NTRODUCTION
In the last years, great advances have occurred in viral hepatitis and many viral agents have been identified. The hepatitis A and E virus, transmitted mainly by oro-fecal route, still present a high prevalence in developing countries, where sanitary and socioeconomic conditions are poor. Hepatitis B frequency has diminished in countries where vaccination against hepatitis B virus (HBV) was implemented, including in some Brazilian areas, prevailing in risk groups and in countries where horizontal in-tra-domiciliary and vertical transmitions are not controlled. Hepatitis C is considered an epidemy due to the great number of diagnosed cases. It is mainly related the high frequency of blood transfusion and hemoderivates contamined transmitions before 1993, but also it is due to the lack of a vaccine against hepatitis C virus (HCV). The hepatitis delta virus prevails where the HBV is endemic and may induce fulminant hepatitis or chronic liver disease in HBV carriers. The transfusion transmitted virus and the hepatitis G virus have been described since 1995 as possible post-transfusional hepatitis agents. However, its pathogenicity still needs elucidation. This review has the objective to discuss the epidemiological aspects of the enterically transmitted hepatitis A and E in Brazil, a huge country with an extension of 8,514,876 km2 , and an heterogeneous estimated population of 179 million inhabitants from the ethnic miscigenation and socioeconomic point of view (fig. 1)1 .
HEPATITIS AHepatitis A virus (HAV) is the prototype of the genus hepatovirus in the family Picornaviridae and has been considered the main etiological agent of acute hepatitis throughout the world. The endemicity of the infection varies among developing and developed countries. In Brazil, although hepatitis A is considered an endemic di-sease 2,3 , data published in recent investigations revealed an HAV infection endemicity that has decreased from high to intermediate level, probably due to improvements in sanitary conditions 4-6 . As a consequence, an increasing proportion of the population, mainly young people living in urban areas, is susceptible to infection and disease outbreaks are frequent 7-9 .
Fig. 1. Brazilian map with its 5 different geographic regions.
Genotypes
Genetic analysis of selected genome regions of HAV suggested that distinct genotypes could be found in different geographical regions. At least 7 HAV genotypes have been identified all over the world, including 4 human genotypes (I, II, III and IV) and 3 simian strains (IV, V, and VI)10 . A limited number of HAV isolates from South America have been characterized at the genomic level. IgM anti-HAV positive serum samples collected from patients with hepatitis A living in the 5 regions of Brazil –North (Pará), Northeast (Bahia, Maranhão, Rio Grande do Norte), Central (Goiás), South (Paraná), and Southeast (Rio de Janeiro, Minas Gerais)– were used to obtain HAV isolates and determine their genetic relatedness. Of 232 cases isolates, sequence data were obtained from the VP1/2A junction region of the HAV genome. All isolates were classified in genotype I; 231 belonged to subgenotype IA, and one to subgenotype IB. HAV isolates from 4 states formed distinct clusters of highly related sequences. However, isolates from other states did not cluster and the sequences from those states were intermingled with sequences found in the other states. The amino acid sequences of all but 2 isolates showed a Leu → Ile substitution at position 42 in the 2A protein. This substitution appeared to be a characteristic geographic fingerprint of HAV sequences within Brazil11 . Previous studies have reported the occurrence of the subgenotype IA in Argentina, Chile, Costa Rica, Cuba, El Salvador, and Uruguay, as the population of HAV circulating endemically. Some Brazilian isolates from this study were closely related genetically (or even identical) to isolates originating in other South American countries and overseas, providing firm evidence for epidemiological links between persons who travel to endemic areas11 . Recently, a mixed infection of a child care provider individual with HAV isolates from different subgenotypes IA and IB was described12 .
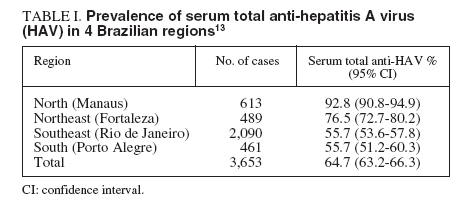
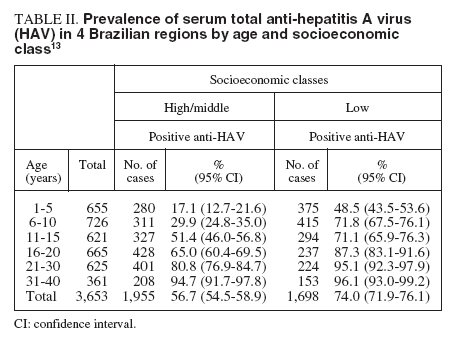
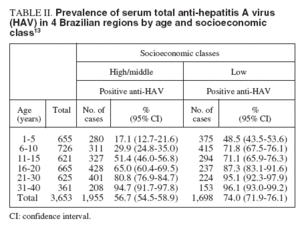
Prevalence of serum antibody to HAVThe prevalence of antibody to hepatitis A was assessed in 3,653 subjects across 4 regions of Brazil (tables I and II). The anti-HAV was detected in 64.7%, with the highest rate seen in the Northern region. In other regions, a seroprevalence over 90% was only reached in the more elderly, indicating an intermmediate endemicity. A significantly higher prevalence was seen in the low socioeconomic group between 1-30 years. The major findings of this study indicate that the pre-adolescent and adolescent population in some Brazilian cities are at the greatest risk for hepatitis A infection13 .
Southeast
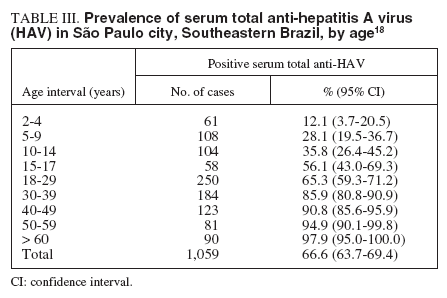
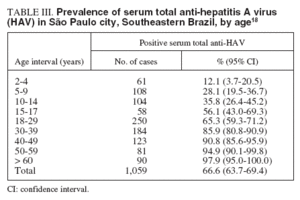
HAV infection in patients with pre-existing chronic liver disease has been associated with increased rate of fulminant hepatitis and mortality. The presence of total and IgM anti-HAV antibodies was investigated in 197 adult patients referred to a reference center in Rio de Janeiro and who were tested positive for anti-HCV antibodies and HCV RNA. One hundred seventy patients (86%) had total, but not IgM anti-HAV antibodies, being therefore, imune to hepatitis A, while 27 (14%) were not. A high proportion (6/27, 22%) of the susceptible patients presented markers of recent HAV infection: one patient was IgM anti-HAV positive, 3 were HAV RNA positive, and 2 presented both markers. By nucleotide sequencing, it was demonstrated that the HAV isolates infecting these patients belonged to subgenotypes IA and IB14 . Despite the notable improvements in sanitary conditions of the population, South America is a continent which is still endemic for hepatitis A, and where outbreaks continue to occur frequently. As an increasing proportion of the population is susceptible to HAV infection, the authors14 conclude that the implementation of hepatitis A vaccination should be considered in HCV infected, HAV susceptible patients. A serological study of hepatitis A was carried out in a low income area of Rio de Janeiro State, the Duque de Caxias city, where environmental conditions are still poor and a major environmental sanitation project is being conducted. The results showed that the risk of infection was greater among households with 2-3 members per room or more (than 3 per room). People living on hilltops, near to open sewers or lacking a kitchen were also at greater risk than others. The number of taps and waterusing fittings in the house was associated with a protective effect. A significant protective association was found with maternal education but not with gender or household income. Ownership of a ceramic water filter was asssociated with a protective effect on the margin of significance, but the practice of boiling drinking-water was not, nor was the type of water source used. The results suggest that the risk of infection with hepatitis A is determined by environmental variables in the domestic and public domains15 . In the same area, environmental interventions may lead to a decrease in the intensity of transmission of HAV and an increase in the average age of first infection in the next few years. This may have public health implications, since hepatitis A is more severe in adults. In this context, specific vaccination programmes may be necessary, as in developed countries16 . The transmission of HAV is primarily by fecal-oral route so the water is an important vehicle of dissemination. Yearly positive rates of serum IgM anti-HAV were 33.7% in 1999, 32.1% in 2000, and 30.6% in 2001 in a metropolitan area in Southeast Brazil. A seasonal variation was recognized with the highest incidence in spring and summer. Furthermore, a seasonal increase in incidence of HAV infection was found during the rainy season (December to March), when the index of rains is very high. Therefore, HAV infections in this area occur all year round with a peak during hot seasons with great number of rains8,9 . Data obtained from Campinas City, in São Paulo State, where the prevalence of anti-HAV in low socioeconomic level subjects demonstrated a rate of 95.0%, whereas in high socioeconomic subjects was only 19.6%, illustrate the duality of the epidemiology of HAV infection in Brazil: anti-HAV prevalence in low socioeconomic subjects is similar to that of developing countries, while in high socioeconomic subjects, a pattern typical of developed countries is found17 . A sample of 1,059 individuals residing in the 5 different districts (North, South, East, West and Central) of the municipality of São Paulo, Southeastern Brazil, was stratified by residence-based, socioeconomic level, randomized in order to assure a representative population of the city, and submitted to determination of the serum anti-HAV IgG antibody. The estimated prevalence in the population studied was 66.6% (confidence interval [CI], 63.7% to 69.4%), with a proportion of HAV occurrence of 65.5% (CI, 61.3% to 69.5%) in the male population and of 67.7% (CI, 63.7% to 71.6%) in the female population. The percentage of positive samples for HAV infection is shown according to age interval groups in table III18 . Although there were overlapping confidence intervals between the age groups, it was clear that the estimated proportions are different, and increased at each age group. The estimated prevalence of antibodies in those under 18 years of age showed a clear difference from the prevalence in those older than 18 years old – 34.3% (95% CI, 29.4% to 80.6%), and 83,3% (95% CI, 39.3% to 86.1%), respectivelly. No significant difference was observed in the prevalence related to the city region of residence (North: 60.3%; South: 65.3%; East: 68.0%; West: 68.9%; Central: 74.9%). The homogeneous distribution of HAV in all areas of the city apparently reflects a similar mixing of social classes in all the quarters. Shantytowns and slums occur in districts formely inhabited by rich classes, and rich businessmen are tending to live in middle class areas of the city. A tendency toward fewer occurrences of HAV infection was observed among students (35.9%; 95% CI, 29.8 to 42.1%), followed by individuals who work and study (58.1%; 95% CI, 46.6 to 69.7%) compared with individuals who had a defined professional occupation (75.6%; 95% CI, 75.6 to 91.7%). A co-infection with HBV and HCV was observed in 8% and 1%, respectivelly. The authors concluded that the circulation of HAV in the São Paulo city population has declined drastically during the last 2 decades and speculated that this decline may be a result of increased use of chlorine in the water supply instituted at the end of the 1980s due to the risk of cholera. This increase in susceptible individuals requires a careful evaluation of the need for HAV active immunoprofilaxis 18 .
TABLE I. Prevalence of serum total anti-hepatitis A virus (HAV) in 4 Brazilian regions13
TABLE II. Prevalence of serum total anti-hepatitis A virus (HAV) in 4 Brazilian regions by age and socioeconomic class 13
TABLE III. Prevalence of serum total anti-hepatitis A virus (HAV) in São Paulo city, Southeastern Brazil, by age 18
Amazon basin
A prevalence of total anti-HAV of 86.4% was observed in 487 children ranging from 2 to 9 years old who were students of nurseries and public schools in a county of the Amazonian region, in Mato Grosso State. The prevalence was high in all ages, suggesting that this is an area of high endemicity for HAV infection. There was no association between the anti-HAV markers and gender, socioeconomic level, parental educational level, hygienic conditions, number or density of residents per room or schools19 . An overall prevalence of HAV infection of 93.7% was observed in communities that live along the Purus and Acre rivers in the States of Acre and Amazonas within the Amazon basin20 . Shellfish is readly contaminated with viruses present in water containing sewage because of the concentration effect of filter feeding. One out of 4 shellfish (oysters) beds in Santa Catarina State, Southern Brazil, was tested positive for HAV RNA21 .
HAV infection is common in homosexual men and intravenous drug users, both being high risk groups for human immunodeficiency virus (HIV) infection. In Campinas, Southeastern Brazil, about 94% of 232 anti-HIV positive Brazilian patients had IgG anti-HAV in their serum22 . Most adult infections are symptomatic and associated with cholestatic acute hepatitis, while among children, hepatitis A is asymptomatic. A study evaluating the evolution of 151 acute hepatitis A cases reported that 96% of the patients presented a classic, 3.3% a polyphasic and 0.7% a cholestatic pattern23 . Our experience with 115 patients with acute hepatitis A, showed an evolution to cure in 54 (46.9%) within 8 weeks, 43 (37.3%) between 9 and 16 weeks, 13 (11.3%) between 17 and 24 weeks, and 5 (4.3%) longer than 24 weeks. We have been observing a higher acute HAV frequency in 157 adults of high socioeconomic level, with a mean age of 22.5 ± 10.7 (range, 362) years. Among these patients, a higher incidence was found between 21 and 30 years. Twenty-one percent of the patients were older than 30 years old. In 39.5% of these patients no epidemiological cause of transmission was found24 .
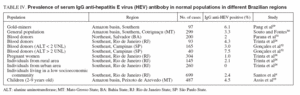
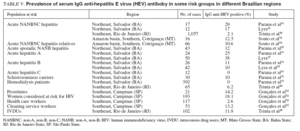
HEPATITIS EHepatitis E virus (HEV) infection is the major cause of enterically transmitted non-A non-B hepatitis in many parts of the world. The infection has been shown to occur in both epidemic and sporadic forms and to be primarily associated with the ingestion of fecally contaminated drinking water25 . Similar to HAV infection, the HEV is transmitted via fecal-oral route, the disease is self limited, and the prevalence of antibodies is closely related to the level of sanitation. However, unlike HAV, HEV appears to affect mainly young adults, which is unusual for a virus transmitted via the fecal-oral route. Also unusual is that secondary cases of HEV infection are much less frequent than with HAV26 . Finally, a major difference with HAV infection is the high mortality rate seen in pregnant women infected with HEV27 . Outbreaks of HEV infection have been reported from Central Asia, Southeast Asia, Middle East, Northern Africa, Sub-Saharan Africa, and Mexico. In developed countries outbreaks have not been recognized. However, sporadic cases have been identified in Mediterranean countries and in the United States, mostly imported from endemic areas25 . In spite of favorable environmental conditions, outbreaks of hepatitis E have never been reported in Brazil28 . Nevertheless, a few reports have demonstrated the evidence of anti-HEV antibodies in some Brazilian regions (tables IV and V).
Prevalence of serum antibody to HEVThe prevalence of serum IgG anti-HEV antibody in normal populations and in some risk groups in different Brazilian regions is shown in tables IV and V.
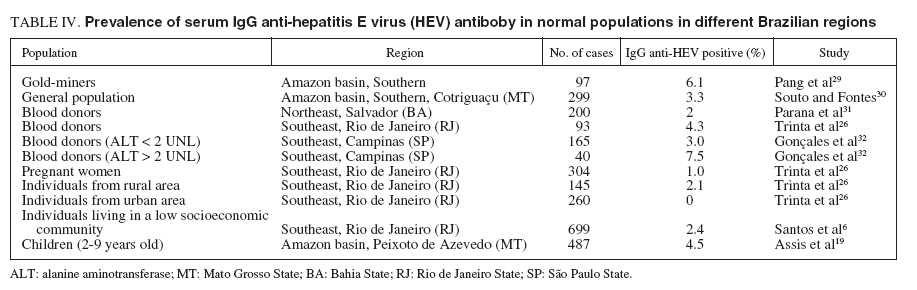
TABLE IV. Prevalence of serum IgG anti-hepatitis E virus (HEV) antiboby in normal populations in different Brazilian regions
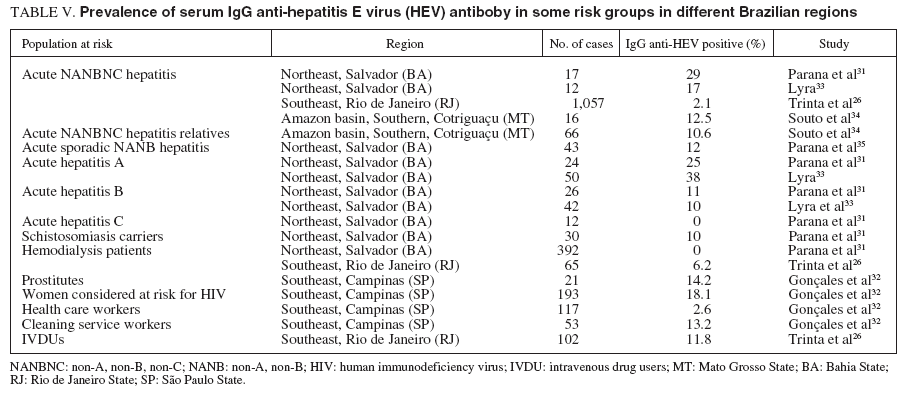
TABLE V. Prevalence of serum IgG anti-hepatitis E virus (HEV) antiboby in some risk groups in different Brazilian regions
Amazon basin
Seroprevalence studies in the Brazilian Amazon showed that 6% of gold-miners had anti-HEV IgG antibodies29 . Also in the Brazilian Amazon, 2 (12.5%) of the 16 acute non-A, non-B and non-C (NANBNC) hepatitis cases were reacted to HEV antibodies of the IgG class. Likewise, sera from 7 (10.6%) of the 66 asymptomatic relatives were positive for anti-HEV34 . However, the lack of randomness in the selection of subjects for the studies means that their conclusions must be treated with caution. The same group30 performed a randomized survey of HEV infection among the general population of Cotriguaçu, a county in the North-Western part of the state of Mato Grosso, in the Southern Amazon basin, with an estimated population of 3,046 individuals. In this county, the first inhabitants arrived in the early 1980s and settled in a place that was to become the current main village. About 1,500 now live in this village, in 487 houses. Each house has its own shallow well as a source of water and many have a rudimentary sewage-disposal system near the water source. Seventy-four houses in the main village were randomly chosen to be visited and all the 238 villagers aged > 2 years who lived in these houses were invited to participate. Verbal informed consent was obtained from the 212 villagers (or their parents), who were then interviewed and bled. Sera were similarly collected from 87 subjects who were randomly selected from the 450 inhabitants of Nova Esperança, a relatively small and poor community in Cotriguaçu county, which was established in 1993 and is situated 60 km from the main village. The 299 subjects were aged 2-74 years (mean: 25.2 years). Ten (3.3%; 95% CI, 1.6% to 6.1%) of the individuals were found to be anti-HEV IgG positive (4 from the main village and 6 from Nova Esperança) and lived in 10 different households. Nine of the 10 subjects had never left Brazil; the exception had been to Paraguay several years prior to the study. The prevalence of anti-HEV was similar by gender, with a mean age of 31.3 years old (range, 2-73) and, with a mean annual income of the families of seropositive subjects not differently from the families of the seronegative ones. The authors mention that although HEV infection appears to be endemic in many poor populations of tropical and sub-tropical regions, the level of exposure in their studied area appears to be low. In fact, asymptomatic HEV infection and acute cases seem to be generally uncommon in the Amazon basin29,30,34 . Person-to-person transmission does not seem to be important in the spread of HEV. Epidemics of hepatitis E have been attributed to exposure to the same contaminated water sources36 . There is such an abundance of natural sources of water in the Amazon basin that the spread of any virus by the sharing of a contaminated water source is perhaps much more unlikely there than in other tropical regions30 . The assessment of serum IgG anti-HEV in 487 children ranging from 2 to 9 years old who were students in nurseries and public schools in Peixoto de Azevedo county, in Mato Grosso State, showed a low prevalence of 4.5% (CI 95%, 2.9% to 6.9%), similar to that found among adults in other Brazilian regions19 .
Northeast
A report from Salvador, Bahia State, showed prevalence rates of 2% among blood donors, 10% in schistosomiasis carriers, and 0% in the hemodialyzed patients. Among patients with acute viral hepatitis, the anti-HEV antibodies were present in 25% with HAV, 11% with HBV, and 29% with NANBNC hepatitis viruses31,35 . The authors concluded that HEV is prevalent in Northeastern Brazil, and the higher prevalence in patients compared with blood donors could be explained by the lower social condition of patients who sought public health service in this area, in contrast to the heterogeneous socioeconomic distribution of blood donors. Patients with acute viral hepatitis due to hepatitis A had a greater frequency of anti-HEV, probably because of similar routes of transmission for both HAV and HEV. Finally, the absence of anti-HEV in the hemodialyzed group could be explained by a lower immunologic response found in patients with chronic renal failure. Acute viral hepatitis still constitutes a major cause of morbidity in Northeastern Brazil. In a study performed in Salvador, Bahia State, analyzing the serum of 94 patients with acute hepatitis, found positivity to anti-HEV IgG antibody in 38% of 40 hepatitis A patients, in 10% of 42 hepatitis B patients and in 17% of 12 acute hepatitis C. Using the anti-HEV IgM for the first time, co-infection of HEV was detected in 4 patients with acute hepatitis A and in one patient with acute NANBNC hepatitis33,37 .
Southeast
The seroprevalence of IgG anti-HEV was investigated in 205 volunteer blood donors, 214 women who attended a center for anonymous testing for HIV infection, and 170 hospital employees in Campinas, a city in Southeastern Brazil. The prevalence of anti-HEV ranged from 2.6% in health care professionals to 17.7% in women who considered themselves at risk for HIV. The prevalence of anti-HEV in health care professionals was not significantly different from that in healthy blood donors (3.0%) and blood donors with raised alanine aminotransferase levels (7.5%). The prevalence of 13.2% in cleaning service workers at a University Hospital was similar to that among women at risk for HIV infection. These observations suggest that HEV is found in Southeastern Brazil and that low socioeconomic status is an important risk factor for HEV infection in this region32 .
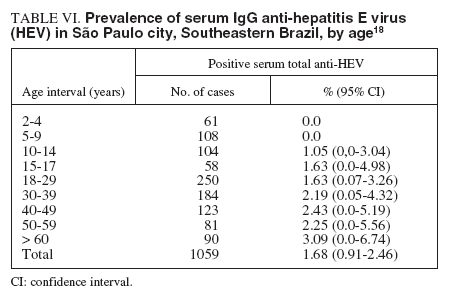
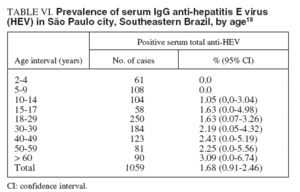
TABLE VI. Prevalence of serum IgG anti-hepatitis E virus (HEV) in São Paulo city, Southeastern Brazil, by age18
A sample of 1059 individuals residing in the 5 different districts (North, South, East, West and Central) of the municipality of São Paulo, Southeastern Brazil, was stratified by residence-based, socioeconomic level, and randomized in order to assure a representative population of the city and submitted to determination of the serum anti-HEV IgG antibody. The estimated prevalence in the population studied was 1.68% (95% CI, 0.91% to 2.46%), with a similar proportion of HEV occurrence of 2.35% (CI, 1.03% to 3.67%) in the male population and of 1.06% (CI, 0.2% to 1.9%) in the female population. The percentage of positive samples for HAV infection is shown according to age interval groups in table VI18 . HEV prevalence tended to be zero in those less than the age 9 years old, followed by a slight increase from the age 10 years old, but without statistical significance. There was no difference in HEV related to educational level or in association with professional activities. There was a tendency toward higher prevalences in the West and downtown areas of the city (3.31%, and 3.26%, respectively), compared with the East, South and North areas (1.86%, 0.98%, and 0.59%, respectively). The HEV positive samples were simultaneously positive for HBV in 10% of cases and HCV in 5% of cases. No cases of co-in-fection with HAV was observed in this study18 . In Rio de Janeiro, Southeastern Brazil, an age-specific prevalence study of anti-HEV IgG among 699 individuals living in the Manguinhos community, a low socioeconomic level area, showed a 2.4% positive rate. The highest prevalence rate was observed in the group ranging from 41-50 years old and over 60 years (p = 0.002), and in the male group (5.7%) as compared with the female group (1.7%) (p = 0.002). The serum anti-HEV IgG prevalence found in this population was similar to the one found in non-endemic areas6 . In the same metropolitan area of Rio de Janeiro, another study was conducted among 1,115 selected subjects. The serum anti-HEV IgG prevalence among the different groups were: 2.1% in 146 patients with acute NANBNC viral hepatitis, 6.2% in 65 hemodialysis patients, 4.3% in 93 blood donors, 11.8% in 102 intravenous drug users, 1% in 304 pregnant women, and 2.1% in 145 individuals living in the rural area. Among individuals living in the urban area they did not find a single positive serum sample. As a general conclusion, the overall prevalence of anti-HEV in Rio de Janeiro was much lower than expected26 . The HEV infection in animals has never been reported in our country, but since data were collected from diverse geographically areas and hepatitis E cases were already described in the Amazon basin, Bahia, São Paulo and Rio de Janeiro, it seems that HEV may be present in all Brazilian regions and animal infection by HEV must be studied38 . Phylogenetic studies showed a great similarity among HEV strains observed in animals and human beings, suggesting that HEV transmission between different species is a common event and that HEV infection may be considered a zoonosis39 . Therefore, new knowledge on HEV transmission between humans and other animals, specially pigs and urban rodents, certainly will have an important impact on epidemiological decisions to control this infection38 .
Correspondencia: Dr. F.J. Carrilho. Rua Silva Correia, 153, ap. 41. 04537-040 São Paulo. Brazil. Correo electrónico: fjcarril@usp.br
Recibido el 5-7-2004; aceptado para su publicación el 20-9-2004.