Human respiratory syncytial virus (RSV) is the most commonly identified virus associated with lower respiratory tract infections. The monoclonal antibody nirsevimab immunization campaign began in our country in October 2023.
MethodsThis study was conducted in the Pediatric Emergency Department (PED) of a tertiary care center in Madrid, Spain. The aim was to compare PED visits of patients eligible for immunization with nirsevimab who attended between weeks 40 and 52 of 2022 and 2023 and who had a confirmed diagnosis of RSV infection.
ResultsDuring the study period, 264 out of 765 patients with confirmed RSV infection who attended the PED were eligible for immunization with nirsevimab and were selected for our analysis. The PED attendance was 80.3% in 2022 and 19.7% in 2023. The number of RSV-positive cases increased from week 42 in both analyzed periods, with a peak of maximum incidence between weeks 46 and 48. In 2022, the morphology of the case curve in the group of children eligible for immunization was similar to the overall curve. However, in 2023, we did not observe a similar increase in cases among patients eligible for immunization.
ConclusionImmunization with nirsevimab during the 2023 RSV epidemic season had a beneficial effect, reducing the number of PED consultations for RSV infection.
El virus respiratorio sincitial humano (VRS) es el virus más frecuentemente identificado en niños con infección respiratoria de vías bajas. A partir de octubre de 2023, comenzó en nuestro país la campaña de inmunización con el anticuerpo monoclonal nirsevimab.
Material y métodosEstudio realizado en el servicio de urgencias pediátricas de un hospital de tercer nivel de la Comunidad de Madrid (España). Comparamos los pacientes pediátricos en edad susceptible de recibir inmunización con nirsevimab, atendidos entre las semanas 40 y 52 de los años 2022 y 2023, con un diagnóstico de infección confirmada por VRS.
ResultadosDurante el periodo de estudio, atendimos 765 pacientes con infección confirmada por VRS. Del total, 264 eran susceptibles de haber recibido inmunización con nirsevimab y por tanto fueron seleccionados para nuestro análisis. El 80,3% (212/264) se atendieron en 2022 y el 19,7% (52/264) en 2023. El número de casos VRS positivos, se incrementó a partir de la semana 42 en ambos periodos analizados, observando un pico de máxima incidencia entre la semana 46 y la 48. En el año 2022, la morfología de la curva de casos del grupo de niños susceptibles de ser inmunizados fue muy similar a la curva global. Por el contrario, en 2023 no registramos dicho ascenso de casos en los pacientes susceptibles de ser inmunizados.
ConclusiónLa inmunización con nirsevimab en la temporada epidémica del VRS de 2023, ha tenido un impacto beneficioso, registrándose una reducción del número de visitas a urgencias por infección debida a VRS.
Lower respiratory tract infection (LRTI) is one of the leading causes of morbidity and mortality in children under five years of age worldwide.1 Human respiratory syncytial virus (RSV) is the most frequently identified virus in children with LRTI and the leading cause of hospitalisation.1 Most children become infected with RSV before the age of two, and up to 14% require medical attention for RSV in their first year of life. Worldwide, RSV causes one in 28 deaths in infants aged 28 days to six months, and is responsible for three in every four hospital admissions in healthy infants.2 The incidence of RSV-related hospitalisation in Europe is approximately one per 56 healthy term newborns. Vaccination of healthy pregnant women and term newborns during their first winter season could have a significant impact on the burden of care and healthcare expenditure.3
Until recently, the only preventive tool against RSV was palivizumab, a monoclonal antibody indicated in at-risk patients (preterm infants, haemodynamically significant heart disease and/or chronic respiratory disease).4,5 In recent years there have been significant advances in RSV prevention,2 and Europe and Spain have recently authorised the use of nirsevimab in infants under one year of age. Nirsevimab is a recombinant monoclonal antibody that binds to the F1 and F2 subunits of the RSV fusion (F) protein, blocking it and preventing the virus from entering the host cell.6
In September 2023 the Sociedad Española de Infectología Pediátrica (SEIP) [Spanish Society of Paediatric Infectious Diseases] published its position on the use of nirsevimab for the prevention of RSV infection. Its recommendations include the administration of nirsevimab to all infants born during the winter season and to infants under six months of age at the beginning of the winter season.6 Based on these recommendations, Madrid Region started the immunisation campaign with nirsevimab on 1 October 2023, with infants under six months of age at the beginning of the season (born from 1 April to 30 September 2023) and newborns born during the season (from 1 October 2023 to 31 March 2024) being eligible.
The main aim of our study was to describe the impact of nirsevimab immunisation on RSV infections seen in paediatric Accident and Emergency departments (A&E) during the first quarter of the 2023 RSV season in Madrid, and to compare the results with the same period in 2022.
Patients and methodsSingle-centre, ambispective observational study conducted in a tertiary hospital paediatric A&E in the Autonomous Region of Madrid. We selected all paediatric patients seen during the period from week 40 to week 52 of 2022 and 2023 with a diagnosis of RSV infection. Samples obtained in A&E were processed in our hospital's microbiology department. A commercial multiplex RT-PCR (polymerase chain reaction) (Influenza A&B and RSV, Cobas Liat, Roche), which detects the presence of influenza A and B viruses and RSV in 20 minutes, was used to confirm diagnosis.
Our study included two groups of patients, which we compared with each other. The first group consisted of patients born between 1 April 2023 and 31 December 2023, and who were therefore eligible for immunisation with nirsevimab. The second group included those born from 1 April 2022 to 31 December 2022. The variables collected included: demographic data; reason for consultation; triage level; previous medical history; history of immunisation with nirsevimab; level of respiratory distress according to the Pulmonary Score scale in case of bronchospasm or the San Juan de Dios scale7 in case of bronchiolitis; need for oxygen therapy (conventional or high flow [HFO]); treatments administered; tests performed; need for assessment by the paediatric intensive care unit (PICU); and, lastly, the patient’s destination.
IBM® SPSS version 21 software was used for statistical data processing. The normality of the variables was tested using the Kolmogorov-Smirnov test. The descriptive analysis of the data is presented graphically and numerically, using the median as the measure of central tendency and the interquartile range (IQR) as the measure of dispersion. For hypothesis testing, the non-parametric Mann Whitney U test was used to compare median age between study groups, and the chi-square test or Fisher’s exact test, as appropriate, was used to compare qualitative variables. Differences with p values <0.05 were considered statistically significant.
The study was approved by our centre’s Independent Ethics Committee (PI-5938).
ResultsDuring the study period, 765 patients with confirmed RSV infection were seen in A&E, generating a total of 991 visits: 765 (77.1%) were first visits; and 226 (22.8%) were repeat consultations. Of these patients, 62.7% (472/765) attended in 2022, and 38.3% in 2023 (293/765), a decrease of 38%. In relation to the total number of visits, 624/991 were registered in 2022 (62.9%), and 367/991 in 2023 (37%). The A&E repeat consultation rate in 2022 was 24.4% (152/624), and in 2023, it was 20.2% (74/367), a decrease of 4.2% (p = 0.137). The median age of patients seen in 2022 was 9.6 months (IQR: 3.2–25.1), increasing to 15.6 months (IQR: 8.9–33) in 2023 (p <0.001).
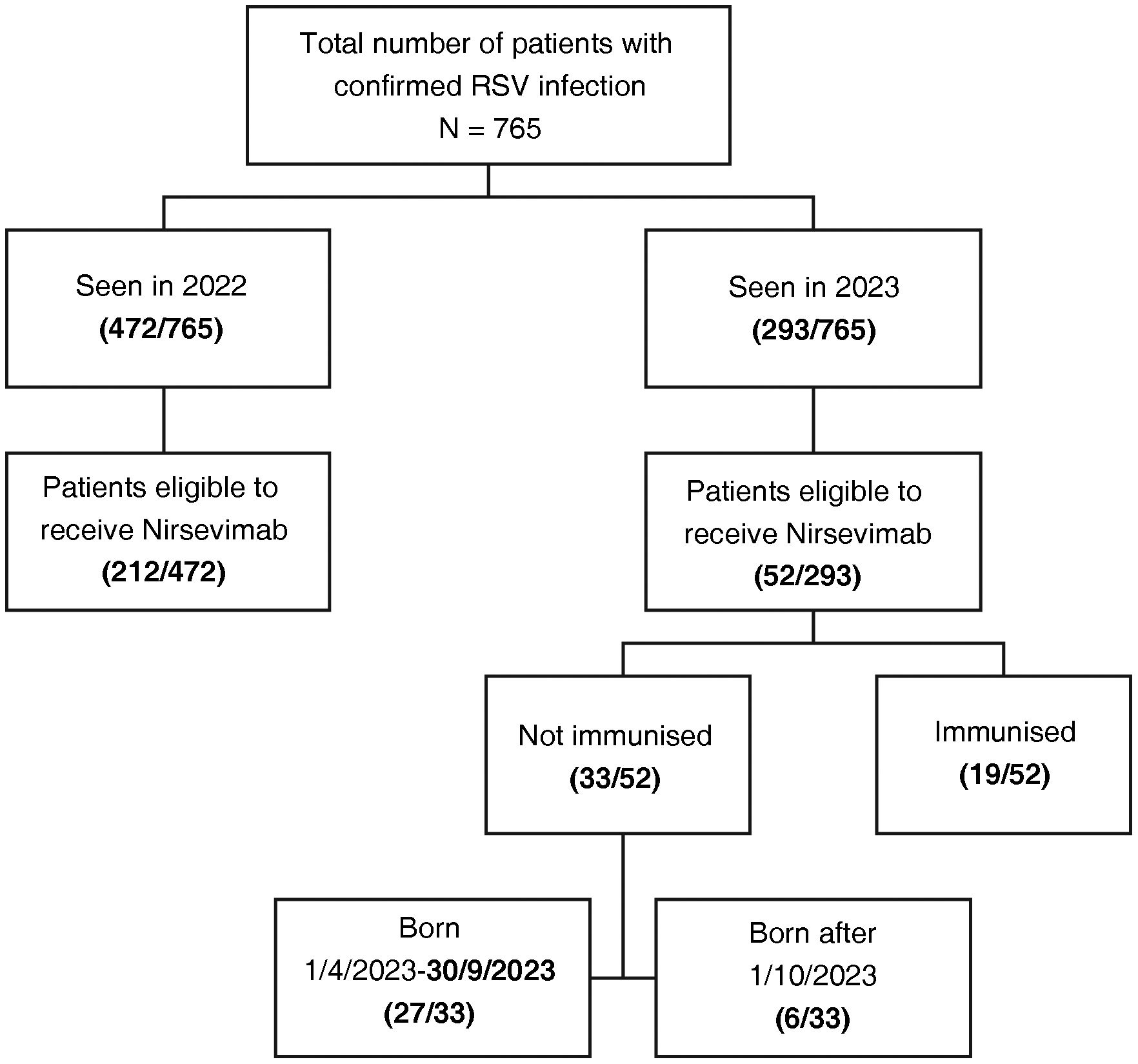
Of the total number of patients seen, 264/765 were infants eligible to have received nirsevimab immunisation, and were therefore selected for our analysis; 80.3% (212/264) had been seen in 2022 and 19.7% (52/264) in 2023. Of the 52 patients seen in 2023, 36.5% (19/52) had received nirsevimab immunisation as prophylaxis. Of the patients not immunised in 2023 (33/52; 63.5%), 6/33 were born on or after 1 October 2023 and 27/33 (81.8%) between 1 April and 30 September 2023 (Fig. 1). Eleven patients who had not previously been immunised received nirsevimab during their admission as part of the treatment of their confirmed RSV infection.
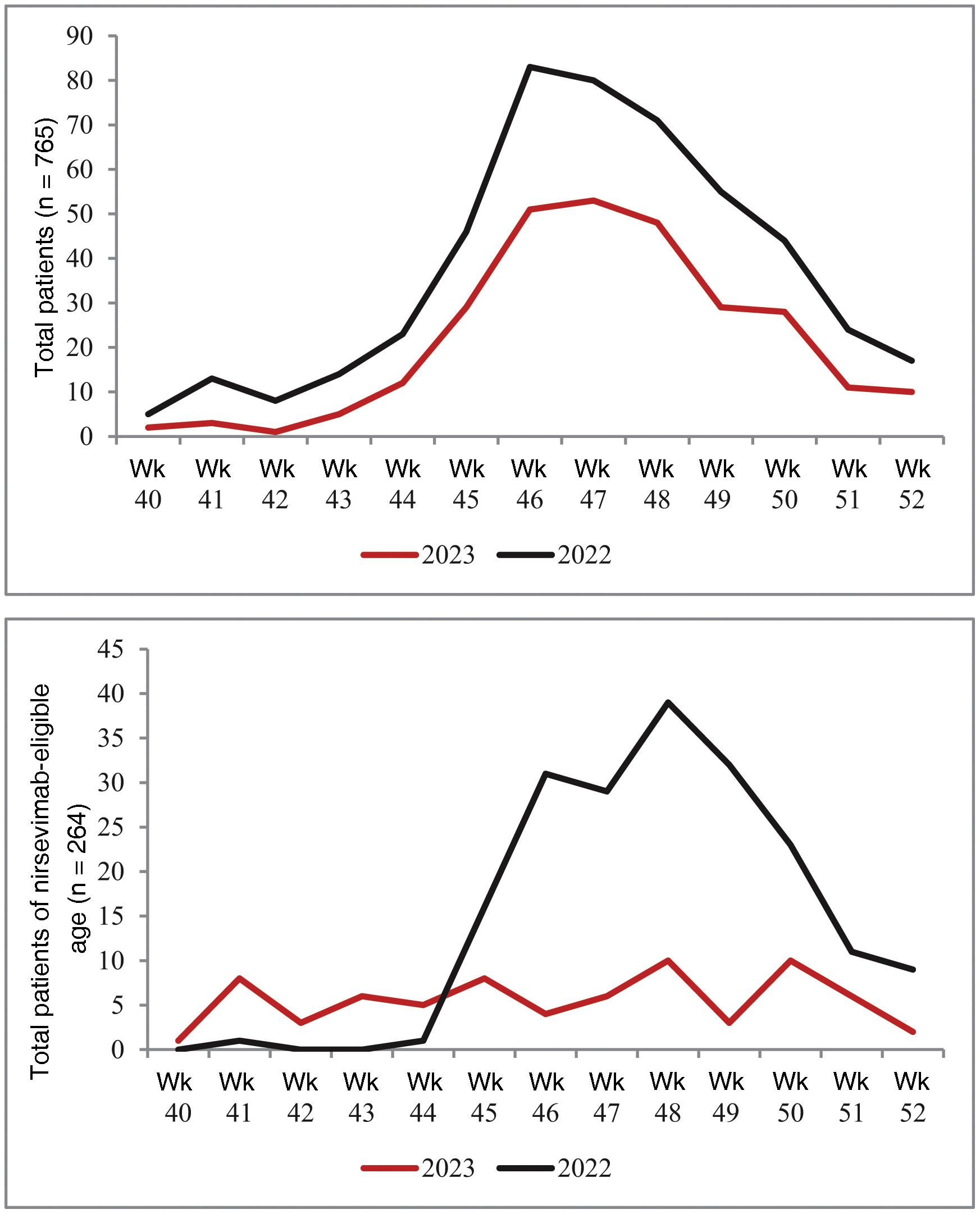
The number of RSV-positive cases increased progressively from week 42 in both periods analysed, with peak incidence between weeks 46 and 48. In 2022, the shape of the case curve for the group of children eligible for immunisation was very similar to the overall curve. In contrast, in 2023, we did not find such an increase in cases in patients eligible for immunisation (Fig. 2).
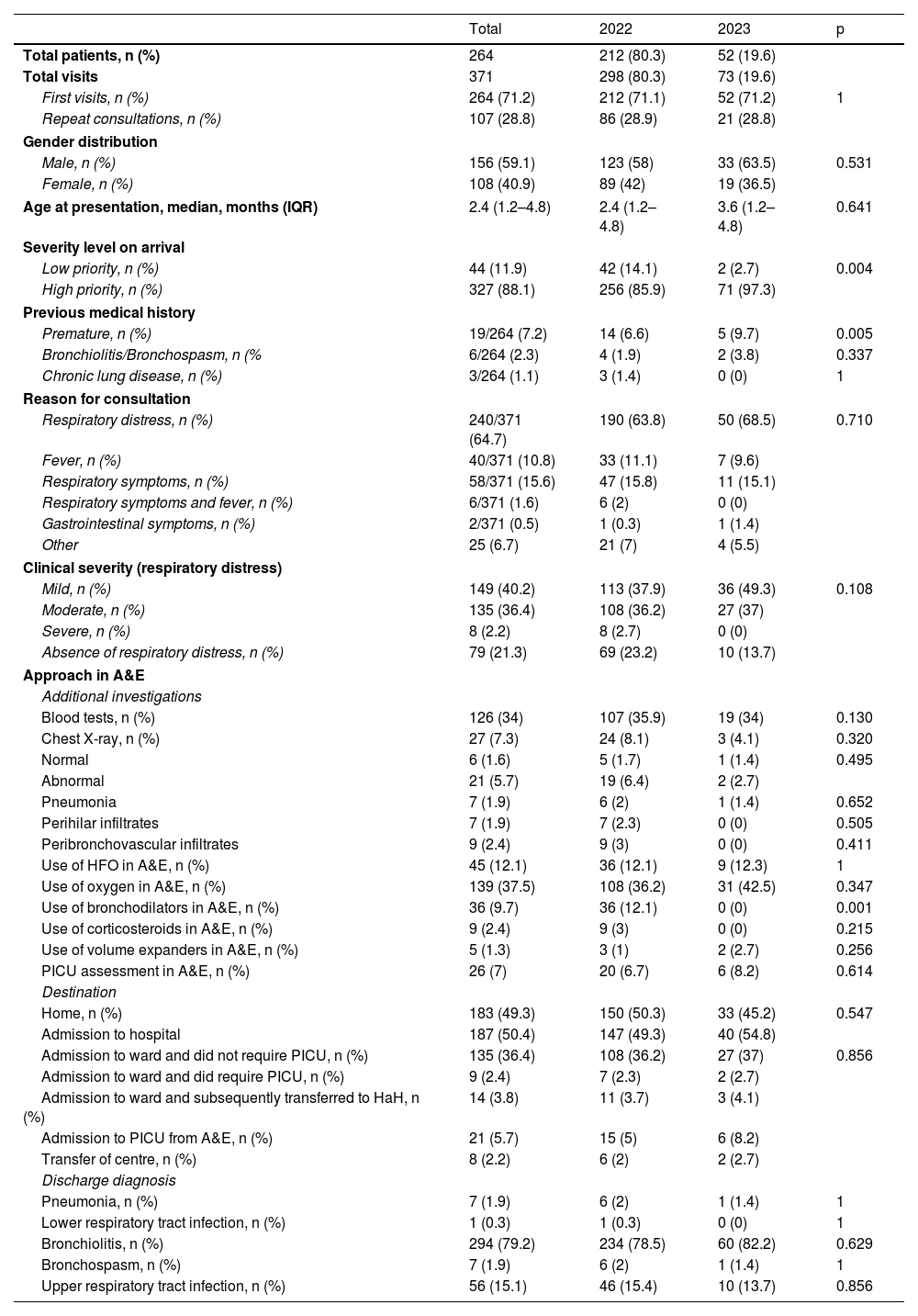
Table 1 shows the demographic and clinical characteristics of the patients included in the study, as well as the therapeutic approach in A&E. We found no differences in gender distribution or age at presentation between the two groups. The main reason for consultation was respiratory distress, followed by respiratory symptoms. Some 88.1% of patients were classified as high priority, with a statistically significant difference between 2022 and 2023. Based on the application of the corresponding respiratory distress severity scales, we found no statistically significant differences when comparing the severity of patients seen in 2022 and 2023. In terms of therapeutic measures applied in A&E, we only found a significant decrease in the use of bronchodilators. There were no differences in the number or results of tests requested, nor in the rates of admission to the ward or intensive care. In one case, relatives refused to admit the patient and opted to self-discharge.
Characteristics of patients of an age eligible for immunisation with nirsevimab who consulted Accident and Emergency for RSV infection. Comparison of 2022 and 2023.
| Total | 2022 | 2023 | p | |
|---|---|---|---|---|
| Total patients, n (%) | 264 | 212 (80.3) | 52 (19.6) | |
| Total visits | 371 | 298 (80.3) | 73 (19.6) | |
| First visits, n (%) | 264 (71.2) | 212 (71.1) | 52 (71.2) | 1 |
| Repeat consultations, n (%) | 107 (28.8) | 86 (28.9) | 21 (28.8) | |
| Gender distribution | ||||
| Male, n (%) | 156 (59.1) | 123 (58) | 33 (63.5) | 0.531 |
| Female, n (%) | 108 (40.9) | 89 (42) | 19 (36.5) | |
| Age at presentation, median, months (IQR) | 2.4 (1.2–4.8) | 2.4 (1.2–4.8) | 3.6 (1.2–4.8) | 0.641 |
| Severity level on arrival | ||||
| Low priority, n (%) | 44 (11.9) | 42 (14.1) | 2 (2.7) | 0.004 |
| High priority, n (%) | 327 (88.1) | 256 (85.9) | 71 (97.3) | |
| Previous medical history | ||||
| Premature, n (%) | 19/264 (7.2) | 14 (6.6) | 5 (9.7) | 0.005 |
| Bronchiolitis/Bronchospasm, n (% | 6/264 (2.3) | 4 (1.9) | 2 (3.8) | 0.337 |
| Chronic lung disease, n (%) | 3/264 (1.1) | 3 (1.4) | 0 (0) | 1 |
| Reason for consultation | ||||
| Respiratory distress, n (%) | 240/371 (64.7) | 190 (63.8) | 50 (68.5) | 0.710 |
| Fever, n (%) | 40/371 (10.8) | 33 (11.1) | 7 (9.6) | |
| Respiratory symptoms, n (%) | 58/371 (15.6) | 47 (15.8) | 11 (15.1) | |
| Respiratory symptoms and fever, n (%) | 6/371 (1.6) | 6 (2) | 0 (0) | |
| Gastrointestinal symptoms, n (%) | 2/371 (0.5) | 1 (0.3) | 1 (1.4) | |
| Other | 25 (6.7) | 21 (7) | 4 (5.5) | |
| Clinical severity (respiratory distress) | ||||
| Mild, n (%) | 149 (40.2) | 113 (37.9) | 36 (49.3) | 0.108 |
| Moderate, n (%) | 135 (36.4) | 108 (36.2) | 27 (37) | |
| Severe, n (%) | 8 (2.2) | 8 (2.7) | 0 (0) | |
| Absence of respiratory distress, n (%) | 79 (21.3) | 69 (23.2) | 10 (13.7) | |
| Approach in A&E | ||||
| Additional investigations | ||||
| Blood tests, n (%) | 126 (34) | 107 (35.9) | 19 (34) | 0.130 |
| Chest X-ray, n (%) | 27 (7.3) | 24 (8.1) | 3 (4.1) | 0.320 |
| Normal | 6 (1.6) | 5 (1.7) | 1 (1.4) | 0.495 |
| Abnormal | 21 (5.7) | 19 (6.4) | 2 (2.7) | |
| Pneumonia | 7 (1.9) | 6 (2) | 1 (1.4) | 0.652 |
| Perihilar infiltrates | 7 (1.9) | 7 (2.3) | 0 (0) | 0.505 |
| Peribronchovascular infiltrates | 9 (2.4) | 9 (3) | 0 (0) | 0.411 |
| Use of HFO in A&E, n (%) | 45 (12.1) | 36 (12.1) | 9 (12.3) | 1 |
| Use of oxygen in A&E, n (%) | 139 (37.5) | 108 (36.2) | 31 (42.5) | 0.347 |
| Use of bronchodilators in A&E, n (%) | 36 (9.7) | 36 (12.1) | 0 (0) | 0.001 |
| Use of corticosteroids in A&E, n (%) | 9 (2.4) | 9 (3) | 0 (0) | 0.215 |
| Use of volume expanders in A&E, n (%) | 5 (1.3) | 3 (1) | 2 (2.7) | 0.256 |
| PICU assessment in A&E, n (%) | 26 (7) | 20 (6.7) | 6 (8.2) | 0.614 |
| Destination | ||||
| Home, n (%) | 183 (49.3) | 150 (50.3) | 33 (45.2) | 0.547 |
| Admission to hospital | 187 (50.4) | 147 (49.3) | 40 (54.8) | |
| Admission to ward and did not require PICU, n (%) | 135 (36.4) | 108 (36.2) | 27 (37) | 0.856 |
| Admission to ward and did require PICU, n (%) | 9 (2.4) | 7 (2.3) | 2 (2.7) | |
| Admission to ward and subsequently transferred to HaH, n (%) | 14 (3.8) | 11 (3.7) | 3 (4.1) | |
| Admission to PICU from A&E, n (%) | 21 (5.7) | 15 (5) | 6 (8.2) | |
| Transfer of centre, n (%) | 8 (2.2) | 6 (2) | 2 (2.7) | |
| Discharge diagnosis | ||||
| Pneumonia, n (%) | 7 (1.9) | 6 (2) | 1 (1.4) | 1 |
| Lower respiratory tract infection, n (%) | 1 (0.3) | 1 (0.3) | 0 (0) | 1 |
| Bronchiolitis, n (%) | 294 (79.2) | 234 (78.5) | 60 (82.2) | 0.629 |
| Bronchospasm, n (%) | 7 (1.9) | 6 (2) | 1 (1.4) | 1 |
| Upper respiratory tract infection, n (%) | 56 (15.1) | 46 (15.4) | 10 (13.7) | 0.856 |
A&E: Accident and Emergency Department; HaH: hospital at home; HFO: high-flow oxygen therapy; PICU: paediatric intensive care unit.
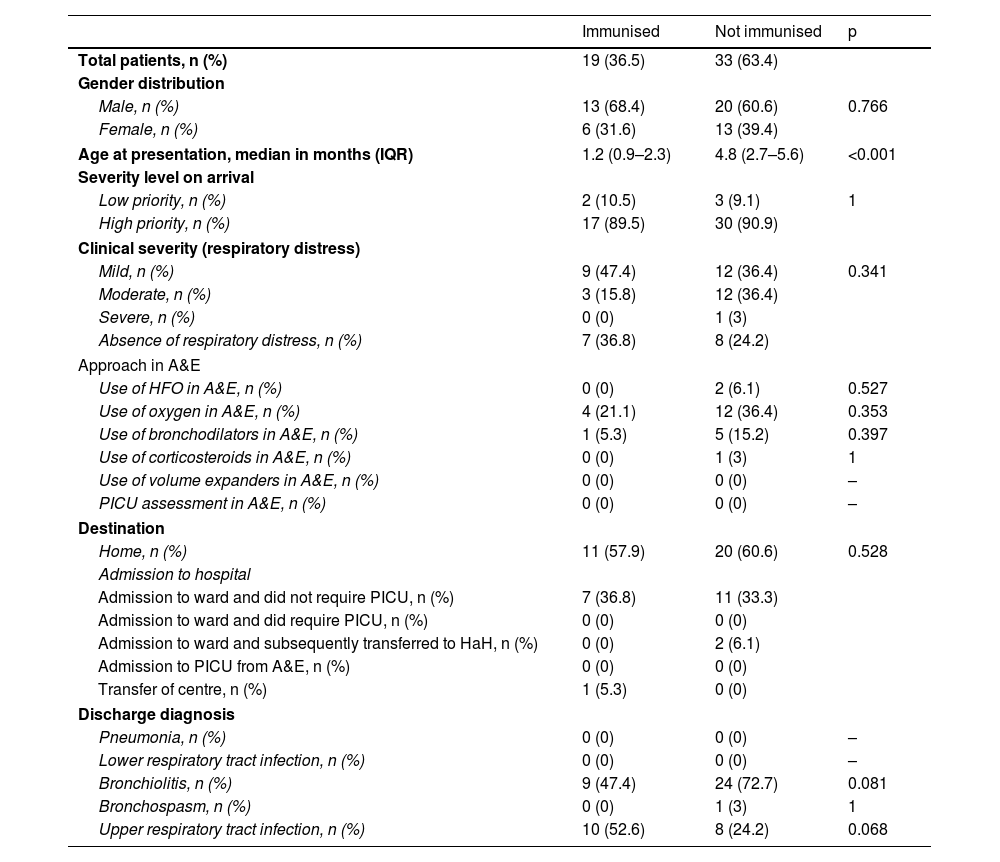
At discharge the most common diagnosis was bronchiolitis (79.2%), followed by upper respiratory tract infection (15.1%). No statistically significant differences were found comparing discharge diagnoses in the two years. We compared the main characteristics of the 52 patients eligible to have received nirsevimab in 2023 (Table 2). The only finding was that the median age of cases who did receive nirsevimab was significantly lower than that of non-immunised cases.
Characteristics of patients of an age eligible for immunisation with nirsevimab who consulted Accident and Emergency for RSV infection in the 2023 season. Comparison between immunised and non-immunised patients (n = 52).
| Immunised | Not immunised | p | |
|---|---|---|---|
| Total patients, n (%) | 19 (36.5) | 33 (63.4) | |
| Gender distribution | |||
| Male, n (%) | 13 (68.4) | 20 (60.6) | 0.766 |
| Female, n (%) | 6 (31.6) | 13 (39.4) | |
| Age at presentation, median in months (IQR) | 1.2 (0.9–2.3) | 4.8 (2.7–5.6) | <0.001 |
| Severity level on arrival | |||
| Low priority, n (%) | 2 (10.5) | 3 (9.1) | 1 |
| High priority, n (%) | 17 (89.5) | 30 (90.9) | |
| Clinical severity (respiratory distress) | |||
| Mild, n (%) | 9 (47.4) | 12 (36.4) | 0.341 |
| Moderate, n (%) | 3 (15.8) | 12 (36.4) | |
| Severe, n (%) | 0 (0) | 1 (3) | |
| Absence of respiratory distress, n (%) | 7 (36.8) | 8 (24.2) | |
| Approach in A&E | |||
| Use of HFO in A&E, n (%) | 0 (0) | 2 (6.1) | 0.527 |
| Use of oxygen in A&E, n (%) | 4 (21.1) | 12 (36.4) | 0.353 |
| Use of bronchodilators in A&E, n (%) | 1 (5.3) | 5 (15.2) | 0.397 |
| Use of corticosteroids in A&E, n (%) | 0 (0) | 1 (3) | 1 |
| Use of volume expanders in A&E, n (%) | 0 (0) | 0 (0) | – |
| PICU assessment in A&E, n (%) | 0 (0) | 0 (0) | – |
| Destination | |||
| Home, n (%) | 11 (57.9) | 20 (60.6) | 0.528 |
| Admission to hospital | |||
| Admission to ward and did not require PICU, n (%) | 7 (36.8) | 11 (33.3) | |
| Admission to ward and did require PICU, n (%) | 0 (0) | 0 (0) | |
| Admission to ward and subsequently transferred to HaH, n (%) | 0 (0) | 2 (6.1) | |
| Admission to PICU from A&E, n (%) | 0 (0) | 0 (0) | |
| Transfer of centre, n (%) | 1 (5.3) | 0 (0) | |
| Discharge diagnosis | |||
| Pneumonia, n (%) | 0 (0) | 0 (0) | – |
| Lower respiratory tract infection, n (%) | 0 (0) | 0 (0) | – |
| Bronchiolitis, n (%) | 9 (47.4) | 24 (72.7) | 0.081 |
| Bronchospasm, n (%) | 0 (0) | 1 (3) | 1 |
| Upper respiratory tract infection, n (%) | 10 (52.6) | 8 (24.2) | 0.068 |
A&E: Accident and Emergency Department; HaH: hospital at home; HFO: high-flow oxygen therapy; PICU: paediatric intensive care unit.
Our study analyses the impact of the recent nirsevimab immunisation campaign on paediatric emergency care. During the first three months of the 2023 RSV epidemic season, the number of patients seen in paediatric A&E with confirmed RSV infection decreased compared to the same period in 2022. This decrease particularly involved the nirsevimab-eligible age group, with a reduction of over 75% compared to the previous season. Although the admission and severity rates, treatments and diagnostic tests performed in this age group were similar to those in the equivalent period of the 2022 season, the absolute number of ward and PICU admissions in 2023 was lower; these findings are consistent with initial results from national8 and international9 studies. This undoubtedly resulted in a decrease in the use of resources, a fact to be taken into account given the significant economic impact of RSV infection in the paediatric population.10
One aspect which could have influenced the results of this study would be if there had been a significant change in the number of births between the two years analysed. However, with the latest data available at the time of writing this analysis,11 the birth rate in Madrid Region remained stable over this period (cumulative annual births in November: 46,739 in 2022 and 47,870 in 2023). Therefore, the decrease in consultations for RSV infection in the nirsevimab-eligible population could be attributed to the protective effect of nirsevimab. That seems even more likely when the number of consultations for RSV infection has remained stable in the non-eligible population, that is patients older than six months at the beginning of the winter season (260 non-eligible patients in 2022 and 241 patients in 2023).
A number of authors agree that one of the effects of the 2023 immunisation campaign has been an increase in the age of RSV cases. A French study12 found that the median age at first RSV infection in the 2023 season compared to the previous season increased from 4.5 to 8.1 months. These authors also found an increase in the age of patients hospitalised for RSV infection from 3.5 months in the 2022–2023 season to 6.2 months by the end of 2023. Similar data were recorded in a study conducted at the National Hospital of Luxembourg, which compared RSV hospitalisations in children under five years of age during the 2022 and 2023 RSV seasons, finding that the mean age of children was significantly higher in 2023 (14.4 months; standard deviation [SD]: 12.9) than in 2022 (7.8 months; SD: 10.1; p <0.001).13 Our data are also consistent with these findings, as the median age of patients seen in our A&E increased significantly in 2023 compared to 2022 (15.6 vs 9.6 months). Focusing the analysis on our study group, in other words infants eligible for immunisation with nirsevimab, we also found a slight increase in median age in 2023 compared to 2022 (3.6 vs 2.4 months), although without reaching statistical significance. The increase in the age of RSV cases could obviously have a significant impact on clinical practice, as it is the young infants who are most vulnerable to severe RSV infections.
One relevant finding in our series was that of the 52 patients with confirmed RSV infection seen in 2023, 63.5% (33/52) had not been immunised with nirsevimab. Most of this group (27/33; 81.8%) were born between 1 April and 30 September, a group which had to make an appointment to be immunised (unlike those born after 1 October, who were offered immunisation before discharge from the maternity ward). According to the data in the report issued on 1 March 2024 by the Madrid Region Department of Health, the immunisation rate in the group of infants born after 1 October is approximately 96%, while in the group born between 1 April and 30 September 2023 it was only 77%.14 The lower coverage rate in this group of patients may explain the results in our study. We suggest that future campaigns should include specific strategies designed to improve the immunisation rate of this group, which could theoretically further reduce the burden of RSV-associated disease.
Immunisation with nirsevimab here in Spain started on 1 October 2023 (week 40); in our case series we found that the number of total confirmed cases decreased dramatically from week 52 on. Considering that the half-life of nirsevimab in vivo is 68.7 days and that the period of effective protection is thought to be at least 150 days,15 this would imply that the theoretical window of protection for the immunised infant would cover the main months of the epidemic, and a single dose therefore seems adequate to ensure protection.
Our study has several limitations. The first of which is the retrospective component of its design, which may have introduced some bias due to the lack of data. Secondly, other variables that may influence the epidemic of RSV infection, such as socio-economic characteristics of the population, geographical location and climatic characteristics, were not analysed. However, the number of cases analysed during the two periods enabled us to identify significant differences in the volume of RSV cases among infants of immunisation-eligible age.
In conclusion, our findings suggest that immunisation with nirsevimab in the RSV epidemic season of 2023 in Madrid Region has had a beneficial impact in reducing the number of visits to A&E for RSV-related LRTI. This decrease in the number of cases was particularly significant among infants. Further studies are needed to confirm these data and to analyse which of the strategies in place to prevent RSV LRTI is most effective or whether different strategies need to be combined to reduce RSV-associated morbidity and mortality.
FundingThis article has received no funding from any public or private entity.
Conflicts of interestThe authors declare that they have no conflicts of interest.