Mycoplasma genitalium (MG) is a sexually transmitted pathogen responsible for 10–35% of non-gonococcal urethritis in men. In women, it is associated with urethritis, cervicitis, endometritis and pelvic inflammatory disease.1 Due to its highly fastidious nature, this infection should be diagnosed using nucleic acid amplification techniques.2 The lack of peptidoglycan in MG precludes the use of antibiotics acting on the cell wall and limits its treatment choice.3 European treatment guidelines recommend azithromycin for the treatment of uncomplicated MG infection or sexual contact, and moxifloxacin as the most commonly used second-line antimicrobial.1 The rapid emergence and spread of antimicrobial resistance in MG is a growing concern.2,3 In Europe, the reported azithromycin resistance rate ranges from 0% to 82%, and from 3 to 11% in the case of moxifloxacin, depending on the population studied.4
The aim of this study was to describe the prevalence of resistance to antibiotics in MG in the sanitary region of the province of Lugo (population of 323,989 inhabitants in 2022) between January 2019 and December 2022. During this time, a total of 4122 specimens from 2622 patients with suspected sexually transmitted infection were tested using RT-PCR Allplex™ STI Essential Assay (Seegene®). Specimens positive in this assay were subsequently tested by the RT-PCR Allplex™ MG & AziR Assay (Seegene®) and RT-PCR Allplex™ MG & MoxiR Assay (Seegene®). The first sample from the first episode was taken for each patient.
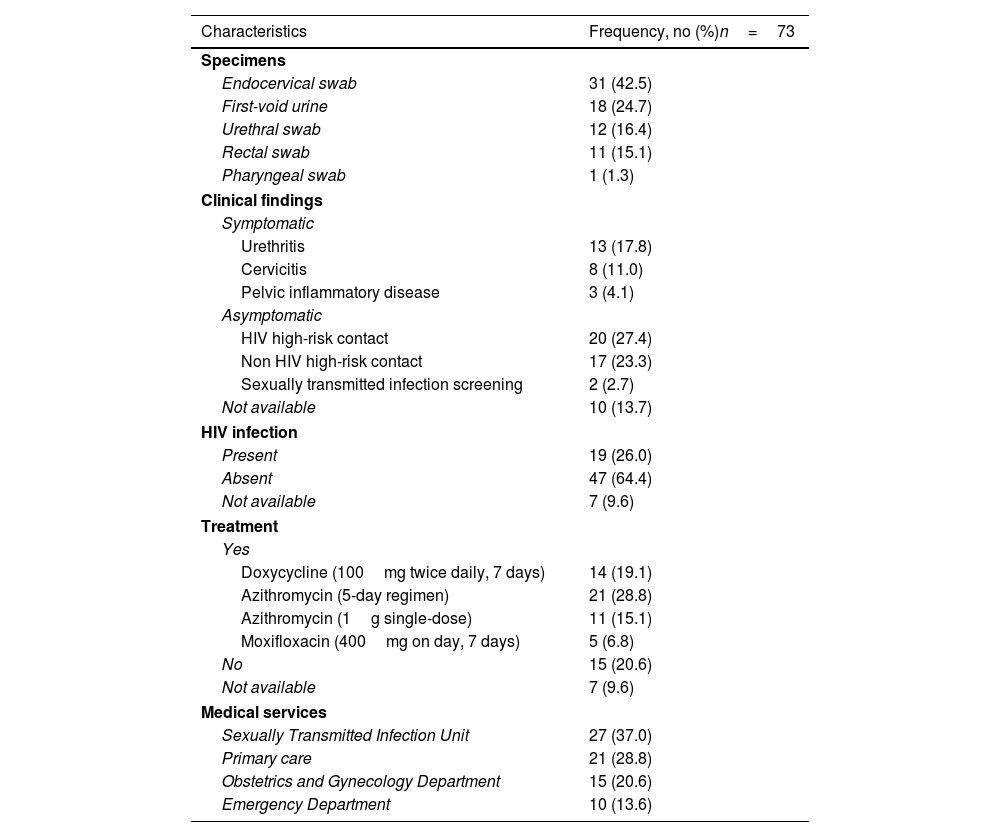
A total of 73/2622 (2.8%, 95% CI 2.2–3.5%) patients tested positive for MG (39 male and 34 female). The median age of all 73 patients was 33.5 years (IQR 26.5–40.5) (males: 37 years (IQR 31–41); females: 28 years (IQR 24–36)). The clinical features and therapeutic management of the 73 patients are described in Table 1. Seven medical records were not completely available. Considering coinfection with other sexually transmitted microorganisms, 20/73 (27.4%) cases were in coinfection with either Chlamydia trachomatis (CT) (16.4%), Neisseria gonorrhoeae (NG) (4.1%), Trichomonas vaginalis (TV) (4.1%), CT and NG (1.4%), CT and TV (1.4%).
Clinical features and therapeutic management in patients with Mycoplasma genitalium infection.
| Characteristics | Frequency, no (%)n=73 |
|---|---|
| Specimens | |
| Endocervical swab | 31 (42.5) |
| First-void urine | 18 (24.7) |
| Urethral swab | 12 (16.4) |
| Rectal swab | 11 (15.1) |
| Pharyngeal swab | 1 (1.3) |
| Clinical findings | |
| Symptomatic | |
| Urethritis | 13 (17.8) |
| Cervicitis | 8 (11.0) |
| Pelvic inflammatory disease | 3 (4.1) |
| Asymptomatic | |
| HIV high-risk contact | 20 (27.4) |
| Non HIV high-risk contact | 17 (23.3) |
| Sexually transmitted infection screening | 2 (2.7) |
| Not available | 10 (13.7) |
| HIV infection | |
| Present | 19 (26.0) |
| Absent | 47 (64.4) |
| Not available | 7 (9.6) |
| Treatment | |
| Yes | |
| Doxycycline (100mg twice daily, 7 days) | 14 (19.1) |
| Azithromycin (5-day regimen) | 21 (28.8) |
| Azithromycin (1g single-dose) | 11 (15.1) |
| Moxifloxacin (400mg on day, 7 days) | 5 (6.8) |
| No | 15 (20.6) |
| Not available | 7 (9.6) |
| Medical services | |
| Sexually Transmitted Infection Unit | 27 (37.0) |
| Primary care | 21 (28.8) |
| Obstetrics and Gynecology Department | 15 (20.6) |
| Emergency Department | 10 (13.6) |
Macrolide resistance-associated mutations were detected in 15/73 (20.6%, 95% CI 12.9–31.2%) of the specimens analysed. This rate is similar to other studies performed in different parts of our country,2,5,6 but lower than in southern Spain with a growing trend in recent years.7,8 In our study, the most prevalent mutations conferring macrolide resistance were A2058G (5/15) (33.3%) and A2059G (5/15) (33.3%), followed by A2058T (4/15) (26.7%) and A2058C (1/15) (6.7%). The most frequent mutations associated with high-level macrolide resistance are A2058G and A2059G.3,6–9 Although the A2058T mutation is very prevalent in the Netherlands, it is not a frequent mutation in other parts of the world.2,3 Our results describe a high rate of resistance (26.7%) and indicate that we must be alert for its possible increase. None of the patients had more than one mutation for macrolide resistance. Fluoroquinolone resistance was detected in 5/73 (6.9%, 95% CI 3.0–15.1%) of the strains. This rate is slightly higher than those described in previous years in our country by other authors.2,7,10 The most frequent fluoroquinolone mutation detected was G259A (80%), while G248T (20%) was in second position. These are the most prevalent mutations described by other authors.2,3 None of the patients had more than one mutation for fluoroquinolone resistance. In our study, azithromycin given as 500mg on day one, followed by 250mg days 2–5 (1.5g total dose) was the most frequent treatment and is recommended as the primary choice for treatment of MG infections.1 Dual mutations conferring resistance to both antimicrobials were found in a total of four resistant strains: two A2058G/G259A, one A2058T/G259A and one A2059G/G248T. The presence of dual mutations could increase treatment failure.7
The main limitation of this study was the incomplete data, like the sexual habits of the patients and the number of patients lost for follow-up and for the analysis of antibiotic failure.
In conclusion, the dissemination of resistant MG strains is a worldwide health problem and supports the importance of detecting macrolide and fluoroquinolone resistance mutations in order to perform targeted treatment and to prevent the spread of resistant strains.