency with which patients have had to postpone or cancel social appointments or commitments due to bowel problems. Finally, the emotional domain evaluates satisfaction and happiness in their personal life. Each item is graded on a scale of 1 (worst HRQoL) to 7 (best quality of life). The total score ranges from 9 to 63 with a higher number indicating a better quality of life.
Physical activityThe Global Physical Activity Questionnaire (GPAQ),20 validated and designed by the World Health Organization (WHO), was used to assess patients’ physical activity. This questionnaire was administered by hospital nursing staff during scheduled outpatient follow-up visits to previously diagnosed patients. This questionnaire consists of 16 questions and collects information on physical activity and sedentary behavior in three contexts: work, commuting and leisure time activity. Leisure time activities include exercise or sport performance. Sedentary behavior evaluates time spent sitting or reclining at work, at home, during commutes, or with friends. The weekly minutes spent in moderate and vigorous physical activity are measured in each context, as well as the weekly minutes of sedentary behavior. Moderate physical activity includes tasks that cause a slight increase in breathing or heart rate, while intense physical activity requires significant physical effort and results in a large increase in heart rate or breathing.
A higher score in any of the specific physical activity categories indicates that the individual is more physically active, as the score reflects the total minutes of moderate or intense activity performed per week. Conversely, lower scores in physical activity and higher sedentary behavior scores suggest greater levels of inactivity.
Statistical analysisStatistical analyses were performed with GraphPad Prism® 10.4.0. Continuous data were presented as the mean and standard error of the mean (SEM), while categorical data were shown as percentages.
Normality tests (Shapiro–Wilk) and homogeneity tests for equality of variances (Levene's test) were performed for continuous variables. To study the relationship between continuous variables, bivariate correlations were performed using Pearson's r coefficient in the variables that followed a normal distribution. Those variables that did not present this type of distribution were evaluated using Spearman's rho coefficient. The Student's t test was used to compare a quantitative variable by groups when these variables followed a normal distribution, while the Mann–Whitney U test was used for those that did not follow this type of distribution. The Chi-square test was used to compare qualitative variables. Effect size was calculated using Cohen's d for normal distributions and Rosenthal's r for non-normal distributions, with both metrics interpreted as low (d<±0.2; r<±0.2), medium (d>±0.5; r>±0.5) and high (d>±0.8; r>±0.8). A p value<0.05 was considered statistically significant, with three levels of statistical significance: p<0.05, p<0.01, p<0.001.
To account for potential confounding variables, multivariate regression models were employed. A multivariate analysis of covariance (Mancova) was conducted to evaluate differences attributable to the treatment, while correlation analyses were used to explore relationships between continuous variables. These statistical procedures were implemented to ensure that the observed effects were not influenced by underlying confounding factors.
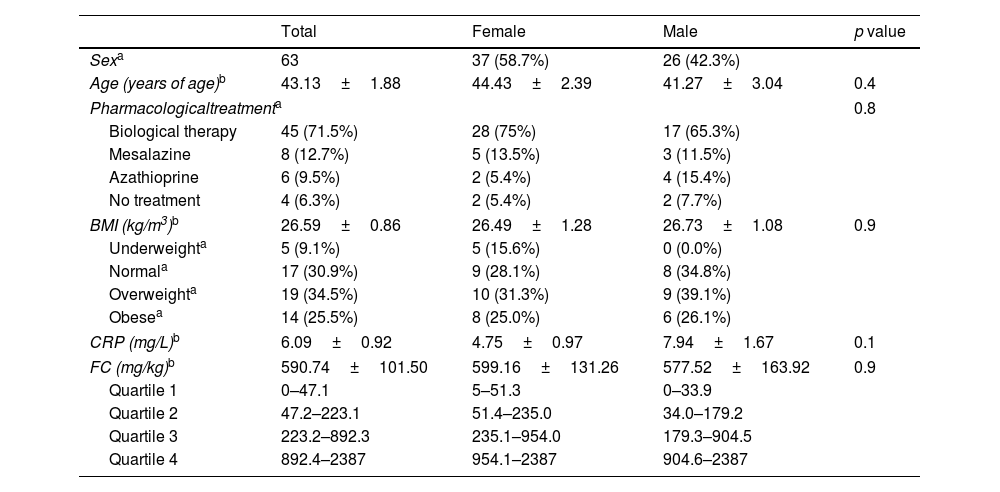
ResultsSociodemographic, anthropometric and clinical data, and inflammatory markersThe sociodemographic, anthropometric and clinical characteristics of the 63 patients included in the study are presented in Table 1. Out of the total sample, 58.7% were female and 41.3% male. BMI values were similar in the female and male groups, with most of the patients being overweight or obese. Additionally, most patients used biological therapy. Conversely, intestinal anti-inflammatory and immunosuppressants drugs were the least frequently used. A small minority did not require pharmacological treatment for disease control. Similar pharmacological treatment patterns were identified in both females and males. Inflammatory markers, including CRP and FC were also analyzed. No differences were identified between sexes.
Anthropometric and clinical data.
| Total | Female | Male | p value | |
|---|---|---|---|---|
| Sexa | 63 | 37 (58.7%) | 26 (42.3%) | |
| Age (years of age)b | 43.13±1.88 | 44.43±2.39 | 41.27±3.04 | 0.4 |
| Pharmacologicaltreatmenta | 0.8 | |||
| Biological therapy | 45 (71.5%) | 28 (75%) | 17 (65.3%) | |
| Mesalazine | 8 (12.7%) | 5 (13.5%) | 3 (11.5%) | |
| Azathioprine | 6 (9.5%) | 2 (5.4%) | 4 (15.4%) | |
| No treatment | 4 (6.3%) | 2 (5.4%) | 2 (7.7%) | |
| BMI (kg/m3)b | 26.59±0.86 | 26.49±1.28 | 26.73±1.08 | 0.9 |
| Underweighta | 5 (9.1%) | 5 (15.6%) | 0 (0.0%) | |
| Normala | 17 (30.9%) | 9 (28.1%) | 8 (34.8%) | |
| Overweighta | 19 (34.5%) | 10 (31.3%) | 9 (39.1%) | |
| Obesea | 14 (25.5%) | 8 (25.0%) | 6 (26.1%) | |
| CRP (mg/L)b | 6.09±0.92 | 4.75±0.97 | 7.94±1.67 | 0.1 |
| FC (mg/kg)b | 590.74±101.50 | 599.16±131.26 | 577.52±163.92 | 0.9 |
| Quartile 1 | 0–47.1 | 5–51.3 | 0–33.9 | |
| Quartile 2 | 47.2–223.1 | 51.4–235.0 | 34.0–179.2 | |
| Quartile 3 | 223.2–892.3 | 235.1–954.0 | 179.3–904.5 | |
| Quartile 4 | 892.4–2387 | 954.1–2387 | 904.6–2387 | |
BMI: body mass index; CRP: C-reactive protein; FC: fecal calprotectin. Student's t test (age, BMI), Chi-square test (pharmacological treatment) and Mann–Whitney U test (CRP, FC); p value female vs. male group.
In addition, the elevated levels of FC observed in both males and females indicate intestinal inflammation; 75% of the patients presented levels above the values considered normal (<50mg/kg) and approximately half of the patients (47.3%) had FC values>250mg/kg, with some even exceeding 2000mg/kg. The distributions were generally similar across groups. The interquartile ranges were 845.2mg/kg for the total sample, 902.7mg kg for females, and 870.5mg/kg for males.
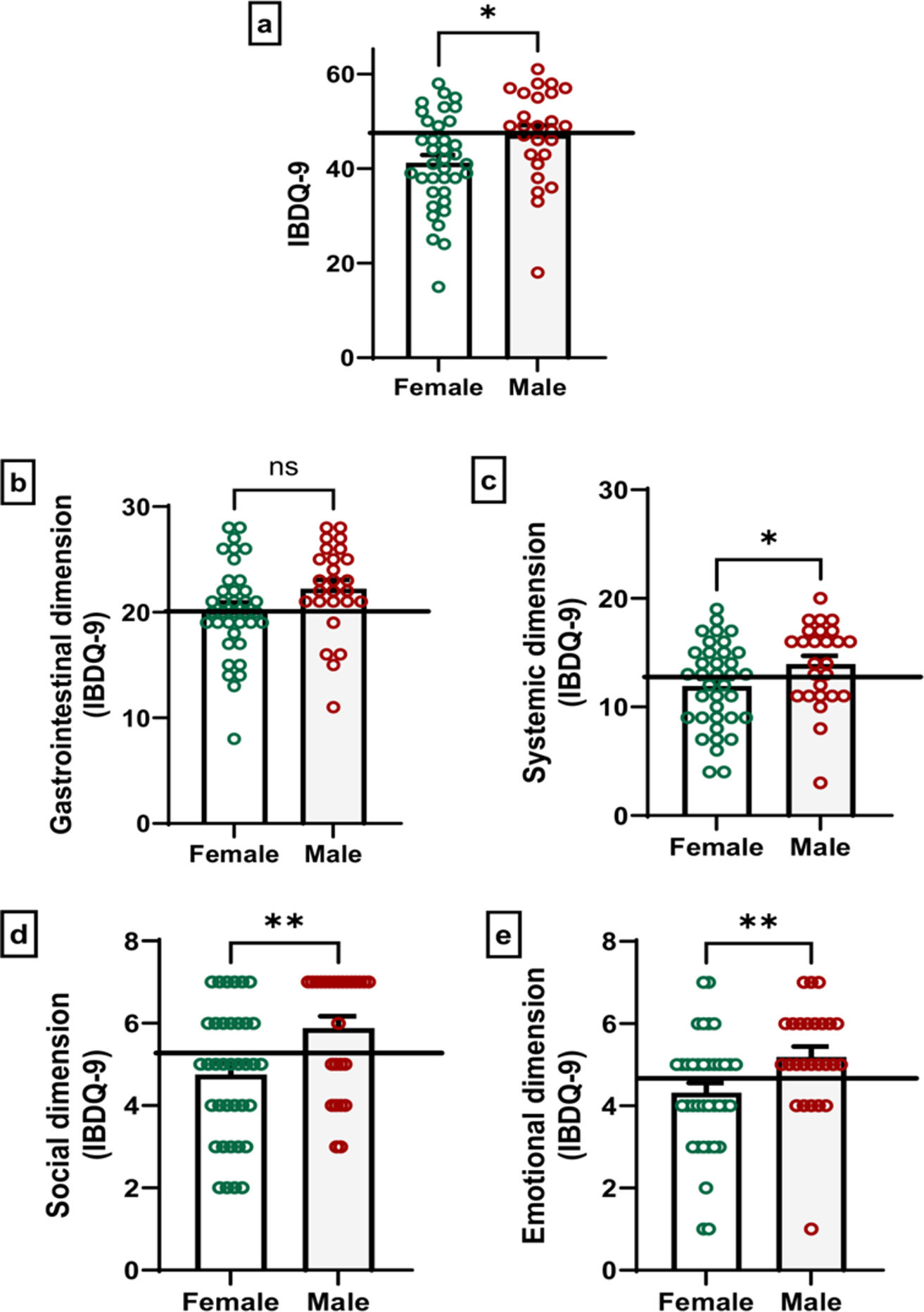
Health-related quality of life in patients with Crohn's diseaseRegarding HRQoL, the minimum IBDQ-9 score was 15 and the maximum was 61. When comparing by sex, males had significantly higher HRQoL scores than females (p=0.01, t=2.40, Cohen's d=0.62), indicating that males perceived a better overall HRQoL.
In the gastrointestinal dimension, males scored higher than females, although the difference did not reach statistical significance (p=0.07, t=1.83, Cohen's d=0.47). Regarding the systemic dimension, which reflects physical symptoms and fatigue, significant differences were observed (p=0.04, t=2.04, Cohen's d=0.52), with men reporting fewer systemic issues. Similarly, the social dimension (p=0.005, U=285.5, Rosenthal's r=−0.35) indicated that men experienced fewer disruptions in their social activities due to the disease. Finally, the emotional dimension, which evaluates the psychological impact of the diseases, also revealed significant differences between the sexes (p=0.007, U=294.0, Rosenthal's r=−0.34), with males reporting greater emotional well-being.
These findings are visualized in Fig. 2, which illustrates the distribution of scores across the different dimensions, highlighting the differences in HRQoL perceptions between males and females.
Differences between IBDQ-9 scores between males and females: IBDQ-9 global scores (a) and the gastrointestinal (b), systemic (c), social (d) and emotional (e) domains. Each point represents the score of each participant. Student's t test (IBDQ-9 global scores, gastrointestinal and systemic dimension) and Mann–Whitney U test (social and emotional dimension); non-significant (ns), *p<0.05 and **p<0.01 male vs. female scores. The horizontal line represents the mean scores of the total sample as a reference value.
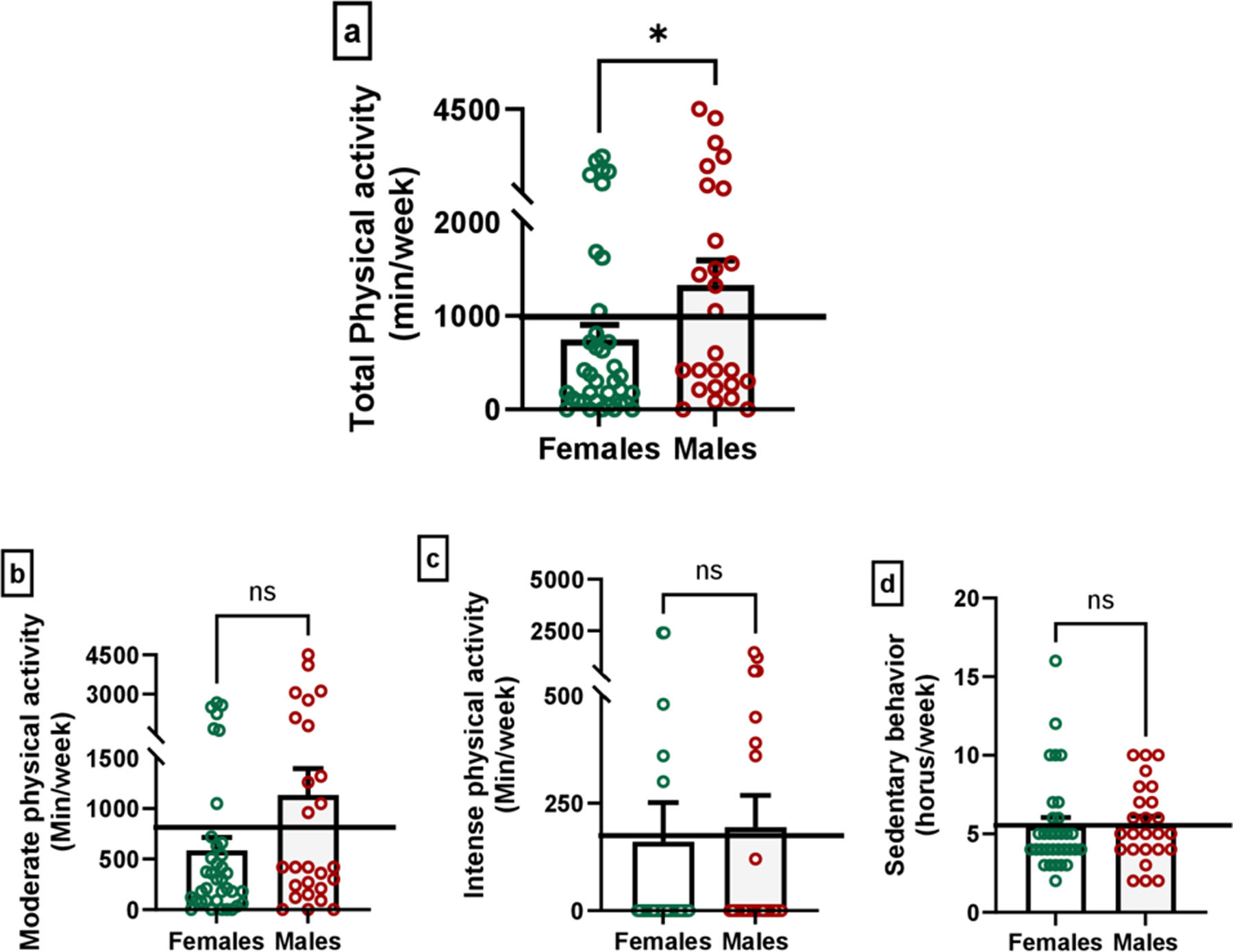
Data from the GPAQ is presented in Fig. 3, showing differences in physical activity levels between males and females. Males engaged in significantly more physical activity than females (p=0.04, U=338.5, Rosenthal's r=−0.25), a result that underscores potential sex differences in activity patterns within the sample. However, when analyzing the specific categories of physical activity intensity, including moderate (p=0.09, U=361.0, Rosenthal's r=−0.21) and intense (p=0.1, U=400.5, Rosenthal's r=−0.20) physical activity, no significant differences were found between the sexes. Similarly, no difference was observed in sedentary behavior (p=0.4, U=352.0, Rosenthal's r=−0.10).
Differences in physical activity between males and females in global physical activity scores (a), moderate physical activity (b), intense physical activity (c) and sedentary behavior (d). Mann–Whitney U test; non-significant (ns), *p<0.05 male vs. female scores. The horizontal line represents the mean scores of the total sample as a reference value.
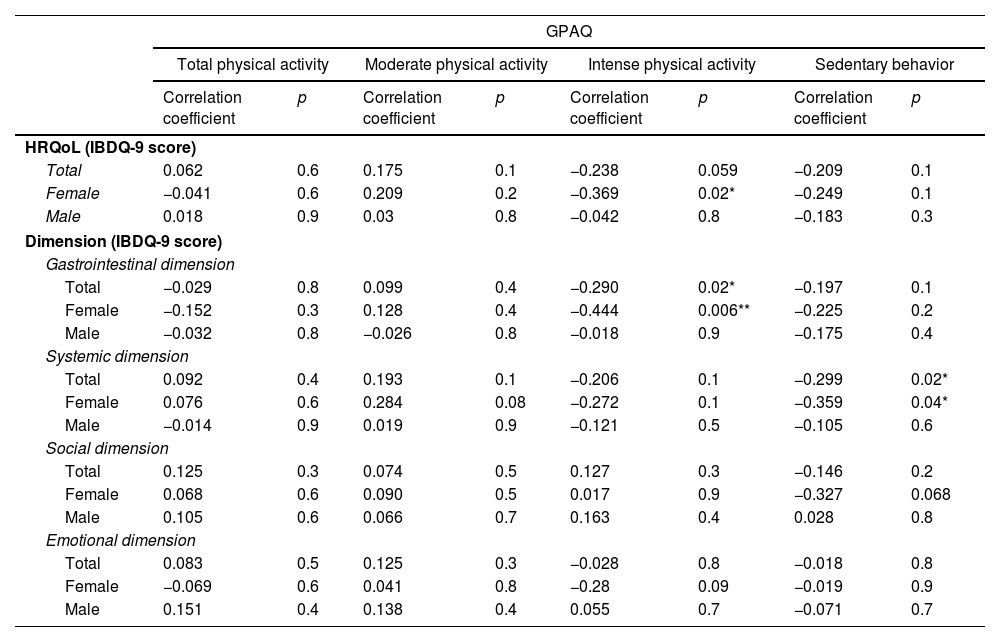
The associations between HRQoL and physical activity are shown in Table 2. Significant associations were found in females, where intense physical activity was negatively correlated with HRQoL (p=0.02, r=−0.369). In contrast, no significant associations were found in males.
Correlations between physical activity and health-related quality of life, in females, males and the overall sample.
| GPAQ | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total physical activity | Moderate physical activity | Intense physical activity | Sedentary behavior | |||||
| Correlation coefficient | p | Correlation coefficient | p | Correlation coefficient | p | Correlation coefficient | p | |
| HRQoL (IBDQ-9 score) | ||||||||
| Total | 0.062 | 0.6 | 0.175 | 0.1 | −0.238 | 0.059 | −0.209 | 0.1 |
| Female | −0.041 | 0.6 | 0.209 | 0.2 | −0.369 | 0.02* | −0.249 | 0.1 |
| Male | 0.018 | 0.9 | 0.03 | 0.8 | −0.042 | 0.8 | −0.183 | 0.3 |
| Dimension (IBDQ-9 score) | ||||||||
| Gastrointestinal dimension | ||||||||
| Total | −0.029 | 0.8 | 0.099 | 0.4 | −0.290 | 0.02* | −0.197 | 0.1 |
| Female | −0.152 | 0.3 | 0.128 | 0.4 | −0.444 | 0.006** | −0.225 | 0.2 |
| Male | −0.032 | 0.8 | −0.026 | 0.8 | −0.018 | 0.9 | −0.175 | 0.4 |
| Systemic dimension | ||||||||
| Total | 0.092 | 0.4 | 0.193 | 0.1 | −0.206 | 0.1 | −0.299 | 0.02* |
| Female | 0.076 | 0.6 | 0.284 | 0.08 | −0.272 | 0.1 | −0.359 | 0.04* |
| Male | −0.014 | 0.9 | 0.019 | 0.9 | −0.121 | 0.5 | −0.105 | 0.6 |
| Social dimension | ||||||||
| Total | 0.125 | 0.3 | 0.074 | 0.5 | 0.127 | 0.3 | −0.146 | 0.2 |
| Female | 0.068 | 0.6 | 0.090 | 0.5 | 0.017 | 0.9 | −0.327 | 0.068 |
| Male | 0.105 | 0.6 | 0.066 | 0.7 | 0.163 | 0.4 | 0.028 | 0.8 |
| Emotional dimension | ||||||||
| Total | 0.083 | 0.5 | 0.125 | 0.3 | −0.028 | 0.8 | −0.018 | 0.8 |
| Female | −0.069 | 0.6 | 0.041 | 0.8 | −0.28 | 0.09 | −0.019 | 0.9 |
| Male | 0.151 | 0.4 | 0.138 | 0.4 | 0.055 | 0.7 | −0.071 | 0.7 |
GPAQ: global physical activity questionnaire; HRQoL: health-related quality of life; IBDQ-9: intestinal bowel disease questionnaire. Pearson's r in HRQoL or gastrointestinal or systemic dimension vs. total, moderate or intense physical activity, or sedentary behavior, in total, female and male groups; Spearman's rho in social or emotional dimension vs. total, moderate or intense physical activity or sedentary behavior, in total, female and male groups.
The analysis of the specific dimensions of the IBDQ-9 showed that intense physical activity was negatively associated with the gastrointestinal dimension in the total sample (p=0.02, r=−0.290) and in females (p=0.006, r=−0.444). In the systemic dimension, there was a statistically significant correlation with sedentary behavior in the total sample (p=0.02, r=−0.299) and in females (p=0.04, r=−0.359). No significant correlations were observed in males for any subcategory of physical activity.
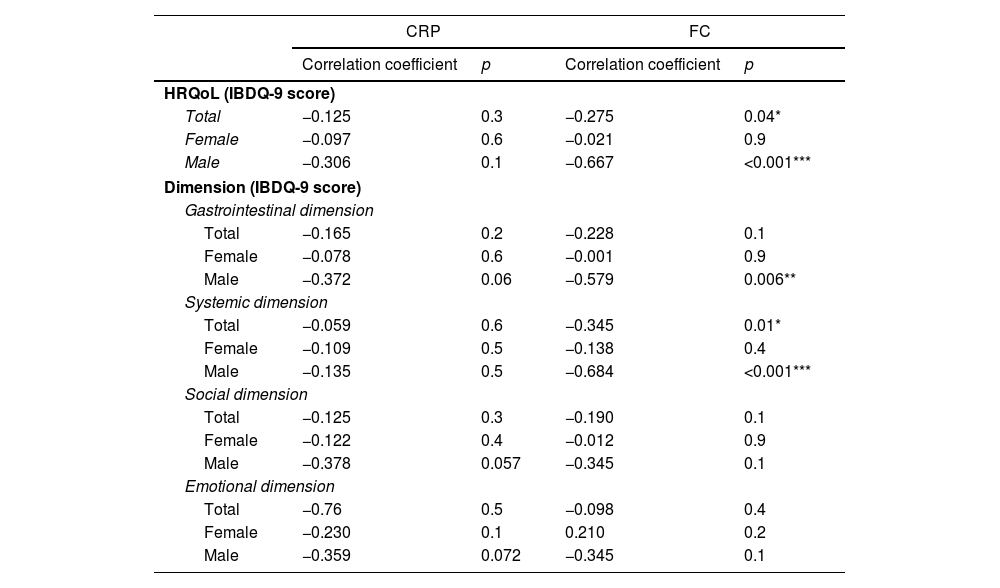
Relationship between health-related quality of life and inflammationResults showing the association study between HRQoL and inflammation are presented in Table 3. The overall results showed no significant association between CRP and HRQoL (p=0.3, r=−0.125), while FC exhibited a statistically significant negative correlation with HRQoL (p=0.04, r=−0.275). When analyzed by sex, FC levels in males were negatively associated with HRQoL (p<0.001, r=−0.667). In contrast, no significant associations were observed in females between HRQoL and FC (p=0.9, r=−0.021).
Correlations between inflammatory markers (C-reactive protein and fecal calprotectin) and health-related quality of life in females, males and the overall sample.
| CRP | FC | |||
|---|---|---|---|---|
| Correlation coefficient | p | Correlation coefficient | p | |
| HRQoL (IBDQ-9 score) | ||||
| Total | −0.125 | 0.3 | −0.275 | 0.04* |
| Female | −0.097 | 0.6 | −0.021 | 0.9 |
| Male | −0.306 | 0.1 | −0.667 | <0.001*** |
| Dimension (IBDQ-9 score) | ||||
| Gastrointestinal dimension | ||||
| Total | −0.165 | 0.2 | −0.228 | 0.1 |
| Female | −0.078 | 0.6 | −0.001 | 0.9 |
| Male | −0.372 | 0.06 | −0.579 | 0.006** |
| Systemic dimension | ||||
| Total | −0.059 | 0.6 | −0.345 | 0.01* |
| Female | −0.109 | 0.5 | −0.138 | 0.4 |
| Male | −0.135 | 0.5 | −0.684 | <0.001*** |
| Social dimension | ||||
| Total | −0.125 | 0.3 | −0.190 | 0.1 |
| Female | −0.122 | 0.4 | −0.012 | 0.9 |
| Male | −0.378 | 0.057 | −0.345 | 0.1 |
| Emotional dimension | ||||
| Total | −0.76 | 0.5 | −0.098 | 0.4 |
| Female | −0.230 | 0.1 | 0.210 | 0.2 |
| Male | −0.359 | 0.072 | −0.345 | 0.1 |
CRP: C-reactive protein; FC: fecal calprotectin; HRQoL: health-related quality of life; IBDQ-9: intestinal bowel disease questionnaire. Pearson's r in HRQoL or gastrointestinal or systemic dimension vs. FC and CRP in total, female and male groups; Spearman's rho in social or emotional dimension vs. FC and CRP, in total, female and male groups.
Regarding specific dimensions of the IBDQ-9, in general, a negative trend was observed between inflammatory parameters and HRQoL dimensions. In the total sample (females and males), there was a significant negative correlation between the systemic dimension and FC (p=0.01, r=−0.345). FC in males showed significant negative associations with the gastrointestinal (p=0.006, r=−0.578) and systemic (p<0.001, r=−0.684) dimensions. In females no associations were identified.
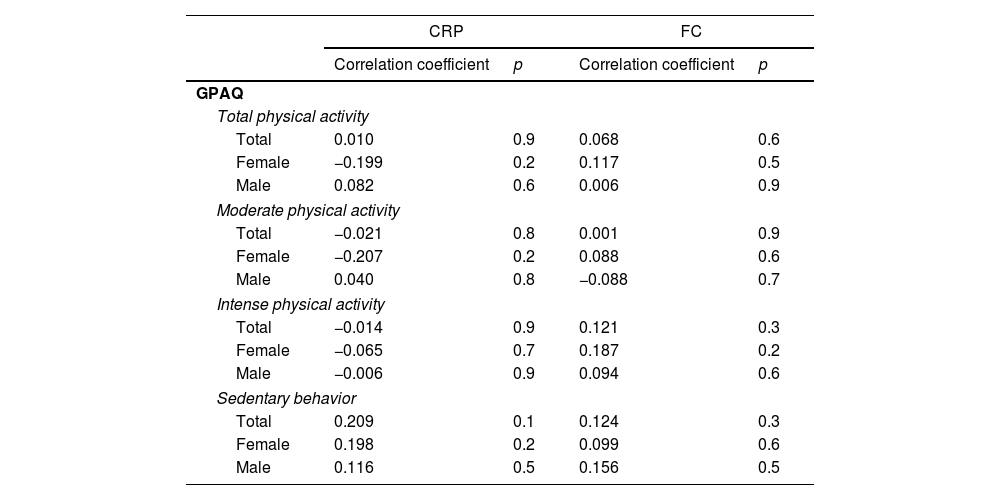
Relationship between physical activity and inflammationNo statistically significant correlations were observed between inflammatory markers and physical activity; however, some trends were observed (Table 4). In general, females exhibited negative trends in the relationships between CRP and physical activity. On the other hand, sedentary behavior showed a positive tendency in the relationship with CRP and with FC.
Correlations between inflammatory markers (C-reactive protein and fecal calprotectin) and physical activity in females, males and the overall sample.
| CRP | FC | |||
|---|---|---|---|---|
| Correlation coefficient | p | Correlation coefficient | p | |
| GPAQ | ||||
| Total physical activity | ||||
| Total | 0.010 | 0.9 | 0.068 | 0.6 |
| Female | −0.199 | 0.2 | 0.117 | 0.5 |
| Male | 0.082 | 0.6 | 0.006 | 0.9 |
| Moderate physical activity | ||||
| Total | −0.021 | 0.8 | 0.001 | 0.9 |
| Female | −0.207 | 0.2 | 0.088 | 0.6 |
| Male | 0.040 | 0.8 | −0.088 | 0.7 |
| Intense physical activity | ||||
| Total | −0.014 | 0.9 | 0.121 | 0.3 |
| Female | −0.065 | 0.7 | 0.187 | 0.2 |
| Male | −0.006 | 0.9 | 0.094 | 0.6 |
| Sedentary behavior | ||||
| Total | 0.209 | 0.1 | 0.124 | 0.3 |
| Female | 0.198 | 0.2 | 0.099 | 0.6 |
| Male | 0.116 | 0.5 | 0.156 | 0.5 |
CRP: C-reactive protein; FC: fecal calprotectin; GPAQ: global physical activity questionnaire. Spearman's rho.
Multivariate statistical analyses were performed using all variables in order to control for potential confounding factors, and no significant differences were observed (data not shown).
PROMs such as HRQoL should be considered as an important target in disease management.18 The burden of CD results in a reduced quality of life compared to that of healthy individuals.21,22 Interestingly, studies have shown that disease activity is an important determinant of HRQoL in CD, but even asymptomatic patients report lower HRQoL, suggesting that other factors also play a role.21
Although quality of life has previously been explored in IBD23,24 and particularly in CD,21 its interrelation with other crucial aspects such as physical activity, inflammation, and sex remains underexamined. Thus, the aim of the present study was to assess the potential influence of those factors in HRQoL in CD. A comprehensive understanding of HRQoL is essential to provide patient-centered care and to develop effective management strategies that address not only the physiological aspects of the disease but also the patient's overall well-being.
First, our results showed that intense physical activity was negatively associated with the gastrointestinal aspects of HRQoL, while sedentary behavior was inversely correlated with the systemic dimension of HRQoL. This demonstrates that sedentary activity has a negative impact on the systemic aspects of the disease (that is, fatigue and malaise), indicating a potential beneficial role for physical activity, regardless of intensity, in better HRQoL outcomes. However, high-intensity physical activity was linked to worse HRQoL when considering gastrointestinal symptoms. This supports the notion that very intense physical activity, including exercise, may have detrimental effects in individuals with CD, altering gastrointestinal parameters and thus potentially exacerbating gastrointestinal clinical manifestations.25 These findings are consistent with previous studies, which reported that higher levels of physical activity were associated with better HRQOL outcomes.14-16,26,27 Taylor and colleagues17 observed that both walking and moderate to vigorous intensity physical activity were independently associated with improved physical, but not mental, HRQoL. Interestingly, one study also noted an inverse correlation between vigorous exercise and HRQoL.14 Moreover, taking into account that physical activity rates in patients with CD appear to be similar to or lower than those in the general population,27 overall, moderate-intensity physical activity (including physical exercise) may be beneficial for improving HRQoL in individuals with CD. However, further research is needed to fully understand its impact on the health and well-being of these patients.28
Regarding the inflammatory biomarkers analyzed, FC and CRP, their relationships with HRQoL and physical activity were assessed. CRP appears to have minimal impact on HRQoL. In contrast, FC (a well-established marker of intestinal inflammatory activity) concentrations showed a negative correlation with overall HRQoL and with the systemic dimension of HRQoL, indicating that lower local inflammation levels in the intestinal tract are associated with better HRQoL in CD patients. In fact, FC concentrations in our CD volunteers were more than ten times higher compared to the reference values of 50mg/kg in healthy individuals.29 Previous research shows inconsistent conclusions: while some studies have also demonstrated an inverse relationship between FC concentrations and HRQoL in CD,30 others have found no significant association between these factors.31 This underscores the pivotal role of chronic inflammation as a determinant in the overall well-being of patients with CD. Notably, this association extends to the systemic aspects of HRQoL, highlighting the broader impact of inflammation beyond the gastrointestinal tract. On the other hand, we did not find a significant relationship between CRP and HRQoL levels in our sample, in accordance with the only previous study available on this subject,31 which could point to intestinal inflammation (reflected by FC) as a more important determinant of HRQoL than systemic inflammation (reflected by CRP). Furthermore, no associations were found between physical activity levels and the inflammatory markers analyzed, which may suggest that, in our sample, physical activity does not directly modulate inflammation as measured by FC and CRP, and therefore, other factors may modulate physical activity in CD; however, further studies are needed to assess causality and safety implications. In the same way, the inflammatory status of the patients does not seem to influence the performance of physical activity or its intensity, which could be attributed, at least in part, to psychological aspects and health perceptions. These findings are in line with previous research showing that FC and CRP were not affected by physical activity performance.32 Nevertheless, other studies have shown that elevated CRP concentrations are associated with poor physical activity in CD.33
However, an important question arises: do these relationships between quality of life, physical activity, and inflammation remain consistent when considering sex as an influencing factor? Sex could play a crucial role in understanding the experience and management of diseases like CD, as it can affect physiological and psychosocial factors, including inflammation levels, HRQoL, mental health, disease progression, and treatment response, among others. Recent studies investigating sex differences in IBD have predominantly focused on areas such as epidemiology, disease behavior, mental health, and treatment adherence,22,34 but evidence on sex-based differences in HRQoL and its influencing factors remains scarce.
First, we will focus on the sex-based differences in the HRQoL, physical activity, and inflammation results, followed by the analysis of the differences in the correlations between these parameters. The findings of this study reveal notable sex-based differences in HRQoL among individuals with CD. In line with prior research, females reported lower HRQoL scores compared to males.35–40 In our sample, this disparity was particularly pronounced in the systemic, social and emotional domains, underscoring the multifaceted impact of CD on females’ lives. Potential contributors to these differences include heightened psychosocial stress (caregiving roles, societal expectations, work-life balance, stigma and social perceptions surrounding chronic illnesses, etc.), emotional burden (including self-image and self-esteem), and greater disease-related concerns among females, leading to an increased impact and experience of disease burden that is reflected in their HRQoL scores. In fact, other works focusing on sex differences in IBD have shown a higher prevalence of anxiety and depression in females with IBD compared to males.39,40 Moreover, results related to physical activity indicated that females were significantly less active than males, highlighting sex-based differences in activity patterns in CD. The vast majority of studies assessing physical activity in CD patients have not conducted separate analyses based on sex.14,17,27,32,41 The work by Lund and collegues42 is in line with our results, since they found higher activity volume in males than in females; however, their sample consisted exclusively of pediatric and young adult patients in remission. Regarding inflammation, in our study there were no differences in inflammatory biomarkers, namely CRP and FC. In fact, FC values were more than ten times higher in comparison with reference values from healthy individuals both in females and males. Data from previous research on the influence of sex on disease activity are conflicting. While some studies did not find differences between males and females,40 others identified either lower or higher disease activity in females.22 In any case, no evidence of sex-based differences in the concentrations of inflammatory biomarkers has been reported to support these findings, in line with our results. Considering the notable disparities observed in HRQoL and physical activity levels between males and females, the absence of differences in inflammatory markers suggests that inflammation may not be the primary factor influencing HRQoL or participation in physical activity. Instead, other factors such as psychosocial determinants and roles, perceptions of illness, and symptom burden could play a more significant role, particularly in females. Altogether, these differences underscore the need for sex-specific analyses in CD to better tailor intervention strategies and improve outcomes.
Secondly, potential correlations between all parameters were assessed separately for each sex. To the best of our knowledge, this study is the first to examine the influence of sex on the complex relationships among different dimensions of HRQoL, physical activity (considering intensity), and inflammatory status in patients with CD. This way, our study provides new insights into how inflammation and physical activity may differently affect HRQoL in male and female individuals, an approach that could be essential for developing more personalized and effective intervention strategies in disease management. Surprisingly, while the global analysis (females and males), revealed a negative relationship between sedentary behavior and systemic HRQoL, this association was observed exclusively in females when analyzed separately. Similarly, the inverse correlation between intense physical activity and the gastrointestinal aspects of the HRQoL was evident only in females. In addition, intense physical activity in females was also linked to lower overall HRQoL. These findings suggest that the impact of physical activity on HRQoL is more pronounced in females compared to males, which should be carefully considered when designing and prescribing individualized exercise programs in CD. Regarding inflammation, higher concentrations of FC were associated with lower HRQoL in the total sample (overall and systemic dimensions) and in male patients (overall, systemic and gastrointestinal dimensions), but not in females. A potential explanation for this finding could lie in sex-based differences in the perception and reporting of symptoms, since the concentrations of the objective biomarker FC are similar in women and men. Males with CD may be more likely to associate inflammation-related symptoms with their overall and physical well-being, whereas females could experience a more complex interplay of factors. Therefore, addressing HRQoL outcomes in CD, particularly in female patients, seems to require consideration of additional factors beyond physiological measures like FC, such as psychological and social aspects of the disease.
Several characteristics of the sample warrant consideration when interpreting our findings. More than half of the participants were overweight or obese, which is consistent with recent data indicating a rising prevalence of obesity in patients with CD.43 Obesity may influence both inflammatory activity and HRQoL and could interact with physical activity patterns in complex ways. Additionally, the mean FC level was over 500mg/kg, suggesting that many participants had ongoing subclinical inflammation, despite the fact that over 70% of them were receiving biological treatment. This may reflect the clinical complexity of CD management and the known challenges in achieving complete biochemical control.
While the study provides relevant insights into the relationships explored, certain limitations should be taken into account. First, its cross-sectional design limits the ability to infer causality between the variables studied. Longitudinal research would be valuable to clarify the direction and dynamics of the observed associations. Secondly, the sample size, though sufficient to explore the primary objectives, may have limited the power to detect more subtle interactions. Moreover, several additional clinical and sociodemographic variables that may influence inflammation, HRQoL, or physical activity were not available. Despite these considerations, this study provides novel and relevant data on an underexplored aspect of CD and highlights the importance of integrating PROMs into disease management. Future prospective studies with larger, more clinically detailed samples will be essential to further explore and confirm these findings. Additionally, it will be necessary to analyze specific factors that shape the influence of sex (hormonal impact, menstrual cycle) in CD, and to delve into the influence of gender (impact of gender identity including roles associated with being a woman or a man, self-perception, and social and cultural norms, behaviors and interactions) in CD.
ConclusionsSex differences influence the relationship between inflammation, physical activity, and HRQoL in CD. Females with CD engage in less physical activity and exhibit lower overall HRQoL than males, with particularly pronounced deficits in systemic, emotional, and social dimensions, despite presenting similar inflammation levels as males. These findings highlight the greater impact of CD on females irrespective of physiological measures of inflammation and underscore the importance of addressing emotional and social factors in disease management.
Overall, HRQoL in CD patients is negatively affected by both intense physical activity and sedentary behavior. This effect is especially evident in females, but not in males, emphasizing the need for personalized physical activity and exercise prescriptions. Non-vigorous physical activity appears to be beneficial for CD patients, particularly for females, as it avoids the detrimental effects associated with intense exercise. Higher intestinal inflammation, indicated by elevated FC concentrations, is associated with lower systemic HRQoL. This relationship is more pronounced in males, whereas no such association is observed in females. Consequently, local inflammation levels could serve as a predictor of poorer HRQoL, especially in male patients.
In conclusion, our findings support considering sex differences in CD in future studies and clinical assessments, which may inform more individualized approaches to improve PROs such as HRQoL.
Author contributionsConceptualization: SHR, LMC. Methodology: SHR, LMC. Formal analysis: SHR, LMC, RM, IG, EO. Investigation resources: SHR, LMC. Data curation: SHR, LMC, RM, IG. Writing – original draft: IG. Writing – review & editing: SH, IG, LMC, EO. Visualization: SH, IG, LMC. Supervision: LMC, RM, IG, EO. Funding acquisition: LMC, IG, EO.
Ethics approvalThis study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the University of Extremadura (201/2022) and the Research Ethics Committee of Cáceres (1092022).
Consent to participateInformed consent was obtained from all individual participants included in the study.
FundingThis study was partially supported by the Gobierno de Extremadura-Fondo Europeo de Desarrollo Regional, Spain (GR21079; GR24071). Funding sources had no role in the study design, collection, analysis, and interpretation of the data or the decision to submit the manuscript for publication.
Conflict of interestsThe authors have no relevant financial or non-financial interests to disclose.
We are grateful to the Gastroenterology Service of the University Hospital of Cáceres, particularly the Inflammatory Bowel Disease outpatient nursing clinic, for their valuable collaboration in the study.