Matrix metalloproteinases (MMPs) are overexpressed at different stages of colorectal carcinogenesis and could serve as early surrogate biomarkers of colorectal neoplasia.
ObjectiveTo assess the utility of plasma MMP2 and MMP9 levels in the detection of advanced colorectal neoplasia and their correlation with tissue levels.
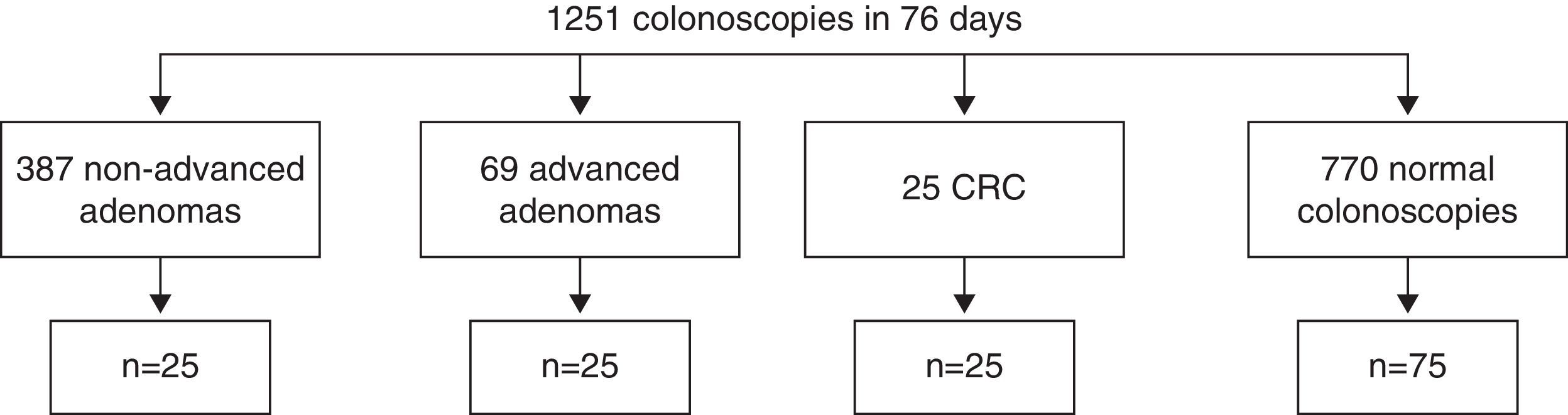
MethodsWe analysed blood and tissue samples from patients with non-advanced adenomas (n=25), advanced adenomas (n=25), colorectal cancer (n=25) and healthy controls (n=75). Plasma and tissue gelatinase levels were determined by Luminex XMAP technology and gelatin zymography. Receiver operating characteristic (ROC) curve analysis was used to calculate the optimum cut-off for the detection of advanced colorectal neoplasia.
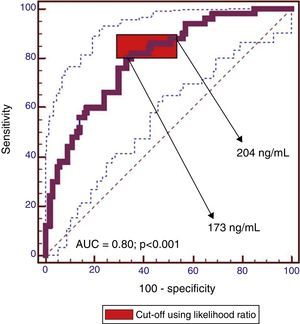
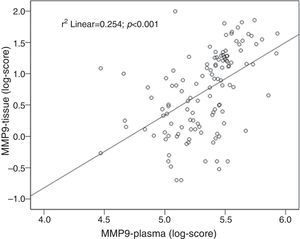
ResultsPlasma MMP2 levels were similar between groups whatever the type of lesion. Plasma MMP9 levels were significantly higher in patients with neoplastic lesions than in healthy controls (median 292.3ng/ml vs. 139.08ng/ml, P<0.001). MMP9 levels were also higher in colorectal cancer than in non-advanced adenomas (median 314.6ng/ml vs. 274.3ng/ml, P=0.03). There was a significant correlation between plasma and tissue levels of MMP9 (r=0.5, P<0.001). The plasma MMP9 cut-off range with the highest diagnostic accuracy was between 173ng/ml and 204ng/ml (AUC=0.80 [95% CI: 0.72–0.86], P<0.001; sensitivity, 80–86% and specificity, 57–67%).
ConclusionPlasma MMP9 could be a surrogate biomarker for the early detection of advanced colorectal neoplasia, although its diagnostic performance could be increased by combination with other biomarkers.
Las metaloproteinasas (MMP) son proteínas que se sobreexpresan en diferentes etapas de la carcinogénesis colorrectal y podrían ser biomarcadores de neoplasia colorrectal.
ObjetivoEvaluar la utilidad de MMP2 y MMP9 en plasma para detectar neoplasia colorrectal avanzada y su correlación con los niveles tisulares.
MétodosSe analizaron muestras de sangre y tejido en pacientes con adenomas no avanzados (n=25), adenomas avanzados (n=25), cáncer colorrectal (n=25) y controles sanos (n=75). Los niveles plasmáticos y en tejido se determinaron mediante tecnología xMAP Luminex y zimografía con gelatina. Se utilizaron curvas ROC para calcular el punto de corte óptimo para neoplasia colorrectal avanzada.
ResultadosLos niveles de MMP2 fueron similares en las distintas lesiones. Los niveles de MMP9 fueron significativamente superiores en los pacientes con lesiones neoplásicas comparados con controles sanos (mediana de 292,3ng/ml vs. 139,08ng/ml; p<0,001). Los niveles de MMP9 fueron más altos en los cánceres colorrectales que en adenomas no avanzados (mediana de 314,6ng/ml vs. 274,3ng/ml; p=0,03). Se observó correlación entre los niveles plasmáticos y tisulares de MMP9 (r=0,5; p<0,001). El rango de MMP9 plasma con mayor precisión diagnóstica fue 173–204ng/ml (AUC=0,80 [IC 95%: 0,72–0,86], p<0,001; sensibilidad 80–86% y especificidad 57–67%).
ConclusiónLos niveles en plasma de MMP9 podrían ser un biomarcador útil para detectar neoplasia colorrectal avanzada. La combinación con otros biomarcadores podría aumentar su rendimiento diagnóstico.
Matrix metalloproteinases (MMPs) are a multigene family of zinc-dependent extracellular matrix (ECM) proteases that play an important role in tissue remodelling. Although they have been implicated in the pathogenesis of various inflammatory diseases,1 they are also involved in different stages of carcinogenesis including ECM degradation associated with tumour growth and angiogenesis, local invasion and extravasation.2 In fact, MMPs are considered the most important factors involved in ECM remodelling, capable of creating a pathway for tumour cells.3
The type IV collagenases, MMP2 (gelatinase A) and MMP9 (gelatinase B), have been extensively studied in epithelial tumours and their expression has been related to the breakdown of the first barrier against invasion, the basement membrane, which has been associated with a high frequency of distant metastasis.4 Both gelatinases also play an important role in the development of the angiogenic phenotype.5,6
In colorectal cancer (CRC), high levels of gelatinases have been associated with increased risk of metastatic disease and poor survival rates.7–10 Several studies have demonstrated overexpression of these enzymes in tissue samples of CRC compared with colorectal adenomas or normal tissue.11,12 Other studies have shown increased levels of gelatinases in serum and plasma of patients with CRC compared with healthy controls (HC), and are considered as potential diagnostic biomarkers.13–16 However, most studies carried out in blood samples have focused on advanced disease13,15 or on testing the usefulness of gelatinase levels as prognostic biomarkers of recurrence after surgery,17,18 but not their potential role in the detection of early stages of CRC (advanced adenoma and carcinoma). In addition, data on gelatinase levels in blood samples as diagnostic biomarkers are scarce and inconsistent between studies.13,14,19,20 Some studies have found an association between certain polymorphisms and the risk of CRC, but the results are not conclusive.21,22 The promoter variant of MMP9, −1562C>T, has been the most extensively studied with contradictory results.21,23,24 The genotype 279R>Q has been associated with early CRC.22
In the current study we hypothesized that MMP2 and or MMP9 may be upregulated in both tissue and plasma samples of patients with advanced colorectal neoplasia, and that elevated gelatinase plasmatic levels could be useful as an early surrogate biomarker of advanced colorectal neoplasia.
The aim of the present study was to assess plasma gelatinase levels in the early stages of the colorectal adenoma–carcinoma sequence and their correlation with tissue levels. In addition, we wished to determine the most accurate plasma MMP cut-off values for detecting advanced neoplasia and whether particular polymorphisms were associated with increased susceptibility to advanced adenoma (AA) or CRC.
MethodsPatientsThe study prospectively included 75 consecutive consenting patients undergoing colonoscopy during a period of two and a half months (September 15–November 29, 2012). Patients were included until we obtained 25 patients for each neoplastic lesion group: non-advanced adenoma (NAA), AA and CRC (Fig. 1). We also included 75 HC (ratio 1:1) (Fig. 1). Neoplastic colorectal polyps were classified as AA (size ≥1cm or presence of high grade dysplasia or villous or tubulo-villous glandular pattern) or NAA.25 Patients with inflammatory bowel disease, history of colorectal neoplasm or hereditary polyposis syndrome were not included in the study. The following variables were collected: demographic characteristics (age and sex), history of cancer or autoimmune diseases, family history of CRC and smoking status. The study protocol was approved by the Clinical Research Ethics Committee of the University Hospital of the Canary Islands. The procedures were in accordance with the World Medical Association's declarations of Helsinki. Informed consent was obtained from all the participants of the study.
Colonoscopic examinationColonoscopies were performed by 4 experienced endoscopists (average 300 colonoscopies per year). Those were performed with conventional colonoscopes (CF-165L and CF-Q145L Olympus Corp., Tokyo). Whenever a flat lesion was suspected, 0.5% indigo carmine was directly applied with a syringe. Polyp size was determined by comparison of the lesion with fully opened biopsy forceps (6mm). Lesions were sampled either with a biopsy forceps before polypectomy, or with a scalpel after polypectomy. Flat lesions were resected by mucosectomy and protruded lesions by snare polypectomy. Paired samples of all lesions and normal surrounding tissue were collected. One of each pair was used for pathological diagnosis on haematoxylin/eosin stained paraffin sections and the other was stored at −80°C until used for protein extraction as described below. The following data were collected: size, histology (neoplastic or hyperplastic), degree of dysplasia (low-grade or high-grade dysplasia) and glandular pattern (villous, tubulo-villous and tubular) assessed using WHO criteria.26 Biopsy specimens were collected after informed patient consent.
Plasma metalloproteinases quantificationBlood samples of all study participants were drawn after colonoscopy. Plasma was separated within 1h after blood collection, aliquoted and stored at −80°C until MMP determination. A second blood sample was obtained from all consenting patients with advanced neoplasia; in order to compare MMP plasma levels before and after treatment.
Two experienced researchers (JT, ES), blinded to the type of lesion, were responsible for MMP determination in tissue or plasma. MMP2 and MMP9 levels were measured using Fluorokine MultiAnalyte Profiling kits from R&D Systems (Minneapolis, MN, USA) and the Luminex 200 system (Luminex corp. Austin, TX, USA). The samples were diluted 20-fold and processed according to manufacturer instructions. The kit contains specific MMP antibodies covalently bound to specific sets of microspheres, each set having a unique spectral signature and a specific MMP antibody. The samples were incubated with the mixture of the two antibody-coated microspheres, and after a washing step, incubated with a mixture of specific biotinylated antibodies for each MMP which bind to the MMPs captured by the microspheres. A final incubation with phycoerythrin–streptavidin produced the final complex microsphere-antibody–MMP-antibody–phycoerythrin for each analyze. The samples were then loaded in the Luminex analyzer, which split the two microspheres signals according to spectral signature, and measured the phycoerythrin intensity for each MMP. The median fluorescence intensity for each measurement was extrapolated to a standard curve produced with known amounts of recombinant MMP.
Tissue gelatinase activityTissue samples were stored at −80°C until analyzed. Biopsy specimens (3–5mg) were homogenized every week in extraction buffer (50mmol/l Tris–HCl pH=7.6, 150mmol/l NaCl) and protease inhibitors (Complete, Roche), sonicated two times for 10s (UP100H, Dr Hielscher, Germany) at 1min intervals and, after 10min on ice, protein extracts were centrifuged at 10,000×g for 10min. The supernatants were removed and assayed for protein concentration (Bicinconinic acid, Sigma, St Louis). Homogenates were stored at −80°C until assayed.
The activity of both gelatinases was analyzed by zymography on tissue homogenates, as previously described.27 Both active and pro-MMP-2 and pro-MMP9 were determined, since the active form of MMP9 (82kDa) is rarely detectable by zymography.28 Briefly, proteins (25μg per lane) were separated in 10% polyacrylamide gel electrophoresis (PAGE) containing 0.2% gelatin (Sigma, Co.) under non-reducing, non-denaturing conditions. Gelatinases were detected using a developing buffer (50mmol/l Tris–HCl pH=7.5, 200mmol/l NaCl, 10mmol/l CaCl2 and 1μmol/l ZnCl2). Serine protease inhibitors (2μg/ml aprotinin) and MMP inhibitors (10mmol/l EDTA or 0.1mmol/l o-phenantroline) were added to the developing buffer, to assess whether the observed gelatinolytic bands were caused by serine protease activity or MMP activity. Human MMP9 and MMP2 standards (Oncogene Research) were run as controls. After 18h development, gels were fixed and stained in 40% methanol, 10% acetic acid and 0.1% (w/v) Coomassie Blue R-250 (Sigma) for 1h and then distained. Relative molecular weights of clear bands were analyzed in comparison to molecular weight standards (DualColor, BioRad) and purified human MMP2 and MMP9. Zymographic activity of each band was measured in terms of optical density/mm2 (OD/mm2) using a calibrated densitometer (GS-800, BioRad) and QuantityOne Quantitation analysis software (BioRad, version 4).
Metalloproteinase-9 expressionSince MMP9 activity is markedly enhanced in homogenates of advanced adenomas and CRC,11 we tested the expression of this gelatinase by western blot analysis.29 Samples were denatured in reducing buffer and loaded (50μg/lane) onto 10% polyacrylamide gels. After SDS-PAGE electrophoresis, proteins were transferred onto nitrocellulose membrane (Protran, Schleider & Schuell) and detected using a rabbit anti-MMP9 (Chemicon) at 1:1000 dilution, a matching secondary antibody (Jackson Immunoresearch) and a chemiluminiscent substrate (Pierce) according to the manufacturer's instructions. The chemiluminiscent signal was imaged on X-OMAT film (Kodak).
Single nucleotide polymorphism (SNP) analysisPolymorphisms were analyzed by one experienced researcher (ES), blinded to the type of lesion. Several SNPs have been described in the MMP9 gene, but we assessed only two, −1572C>T and R279Q, because other polymorphisms are known to be in linkage disequilibrium with these. DNA was prepared from 3ml of peripheral blood using proteinase K treatment, phenol-chloroform extraction and ethanol precipitation. About 100ng DNA were used as template in polymerase chain reactions (PCR) using primers flanking either the −1572C>T SNP (rs3918242) or the R279Q SNP (rs17576) in the MMP9. For −1572C>T SNP, primers 5′-GGCACATAGTAGGCCCTTTAA-3′ and 5′-TCACTCCTTTCTTCCTAGCCA-3′ were used to amplify a 424bp product that was treated with SphI in order to distinguish the C allele (424bp) from the T allele (264+178bp). For R279Q, primers 5′-GAGAGATGGGATGAACTG-3′ and 5′-GTGGTGGAAATGTGGTGT-3′ were used to amplify a 439bp product that was treated with MspI in order to distinguish the Q allele (252+187bp) from the R allele (187+129+123bp).
The amplified DNA was restricted with endonucleases (New England Biolabs, Boston, MA, USA) for two hours at 37°C. The resulting DNA fragments were separated by electrophoresis in 5% acrylamide gels and visualized under ultraviolet light after staining with ethidium bromide. Genotyping was performed in a blinded manner.
Statistical analysisResults are expressed as means and standard deviation, medians and ranges. As plasma and tissue levels of gelatinases did not follow a normal distribution, non-parametric statistical tests were used. Mann–Whitney U test was used to compare plasma concentrations between the different groups (HC, NAA, AA and CRC). The same test was used to compare gelatinase activity in tissue between the different groups. The performance of gelatinases to discriminate patients with advanced neoplasia (AA and CRC) from those without (healthy controls, NAA) was evaluated using Receiver Operating Characteristic (ROC) curve analysis. We calculated areas under the curve and 95% confidence intervals (CI), sensitivities, specificities, predictive values and likelihood ratios.
Pearson's test was used with log-transformed values to assess the correlation between plasma levels of MMP9 and pro-MMP9 activity in tissue. Differences with a P value less than 0.05 were considered statistically significant. Data were analyzed with the Statistical Package for Social Sciences v. 17.0 (SPSS Inc., Chicago, IL, USA) and MedCalc v. 12.0.4 (Mariakerke, Belgium).
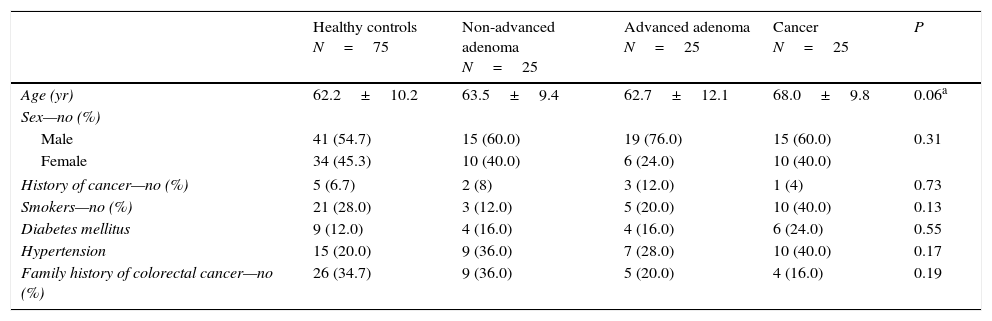
ResultsBaseline participant characteristics including sex, history of cancer, smoking habit, diabetes, hypertension or family history of CRC were similar between the four groups (Table 1). Controls were significantly younger than patients in the CRC group (Table 1). However, no significant difference in age was found between patients stratified into advanced and non-advanced colorectal neoplasm (P=0.13).
Baseline characteristics of the included patients.
| Healthy controls N=75 | Non-advanced adenoma N=25 | Advanced adenoma N=25 | Cancer N=25 | P | |
|---|---|---|---|---|---|
| Age (yr) | 62.2±10.2 | 63.5±9.4 | 62.7±12.1 | 68.0±9.8 | 0.06a |
| Sex—no (%) | |||||
| Male | 41 (54.7) | 15 (60.0) | 19 (76.0) | 15 (60.0) | 0.31 |
| Female | 34 (45.3) | 10 (40.0) | 6 (24.0) | 10 (40.0) | |
| History of cancer—no (%) | 5 (6.7) | 2 (8) | 3 (12.0) | 1 (4) | 0.73 |
| Smokers—no (%) | 21 (28.0) | 3 (12.0) | 5 (20.0) | 10 (40.0) | 0.13 |
| Diabetes mellitus | 9 (12.0) | 4 (16.0) | 4 (16.0) | 6 (24.0) | 0.55 |
| Hypertension | 15 (20.0) | 9 (36.0) | 7 (28.0) | 10 (40.0) | 0.17 |
| Family history of colorectal cancer—no (%) | 26 (34.7) | 9 (36.0) | 5 (20.0) | 4 (16.0) | 0.19 |
Most CRCs were well differentiated (n=18; 72%), located in the distal colon (n=15; 60%) and most patients presented at an advanced stage (24% stage I, 20% stage II, 32% stage III and 20% stage IV). Most AA (median size 17mm, range 10–30mm), were tubular (n=19; 76%), 2 harboured high-grade dysplasia and they were located in the distal colon (n=15; 60%). Regarding patients with NAA (median size 17mm, range 10–30mm), in most cases the lesions were located in the distal colon (44% distal, 20% proximal, 36% both).
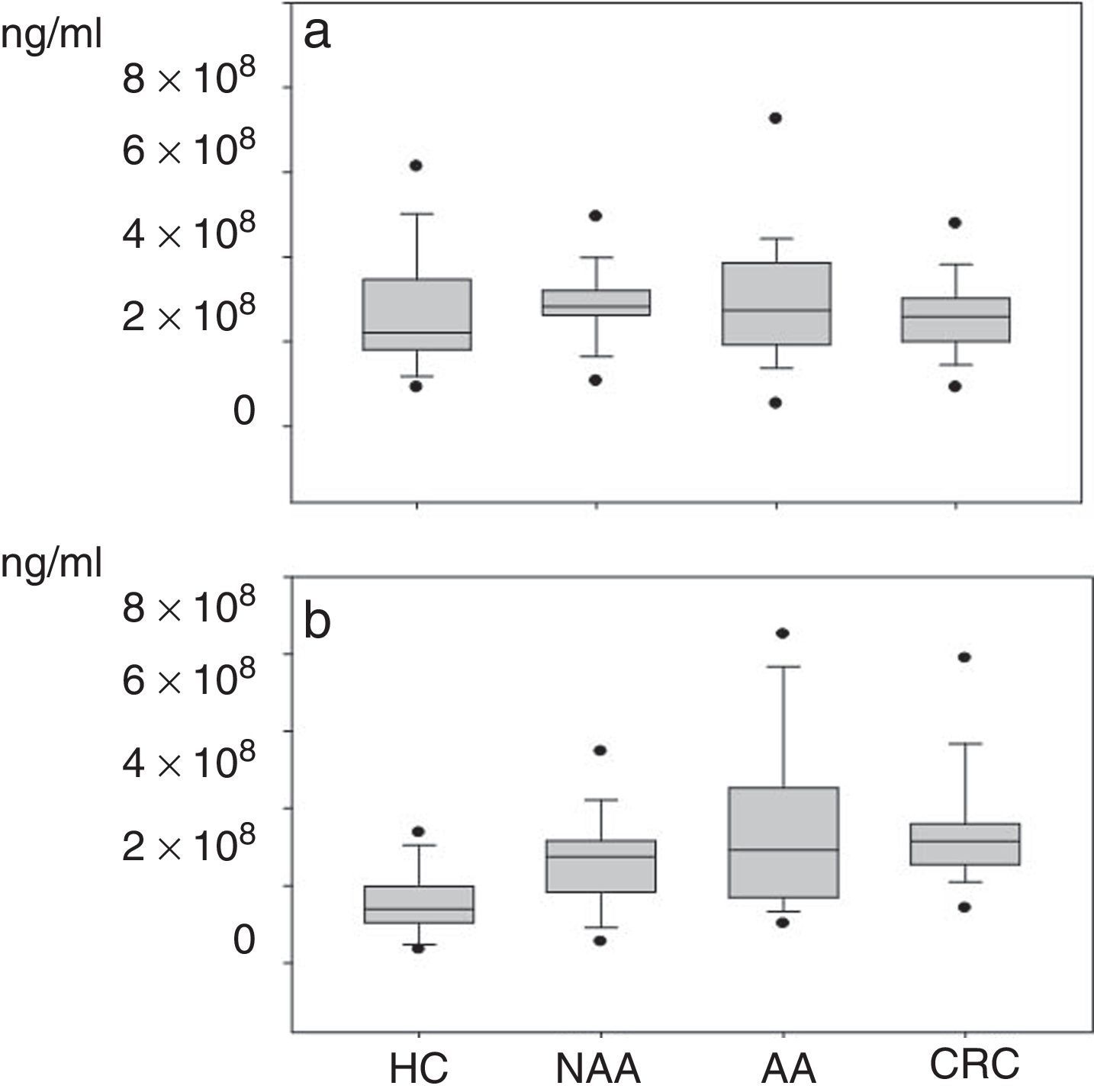
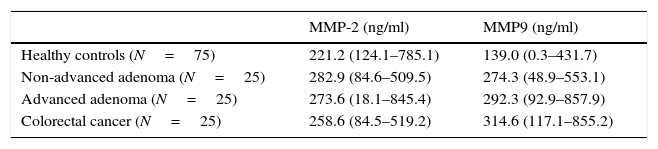
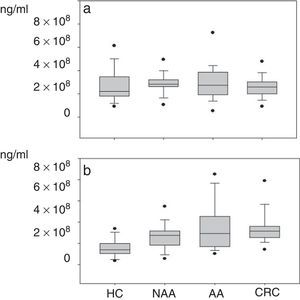
Plasma gelatinase determinationPlasma concentrations of MMP2 and MMP9 in HC and patients with NAA, AA and CRC are shown in Table 2. Median plasma levels of MMP-2 were not significantly different between the four groups (Table 2, Fig. 2a). There were significant differences in MMP9 levels when samples of patients with neoplastic lesions were compared with those of HC (median 292.3ng/ml vs. 139.08ng/ml, P<0.001) (Table 2, Fig. 2b).
Plasma levels of MMP-2 and MMP-9 in healthy controls (HC) and patients with non-advanced adenoma (NAA), advanced adenoma (AA) and colorectal cancer (CRC). Results are expressed as medians (ranges).
| MMP-2 (ng/ml) | MMP9 (ng/ml) | |
|---|---|---|
| Healthy controls (N=75) | 221.2 (124.1–785.1) | 139.0 (0.3–431.7) |
| Non-advanced adenoma (N=25) | 282.9 (84.6–509.5) | 274.3 (48.9–553.1) |
| Advanced adenoma (N=25) | 273.6 (18.1–845.4) | 292.3 (92.9–857.9) |
| Colorectal cancer (N=25) | 258.6 (84.5–519.2) | 314.6 (117.1–855.2) |
MMP-2. HC vs. NAA: P=0.07; HC vs. AA: P=0.19; HC vs. CRC: P=0.50; NAA vs. AA: P=0.9; NAA vs. CRC: P=0.09; AA vs. CRC: P=0.18.
MMP-9. HC vs. NAA: P=0.001; HC vs. AA: P<0.001; HC vs. CRC: P<0.001; NAA vs. AA: P=0.38; NAA vs. CRC: P=0.03; AA vs. CRC: P=0.52.
(a) Plasma levels of MMP-2*: NAA vs. HC: P=0.07; AA vs. HC: P=0.20; CRC vs. HC: P=0.50; NAA vs. AA: P=0.9; NAA vs. CRC: P=0.09; AA vs. CRC: P=0.18. (b) Plasma levels of MMP-9: NAA>HC: P=0.001; AA>HC: P<0.001; CRC>HC: P<0.001; CRC NAA: P=0.03; no significant differences between NAA vs. AA (P=0.38) and AA vs. CRC (P=0.51). *Healthy controls (HC); non-advanced-adenoma (NAA); advanced adenoma (AA); colorectal cancer (CRC).
Although progressive up-regulation of MMP9 was found in NAA, AA and CRC, statistically significant differences were only demonstrated in the CRC group compared with the NAA group. Patients with advanced neoplasia showed significantly higher MMP9 levels than NAA and HC (median 316.7ng/ml vs. 162.4ng/ml respectively, P<0.001). However, no significant differences were found regarding MMP2 levels (median 260.6ng/ml vs. 247.8ng/ml respectively, P=0.54). When MMP9 levels were compared between patients with advanced neoplasias located in the proximal and distal colon no significant differences were found either (326.8ng/ml vs. 302.8ng/ml respectively, P=0.686)
Blood samples were collected either after surgery or polypectomy treatment (median interval between treatment and second blood sample: 9.5 months, range 6–12 months) in 16 willing participants (8 with CRC and 8 with AA). Plasma MMP9 and MMP2 levels reduced markedly after treatment (median MMP9 levels decreased from 359.9ng/ml to 29.0ng/ml and median MMP2 levels from 240.4ng/ml to 124.8ng/ml) in most patients (S1).
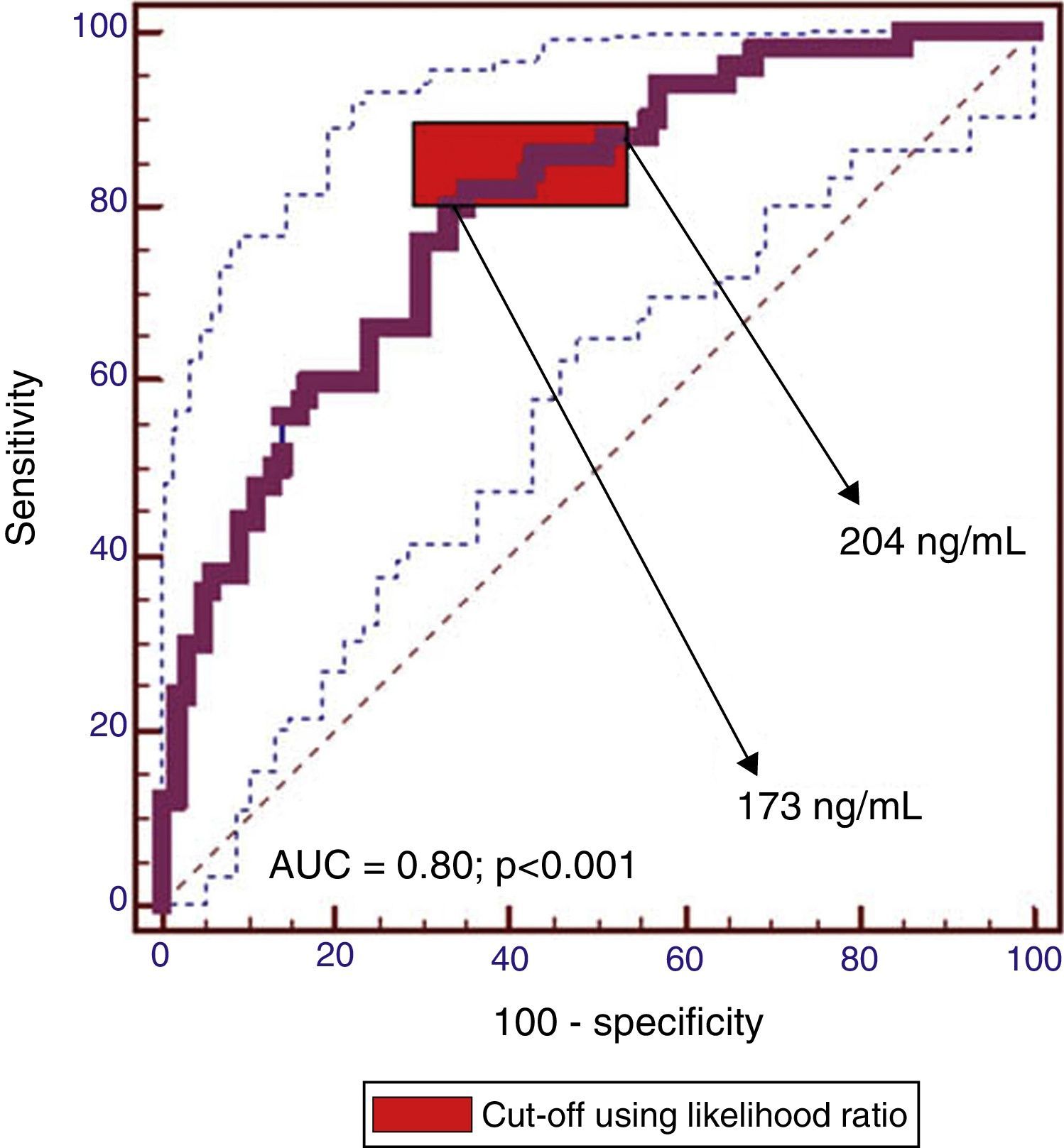
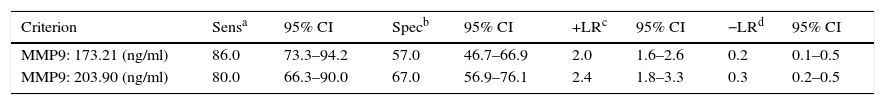
The cut-off range corresponding to the highest diagnostic accuracy was between 173ng/ml and 204ng/ml (AUC=0.80 [IC95%: 0.72–0.86], P<0.001) (Fig. 3, Table 3).
Performance of plasma MMP9 to discriminate between patients with advanced neoplasia (advanced adenoma and CRC) and without (healthy controls, non-advanced adenoma).
The cut-off value corresponding to 90% sensitivity was 151ng/ml, but specificity dropped to 44%. Conversely, for a specificity of 90%, the optimum cut-off value was 319ng/ml, but at the expense of a decrease in sensitivity to 44%.
Single nucleotide polymorphism analysisThe frequency of MMP9 −1562C>T and 279R>Q genotypes showed no significant difference between patients with advanced neoplasia and the rest (data not shown). The most frequent genotype for the −1562C>T gene was CC, whereas for the 279R>Q gene it was QR and QQ.
Tissue gelatinase determinationMMP9 and MMP2 activities were quantified in homogenates of normal tissue, NAA, AA and CRC by gelatin zymography in order to determine the activity of MMP2 and MMP9 in the different tissue types (S3).
The median level of pro-MMP2 activity was significantly higher in CRC samples (7.5OD/mm2 (range 1.0–18.5OD/mm2) than in normal mucosa (3.2OD/mm2 (range 0–10.0OD/mm2, P=0.001), but the difference was not statistically significant when compared with NAA (4.1OD/mm2 (range 1.4–12.8OD/mm2, P=0.06) or AA (8.3OD/mm2 (range 1.5–25.5OD/mm2)(S4a). Pro-MMP2 activity was significantly higher in AA compared with normal tissue (P<0.001) or NAA (P=0.003).
In addition, the median activity level of active-MMP2 (S4b) in CRC samples (3.3OD/mm2 (range 0.0–11.5OD/mm2) was higher compared with normal tissue (0.2OD/mm2 (range 0.0–0.6OD/mm2, P<0.001), NAA (0.2OD/mm2 (range 0.0–3.6OD/mm2, P<0.001) or AA (0.3OD/mm2 (range 0.0–12.0OD/mm2, P=0.002)
The activity of pro-MMP9 in neoplastic and non-neoplastic tissues is shown in S4c. There was a significant and progressive activation of the latent form of MMP9 in the different types of colorectal neoplasms. The median level of pro-MMP9 activity in CRC samples (19.2OD/mm2 (range 9.7–63.9OD/mm2), AA (16.8OD/mm2 (range 0.0–99.4OD/mm2) and NAA (2.7OD/mm2 (range 0.0–71.7OD/mm2) was significantly higher than in normal mucosa (1.0OD/mm2 (range 0.0–18.9OD/mm2, P<0.001). For neoplastic polyps, AA presented significantly higher pro-MMP9 activity than NAA (P<0.001) and pro-MMP9 activity in CRC samples was also higher than in AA and NAA samples (P<0.001). Western blot analysis confirmed the expression of pro-MMP9 protein in advanced colorectal neoplasms, as shown by the immunoreactive band migrating at 92kDa (S3)
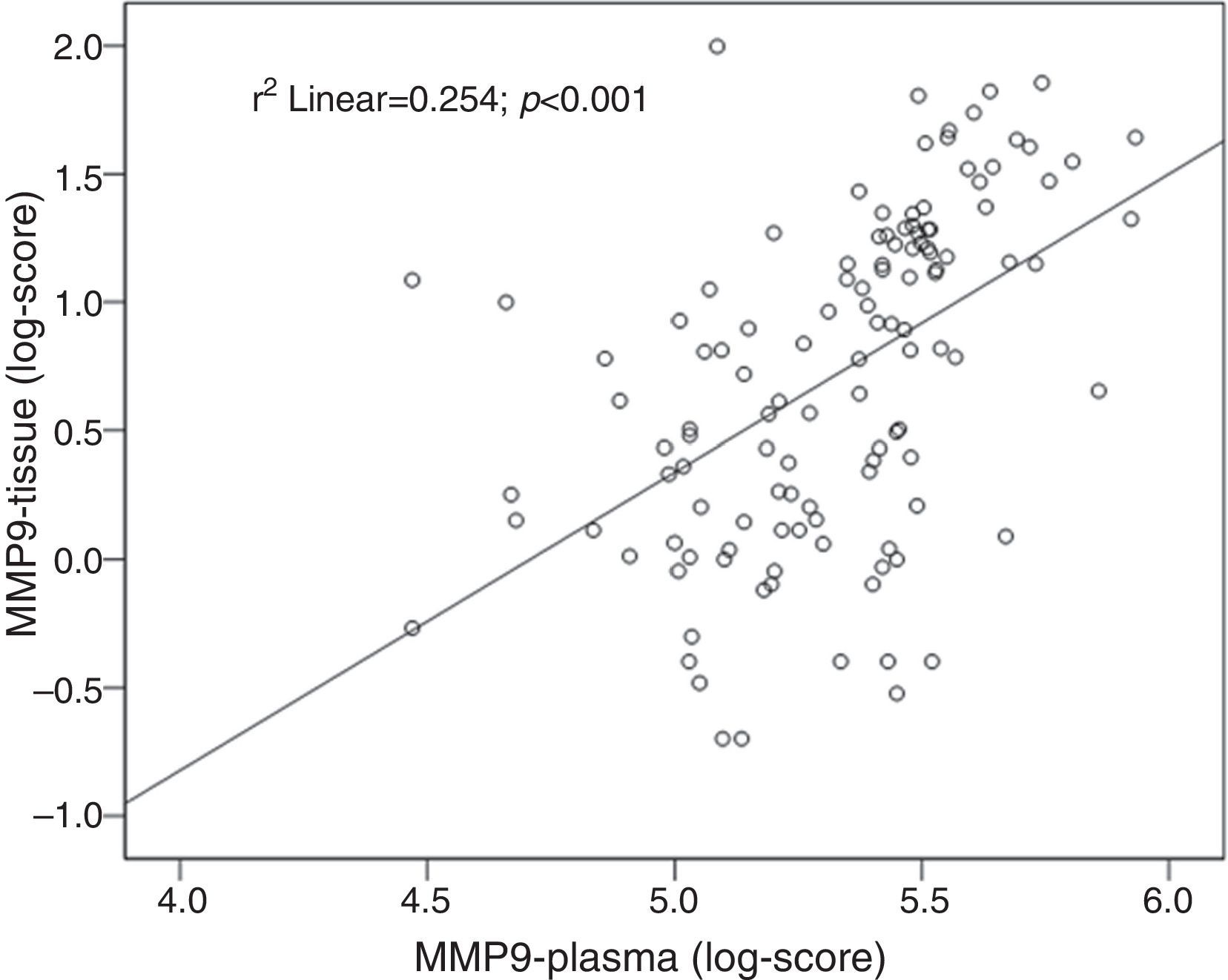
Tissue and plasma MMP9 correlationA positive correlation between tissue and plasma levels of MMP9 was found: r=0.50; P<0.001 (Fig. 4).
DiscussionStructural tests (colonoscopy, sigmoidoscopy or barium enema) or faecal occult blood tests are recommended as screening strategies for the early detection of advanced colorectal neoplasms in family-risk and average-risk populations.30 However, these tests have several drawbacks, including lack of widespread availability, risk of severe complications, and patient discomfort which could interfere with compliance. Tests based on blood sample analysis may be more convenient and acceptable, avoiding direct risks and user manipulation.31
The present case-controlled study showed that plasma MMP9 could be a useful biomarker of advanced colorectal neoplasms. Although complex formation, degradation and proform activation of MMPs may be different in plasma and tissue, we found a positive correlation between plasma and tissue levels, which supports the hypothesis that the main origin of elevated MMPs is the presence of colorectal neoplasm. Moreover, in a sample of patients with advanced neoplasia, a dramatic reduction of plasma levels of both MMPs was found after treatment. Therefore, if increasing levels of plasma MMP9 are indicative of advancing neoplasia, they should also arouse suspicion of recurrence in treated patients.
Few studies have assessed MMPs in blood samples as diagnostic biomarkers.13,15,16,19,20,32,33 In most, MMP9 levels in serum or plasma samples were increased in CRC compared with HC.13,16,32,33 However, the difference was less clear on comparing CRC with adenomas, or adenomas with HC.14,16,32 Tutton et al.,16 compared MMP9 levels in 132 CRC, 13 adenomas and 47 HC; they found higher levels in CRC compared with adenoma or HC but similar levels when adenomas and HC were compared. However, in another study including 63 CRC, 27 AA and 46 HC,32 higher levels were found in neoplastic lesion samples, with no difference between CRC and AA. In a third study,14 35 adenomas, 75 CRC and 70 HC were included. In this case, MMP levels were higher in CRC compared with adenomas or HC and in adenomas compared with HC.
To our knowledge, only two recent studies have determined MMP9 levels at different stages of colorectal carcinogenesis, including advanced colorectal neoplasms (CRC and AA) and NAA.34,35 This is particularly interesting since the major goal of screening is the detection of advanced colorectal neoplasms, including not only CRC but also AA.25 Small NAA present low risk of transformation into CRC and are not considered an issue in CRC screening.36 In the first study, 68 CRC, 28 adenomas (17 tubulovillous and 11 tubular adenomas) and 17 healthy blood donors were included and serum MMP9 was assessed by zymography. MMP-9 values in patients with adenomas were similar to those of the controls, and only in the most advanced stages of CRC were they significantly increased.34 Conversely, and in accordance with our results, a large study including 3 CRC, 43 AA and 165 NAA by Wilson et al. showed higher levels of serum MMP9 in the patients with advanced neoplasia than in those with non-advanced neoplasia, with a sensitivity and specificity of 79% and 63%, respectively.35
Discrepancies between studies may be related to the number of patients included and the type of samples used, as some authors have pointed out.13 Indeed, either serum14,32 or plasma samples16 have been used. The use of serum samples has been criticized because serum mostly contains MMPs released in vitro either by platelets and leukocytes during coagulation or induced by the clot activator itself.37,38 For this reason, plasma MMP9 may offer better diagnostic accuracy.39 Plasma samples were used in our study, following the recommendations of some authors.37 Results using the same technique may also differ depending on the assay with different substrate specificities.20 Although enzyme-linked immunosorbent assay (ELISA) has traditionally been used to identify MMPs in serum or plasma samples, a different technique was used in the present study. Luminex technology is increasingly used in different settings to assess MMP levels, although not in CRC studies.40,41 This technology allows multiplexing MMP measurements in a single assay with greater flexibility, reduced sample volume and lower cost than ELISA. These features are advantageous when several analyses are being performed, in which case the labour cost for ELISA is much higher. In addition, the use of microspheres in solution increases the surface area in direct contact with the sample, thus increasing the sensitivity of the technique. Another possible reason for discrepancy is the lack of stratification of adenomas (NAA vs. AA) in most studies. In this regard, our group demonstrated a progressive up-regulation of pro-MMP9 activity from normal mucosa through to CRC11 tissue samples, suggesting that NAA and AA have a different gelatinase profile. Using gelatin zymography, the present study also reproduced these previous findings.
In the current study, plasma MMP2 concentration was significantly higher in patients with neoplastic lesions compared with HC. Contradictory results have been reported regarding MMP2 values,15,16,18,19 but our findings indicate that MMP2 determination in plasma is not useful for predicting CRC.13,19 Despite our small sample size, none of the MMP9 polymorphisms showed enhanced susceptibility to advanced neoplasia, which is consistent with the results of a recent meta-analysis.42
The present study has certain limitations. Firstly, the isolated determination of this enzyme showed only moderate diagnostic performance and was not accurate enough to be recommended in clinical practice. However, recent evidence supports the usefulness of type 1 tissue inhibitor of matrix metalloproteinase (TIMP-1) in the diagnosis of CRC, with encouraging results.14 Unfortunately, TIMP-1 measurement was not included in the present study. The combined use of both markers may increase predictive accuracy for advanced neoplasms. Secondly, patient participants were subjects with a scheduled colonoscopy and therefore the sample is not representative of the average-risk population for CRC. However, the purpose of the study was only to determine the gelatinase profile of different types of lesions rather than its usefulness as a screening technique. Thirdly, Luminex technology and gelatin zymography measure different characteristics of MMPs, and therefore, a perfect correlation was not expected. Still, we did find a fair correlation between the two methods and this, we believe, is a strength of the study. Fourthly, we are aware that most CRCs included were diagnosed in an advanced stage. For the purpose of this study, a more representative sample of early CRCs would have been desirable. However, the increased MMP9 levels in AA suggested that this biomarker might be raised before CRC development. Finally, our group of patients with advanced neoplasms was somehow heterogeneous. Although the defining characteristics of the AA (size, villous pattern and high-grade dysplasia) may have a different influence on MMP9 levels, they frequently coexist in the same lesion. On the other hand, a more homogeneous sample of CRC regarding tumour differentiation and stage would have been desirable.
In conclusion, this study using Luminex technology suggests that plasma MMP9 is increased in advanced neoplasia and could be useful as a surrogate biomarker in CRC screening programmes. However, in isolation it lacks sufficient diagnostic accuracy to be able to recommend its use in clinical practice. Studies assessing its diagnostic performance in screening settings are warranted, especially in combination with other biomarkers.
Source of fundingThis work was supported by a grant from the Fundación Canaria de Investigación y Salud (convocatoria 2008). It had no involvement in study design, collection, analysis, interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
Conflict of interestThe authors declare no conflict of interest.