Standard bowel cleansing for colon capsule endoscopy (CCE) requires a liquid diet and bowel laxatives for at least 2 days, which is a major drawback of this procedure and affects tolerance and acceptability.
ObjectiveTo compare the quality of colon cleanliness achieved with one-day versus two-day bowel preparation in outpatients undergoing CCE.
MethodsPatients were randomly assigned to one of two groups: group I (one-day schedule, n=20) received a fiber-free diet and 3L of polyethylene glycol (PEG) on day 0; group II (two-day schedule, n=20) received a liquid diet and 3L of PEG in the evening of day −1, and 1L of PEG in the early morning of day 0. In both groups, the patients received 15mg bisacodyl on day −1 and one or two additional sodium phosphate (NaP) boosters following capsule ingestion. Each colon segment was assessed for cleanliness using a four-point grading scale (excellent=1, good=2, fair=3, and poor=4). For the final analysis, colon cleanliness was rated as adequate (good or excellent) or inadequate (fair or poor).
ResultsOverall colon cleanliness was adequate in 94% (CI 91–97) of patients in group I versus 80% (CI 72–88) in group II (P=0.27). No significant differences were observed in the per-segment quality of colon cleansing between the two groups. CCE reached the rectum in 80% (CI 73–87) of patients in group I versus 75% (CI 67–83) in group II (p=0.59).
ConclusionThe quality of colon cleanliness achieved with one-day bowel preparation is equivalent to that of the standard two-day schedule in patients undergoing CCE.
El inconveniente principal de la cápsula endoscópica de colon (CEC) es una preparación intestinal que exige una dieta estricta y la toma de soluciones evacuantes durante al menos los 2 días previos a la prueba, limitando su aceptabilidad.
ObjetivoComparar la calidad de la preparación intestinal administrada en un día respecto a la de 2 días para la CEC.
MétodosSe aleatorizan 2 grupos: Grupo I (preparación-1 día, n=20) dieta sin fibra con 3L de polietilenglicol (PEG) el día 0; Grupo II (preparación-2 días, n=20) dieta líquida y 3L de PEG la noche previa (día 1) y 1L de PEG el día 0. En ambos grupos se administra 15mg de bisacodilo el día 1 y fosfato sódico (NaP) como propulsor tras la ingesta de CEC. Cada segmento del colon fue valorado según la escala de limpieza de 4 grados (excelente=1, buena=2, regular=3, y mala=4). Para el análisis final, la limpieza global se cataloga como adecuada (1 y 2) o inadecuada (3 y 4).
ResultadosLa limpieza global fue adecuada en un 94% (IC: 91–97) de pacientes del Grupo I frente al 80% (IC: 72–88) en el Grupo II (P=0,27). No se determinaron diferencias cuando se compara la limpieza por segmentos colónicos. La CEC alcanza el recto en el 80% (IC: 73–87) del Grupo I frente al 75% (IC: 67–83) del Grupo II (p=0,59).
ConclusiónLa calidad de la limpieza colónica para la CEC obtenida con la preparación de un día único es similar a la preparación estándar de 2 días.
Colon capsule endoscopy (CCE) was developed as a safe, minimally invasive, patient-friendly alternative to conventional colonoscopy (CC) for the examination of the colonic mucosa. When compared with CC, CCE has been shown to be effective for detecting polyps, tumors and other colonic lesions. In a recent meta-analysis, pooled data showed that CCE had a per-patient sensitivity of 73% and specificity of 89% for any finding (i.e. report of any polyp found, regardless of size) compared to CC.1
An important pre-requisite for a complete examination of the colonic mucosa in both CC and CCE is adequate bowel preparation. The frequency of inadequate colonic cleansing reported in CC varies between 10% and 75%.2 Several studies have shown a positive correlation between the quality of colon cleansing and the time lapse from the last laxative intake to the beginning of the procedure.2,3 In fact, there is evidence suggesting that a time lapse of 3–4h between the last laxative intake and CC significantly improves the diagnostic yield as compared to the day-before cleansing schedule.3–6 Moreover, some authors have observed significantly higher quality preparation of the right colon with same-day versus day-before cleansing.4
Standard preparation for CCE has been developed on an empirical basis and requires a liquid diet the day before the procedure plus the administration of up to 4L of polyethylene glycol (PEG), distributed between the evening before and the morning of the procedure.1,7 CCE also involves capsule propulsion through the intestinal tract and boosters of sodium phosphate (NaP) or polyethylene glycol (PEG) are required to facilitate capsule propulsion and excretion. However, recent studies have suggested that low volume preparation regimens for bowel cleansing in patients undergoing CCE may achieve adequate colonic cleansing, which could improve patient acceptance of CCE and reduce resource consumption. Since reduced volume regimens given the same day of the procedure have also been proved to be effective in CC,2–4 we hypothesized that bowel preparation performed 3h before CCE ingestion would be equally effective than the standard two-day schedule. Therefore, in the present randomized pilot study we compared the feasibility and efficacy of one-day versus two-day bowel cleansing schedule in outpatients undergoing CCE.
Patients and methodsStudy design, inclusion and exclusion criteriaIn this prospective, randomized pilot trial (EudraCT 2010-019135-35), 44 patients were enrolled between April and December 2011. Patients eligible for inclusion in the study were women and men between 18 and 80 years of age who refused CC or had a recent incomplete CC. Exclusion criteria included prior bowel surgery or obstruction, implantable defibrillator, pregnancy, advanced heart or kidney failure, or contraindications to bowel preparation and/or prokinetic agents used in the study.
The Ethics committee of Hospital Universitario de Canarias approved the study and all participants signed written informed consent. After enrollment, patients were assigned by an independent investigator to one of two groups: Group I (one-day preparation) and Group II (two-days preparation) using a computer generated random number table. Patients received written instructions on the bowel preparation schedule and about the CCE procedure.
Colon preparation before capsule administrationPatients in Group I followed a low-fiber diet the day before the CCE procedure (day −1) and received 15mg of bisacodyl on the evening before. Next morning, participants ingested 3L of polyethylene glycol (PEG) between 6:00 and 7:30 am and were scheduled for CCE at 9:00 am. Patients in Group II were instructed to follow a clear liquid diet and to drink 3L of PEG in the evening of day −1. In the early morning of day 0 these patients received 1 additional liter of PEG between 6:00 and 7.00 am, and were scheduled for CCE at 9:00 am.
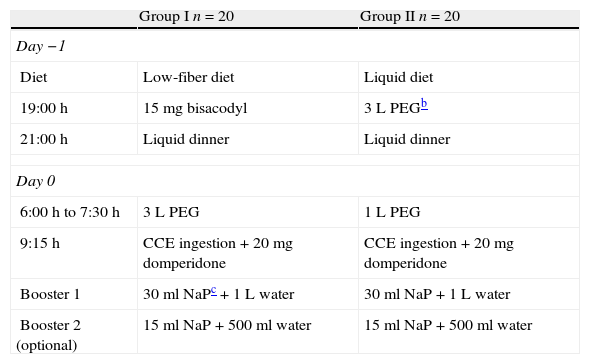
Colon cleansing regimens and study design are summarized in Table 1.
Bowel cleansing regimens for CCEa.
| Group I n=20 | Group II n=20 | |
| Day −1 | ||
| Diet | Low-fiber diet | Liquid diet |
| 19:00h | 15mg bisacodyl | 3L PEGb |
| 21:00h | Liquid dinner | Liquid dinner |
| Day 0 | ||
| 6:00h to 7:30h | 3L PEG | 1L PEG |
| 9:15h | CCE ingestion+20mg domperidone | CCE ingestion+20mg domperidone |
| Booster 1 | 30ml NaPc+1L water | 30ml NaP+1L water |
| Booster 2 (optional) | 15ml NaP+500ml water | 15ml NaP+500ml water |
The characteristics of first generation CCE (PillCam® colon capsule, Given Imaging Inc, Yoqneam, Israel) has been described previously.7 Briefly, this device measures 11mm×32mm and has a dual camera system, allowing image acquisition of four frames per second and a total operating duration of 10h approximately. After initial turn on, the capsule enters a delay mode for about 2h and restarts spontaneously with transmission of images. Endoscopic images are registered in a portable recorder, which has an antenna array and a receiver attached to the body. After completion of the examination, data are downloaded from the recorder to the RAPID® workstation for processing and subsequent viewing.
In the current study, CCE was activated at 8:00 am and in all cases the capsule was ingested at 9:15 am together with 20mg of domperidone. Capsule gastric emptying was checked with the Real Time Viewer© (Rapid® Access Real Time, Given Imaging). When the capsule reached the small bowel, an oral sodium phosphate (NaP) booster (30ml) followed by one liter of clear water were administered. If the capsule had not been excreted 3h later, a second booster was added (15ml of oral NaP and 500ml of clear water). If the colon capsule had not been excreted after 5h, a 10mg bisacodyl suppository was administered (Table 1).
Assessment of bowel cleanliness, colonic findings and adverse effectsColon cleanliness was graded for each of the following intestinal segments: right colon (cecum and ascending colon), transverse colon, left colon (descending and sigmoid colon) and rectum. A four-point grading scale was used to assess colon cleanliness as previously described7: excellent (1 point) if only small traces of feces are seen in the intestinal lumen; good (2 points) refers to a small amount of stool or dark content; fair (3 points), when there is enough feces or dark content to interfere with the examination; and poor (4 points) if a large amount of fecal residue is present. The primary aim was to compare the two regimens (one-day versus two-day schedule) in terms of adequate cleansing. For this purpose a colon cleansing rated as “excellent” or “good” was considered as “adequate” whereas colon cleansing rated as “fair” and “poor” was classified as “inadequate”.
CCE images were viewed at a speed lower than 10 frames per second by two independent blinded observers (AZG and OA), with extensive experience in small bowel and colon capsule endoscopy (more than 50 examination for each procedure). Images were captured separately by each head of the capsule and finally combined. Anatomical landmarks were defined by thumbnail images of the cecum, the hepatic and splenic flexures, rectosigmoid junction, anal canal (defined as clearly observed venous plexus, anal papillae or both), and finally at expulsion from the body if it occurred during recording time. Each finding was reported separately. Agreement between the two viewers on the quality of colonic cleansing was required. A third reader (LRL) made the decision in cases of disagreement between the two main viewers.
CCE findings for each segment were collected and a global lesion evaluation for each patient was recorded as: normal (no lesions), polyps, diverticula, cancer or miscellaneous.
At least one week after CCE, a telephone questionnaire was completed for every patient in order to evaluate tolerance and adverse effects related with the colonic preparation. We decided to perform this evaluation several days after the colonoscopy-day based on previous studies which shown a more realistic evaluation about a procedure when the questionnaire is delayed.8,9 The interviewer was not implicated in the group assignment or the CCE procedure and the questionnaire provide information about whether or not they experienced gastrointestinal (GI) symptoms such as nausea, bloating, and abdominal discomfort, the amount of solution actually taken, difficulty to complete the preparation (5-point scale), taste (4-point scale) and willingness to repeat the same preparation in the future (yes or no).
Statistical analysisStatistical analysis was performed using SPSS 12.0 for Windows (SPSS Inc., IL, USA). Continuous variables are reported as mean and standard deviation (SD), and categorical variables as percentages. Two-sided t test was used to compare the means of the continuous variables in the two groups, and Chi-square test was used to compare the categorical variables. A value of P<0.05 was considered statistically significant.
ResultsPatientsOf 44 patients enrolled in this study, one was unable to swallow the capsule because of nausea and vomits and in another three cases there was a technical failure: the capsule did not turn on after the delay mode. These patients were re-scheduled for a new test and were excluded from the study. Thus, a total of 40 patients were finally included and randomly assigned to either Group I (n=20 patients) or Group II (n=20 patients).
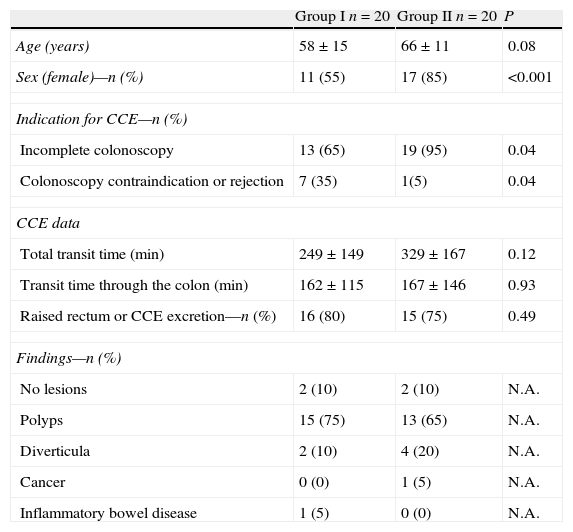
Table 2 shows patient demographic characteristics, indications for CCE and details on CCE performance. There were significant differences between the two groups due to the group assignment by random number table, with women being predominant in Group II, and the indication of CCE for incomplete CC was higher in Group II as compared to Group I, whereas colonoscopy contraindication or rejection was significantly higher in Group I than in Group II.
Demographic characteristics of patients and CCEa related data.
| Group I n=20 | Group II n=20 | P | |
| Age (years) | 58±15 | 66±11 | 0.08 |
| Sex (female)—n (%) | 11 (55) | 17 (85) | <0.001 |
| Indication for CCE—n (%) | |||
| Incomplete colonoscopy | 13 (65) | 19 (95) | 0.04 |
| Colonoscopy contraindication or rejection | 7 (35) | 1(5) | 0.04 |
| CCE data | |||
| Total transit time (min) | 249±149 | 329±167 | 0.12 |
| Transit time through the colon (min) | 162±115 | 167±146 | 0.93 |
| Raised rectum or CCE excretion—n (%) | 16 (80) | 15 (75) | 0.49 |
| Findings—n (%) | |||
| No lesions | 2 (10) | 2 (10) | N.A. |
| Polyps | 15 (75) | 13 (65) | N.A. |
| Diverticula | 2 (10) | 4 (20) | N.A. |
| Cancer | 0 (0) | 1 (5) | N.A. |
| Inflammatory bowel disease | 1 (5) | 0 (0) | N.A. |
As shown in Table 2, there were no significant differences in global or colonic transit time between the two groups. Every patient in each group received the first booster (30ml NaP), and a second booster was administered in 13 (65%) of cases in both groups. CCE reached the rectum enabling examination of the entire colonic tract in most cases in both groups. The capsule was excreted in 13 (65%) patients in Group I and in 11 (55%) patients in Group II (P=0.19). Regarding CCE findings, the most frequent lesions found were colonic polyps, representing 75% of findings in Group I and 65% in Group II (P=0.13) (Table 2).
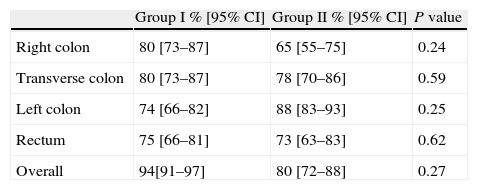
Colon cleanlinessColon cleanliness assessment is shown in Table 3. The comparison of colon cleanliness between the two groups was performed separately for each intestinal segment (right colon, transverse colon, left colon and rectum). In cases in which the cleanliness score of every segment was available we also performed a global cleanliness assessment. Therefore, global cleanliness score was not analyzed in 4 (20%) and 5 (25%) subjects of Group I and 2, respectively.
Adequatea colon cleansing level.
| Group I % [95% CI] | Group II % [95% CI] | P value | |
| Right colon | 80 [73–87] | 65 [55–75] | 0.24 |
| Transverse colon | 80 [73–87] | 78 [70–86] | 0.59 |
| Left colon | 74 [66–82] | 88 [83–93] | 0.25 |
| Rectum | 75 [66–81] | 73 [63–83] | 0.62 |
| Overall | 94[91–97] | 80 [72–88] | 0.27 |
No significant differences were found between the two cleanliness regimens at any colonic segment. Overall colon cleanliness was scored as adequate (good or excellent) in 15/16 (94%) patients in Group I and in 12/15 (80%) patients in Group II (P=0.27).
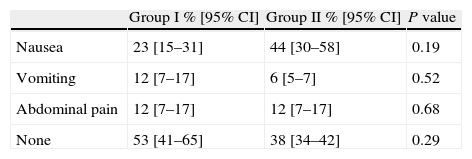
Adverse effects and toleranceOnly mild adverse events occurred and did not prevent the completion of CCE in any patient. As shown in Table 4, nausea was the most commonly reported adverse effect, being less frequent in Group I (23%) than in Group II (44%) without reaching statistical significance (P=0.19). The proportion of patients without any adverse effect was 53% in Group I and 28% in Group II (P=0.29).
DiscussionThe standard bowel preparation regimen recommended for CCE includes a two step process consisting in a low fiber diet plus the administration of 4L of PEG solution to achieve adequate colonic cleanliness, followed by one or two boosters of NaP or PEG to facilitate capsule bowel propulsion. The large volume of fluid as well as the long duration (two days) of the standard regimen entails considerable interference in daily life and working hours of these patients, representing a major drawback for this technology, particularly if it is to be considered a possible alternative to CRC screening in the near future. This is why it is of major interest to simplify the methodology related to the whole process. In the current study, we show that a one-day regimen before CCE yielded similar global and per-segment colon cleanliness compared to the standard two-day regimen, which could simplify the process of bowel preparation and increase patient acceptability.
To date, few studies have challenged standard bowel preparation in patients undergoing CCE. Two recent reports have assessed the efficacy of low volume bowel preparation. In the first, Hartmann et al.10, compared the administration of 1750ml versus 2000ml of PEG, plus ascorbic acid solution administered during two days, associated with 2 boosters of 0.5 and 0.25L of PEG. In that study, the overall cleanliness was good or excellent in more than 80% of patients in each group. However, this low-volume preparation was associated with inadequate cleansing of the cecum in about half of the patients, a result similar to that observed in CC when the volume solution is administered during more than eight hours.4 In addition, the study had methodological flaws, including non-randomization of patients and comparison with the standard regimen was not performed. The second study, by Kakugawa et al.,11 proposed a new reduced volume regimen using 24mg of sennoside the day before and 2L of PEG with 400mg of simethicone on the day of the CCE procedure. They used magnesium citrate (50g) and clear water (900ml) as boosters. When compared to the standard regimen, they obtained adequate bowel preparation in 94% of cases and 71% of cases excreted the capsule, without differences compared to the conventional volume method given during 2 days. These results are in keeping with the findings observed in our study, in which the efficacy of one-day bowel preparation on quality of bowel cleansing was not different from that achieved with the standard two-day regimen. Interestingly, our study also corroborated that when the volume solution is given three hours before CCE ingestion, good or excellent bowel preparation is achieved at all colonic segments. These three studies used different boosters following oral ingestion of CCE with similar rates of capsule excretion, suggesting that the performance of this new CCE preparation regimen depends more on the time lapse between volume solution and capsule ingestion than on the type of booster used.
The use of NaP in bowel preparation of CC has been associated with major complications, such as electrolyte disturbance, acute nephropathy, and kidney failure,12 raising safety concerns about it use in CCE. However, the only randomized study that assessed the feasibility of substituting low dose NaP (45ml) with PEG-boosters in the protocol preparation for CCE showed that there were no significant differences in the rate of adverse effects between the two booster preparations, but PEG-boosters were ineffective at achieving a reasonable capsule excretion rate and quality of bowel cleansing.13 Furthermore, hundreds of patients have received NaP boosters for CCE procedures, and no adverse effect has ever been reported,7,13–16 as in our study. All together, the evidence against the use of NaP-boosters in patients undergoing CCE is very weak and current guidelines still recommend them in the standard protocol of preparation for CCE.17
The strength of this randomized pilot study is that it is the first to assess the efficacy of a one-day preparation regimen using Nap as a booster compared with the standard recommended preparation17 as a control. In fact, it is the first randomized study in Europe to assess the feasibility of one-day bowel preparation for CCR. However, the study has certain limitations. First, the relatively small sample size means that the findings may not be extrapolated to all referral causes for CCE. However, statistical analysis was robust and blinded inter-observer agreement on the interpretation of colonic cleansing was required. Second, these results should be taken with caution since they were obtained in a single center. However, as stated above, a recent multi-center study carried out in Japan11 showed similar results regarding the efficacy of a one-day preparation regimen, despite using a different booster. Future prospective multicenter studies including a greater number of patients are warrant to investigate whether one-day colonic preparation regimen improve the performance of CCE.
Moreover, the growing development of new colonic preparations in CC, as the sodium picosulphate/magnesium oxide/anhydrous citric acid (2-L Citrafleet®) regimen or low-volume polyethylene glycol (2-L Moviprep; Norgine Pharmaceuticals), appear as a new and promising field for CCE procedure. These regimens determine a reduced volumen ingestion and a well tolerated taste but their use must be tested in the performance of CCE.
Finally, several studies have associated the diagnostic yield of CCE with the quality of the bowel preparation. Van Gossum et al.14 confirmed that the sensitivity of CCE for colorectal neoplasia detection was significantly higher in patients with adequate colonic cleansing, as compared to those with fair or poor bowel preparation, whereas specificity was minimally affected by the quality of bowel preparation. In our study, the diagnostic yield of CCE in patients following the one-day regimen was similar to that of those undergoing the two-day regimen. Furthermore, there were similar few side effects in both regimens. We can consider to use of one-day or two-days preparation considering the patient situation; if the patients live far away from the hospital may feel comfortable by using two-days preparation but the hospitalized patients could be benefited by using one-day regimen.
ConclusionsIn summary, the present study shows that patients undergoing CCE with one-day bowel preparation achieved similar rates of adequate colonic cleansing, diagnostic yield and adverse effects than those with the standard two-day preparation. Therefore, bowel preparation for CCE may be simplified to a one-day regimen without affecting test performance, which could facilitate its use and acceptability.
FundingThis work was supported by the European Society of Gastrointestinal Endoscopy in cooperation with Given Imaging.
Authors’ contributionsEQ designed the experiments, analyzed the data and wrote the paper; OA-F, designed the experiments, performed the colon capsule lecture, analyzed the data and wrote the paper; LR, recruited the participants, performed the colon capsule procedure and lecture, created/maintained the participants database, analyzed the data and wrote the paper; Z A de G, recruited the participants, performed the colon capsule procedure and maintained the participants database; AZ G-G and D N-L, performed the colon capsule procedure; A J, analyzed the data.
All authors discussed the results and implications on the manuscript at all stage.
Conflict of interestThe authors disclose no conflicts.