Carbapenemase-producing Enterobacterales (CPE) is a global threat. We evaluate the prevalence of CPE among isolates categorized as meropenem-susceptible, but that meet the European Committee on Antimicrobial Susceptibility Testing (EUCAST) screening cut-off values for carbapenemase detection, and analyze the susceptibility of these isolates to new available drugs.
MethodsWe analyzed 257 isolates from patients hospitalized in a tertiary hospital in Brazil, from July 2022 to April 2023. Only isolates that met the screening cut-off values established by EUCAST for detection of carbapenemases were analyzed (i.e. meropenem inhibition zones of 25–27mm by disk diffusion). The detection of carbapenemases was performed by immnunochromatographic testing and confirmed by high-resolution melting-PCR (HRM-qPCR).
ResultsWe identified 12 (4.7%) CPE including 7 KPC, 4 NDM, and 1 OXA-48-like. The isolates were susceptible to ceftazidime–avibactam (72.7%), meropenem–vaborbactam (100%), imipenem–relebactam (63.6%) and ceftolozane–tazobactam (36.4%).
ConclusionWe highlight the importance of tracking carbapenemases for epidemiological control and therapeutic guidance.
Las Enterobacterales productoras de carbapenemasas (EPC) constituyen una amenaza mundial. Evaluamos la prevalencia de EPC entre cepas categorizadas como sensibles a meropenem, pero que cumplen los criterios del European Committee on Antimicrobial Susceptibility Testing (EUCAST) para la detección de EPC, y su sensibilidad a nuevos antimicrobianos.
MétodosAnalizamos 257 aislamientos de pacientes hospitalizados en un hospital terciario de Brasil (julio- 2022 a abril-2023). Solo se analizaron los aislados que cumplían los valores de puntos de corte establecidos por el EUCAST para la detección de carbapenemasas (halo de inhibición de meropenem de 25-27mm por difusión con disco). La detección de carbapenemasas se realizó mediante inmunocromatografía y se confirmó mediante análisis de PCR de alta resolución (HRM-qPCR).
ResultadosIdentificamos 12 (4,7%) EPC, incluyendo 7 KPC, 4 NDM y 1 OXA-48-like. Los aislados eran sensibles a ceftazidima-avibactam (72,7%), meropenem-vaborbactam (100%), imipenem-relebactam (63,6%) y ceftolozano-tazobactam.
ConclusiónDestacamos la importancia del seguimiento de las carbapenemasas para el control epidemiológico y orientación terapéutica.
Bacterial resistance to antimicrobials has become a global threat to public health. The World Health Organization (WHO) points to carbapenem-resistant Enterobacterales (CRE) as one of the main problems, since they are frequently related to therapeutic failures, longer hospital stays, higher healthcare costs, and greater mortality.1,2 Among resistance mechanisms, the production of carbapenemase enzymes is the most clinically relevant. The dissemination of carbapenemases in Enterobacterales is becoming a major problem as infections caused by these bacteria are in general associated with limited therapeutic options or, even in some cases, the absence of effective antimicrobial therapy, leaving only combinations of drugs available with different side effects.3 Active surveillance of carbapenemase-producing Enterobacterales (CPE) among high-risk populations can facilitate early detection and cohorting, limiting the risk of transmission as antimicrobial stewardship programs should be part of all healthcare settings, being associated with a 50% reduction in the incidence of CRE in hospitals.4
However, the presence of carbapenemases in Enterobacterales does not always confer clinical resistance due to low-level expression or even not being expressed. Certain infections caused by these isolates could be treated with carbapenems, but the additional detection of these mechanisms may be relevant for infection control and public health. Therefore, this study aimed to evaluate the prevalence of CPE among phenotypically meropenem-susceptible Enterobacterales (clinically susceptible), but that meet the European Committee on Antimicrobial Susceptibility Testing (EUCAST)5 screening cut-off values for carbapenemase detection. In addition, we evaluate the susceptibility profile of these isolates to new available drugs (beta-lactam/beta-lactamase inhibitor combinations).
Material and methodsBacterial strains and susceptibility profileConsecutive, non-duplicate isolates recovered from clinical samples from patients admitted to the Hospital de Clínicas de Porto Alegre (HCPA) (Porto Alegre, Brazil) from July 2022 to April 2023 were analyzed (approved by the local ethic committee). Only isolates that met the screening cut-off values established by EUCAST5 for detection of carbapenemase enzymes were analyzed, i.e., isolates that were susceptible to meropenem (10μg – OXOID, United Kingdom) with an inhibition zone of 25–27mm and resistant to piperacillin/tazobactam (36μg – OXOID), and isolates with meropenem inhibition zones of <25mm (regardless of susceptibility to piperacillin–tazobactam) by disk diffusion.6 The isolates were previously identified by MALDI–TOF MS (VITEK® MS System, bioMérieux, France).
The susceptibility profile of carbapenemase-producing isolates was obtained by gradient diffusion strips (Liofilchem®, Italy) for meropenem, ceftazidime–avibactam (CZA), meropenem/vaborbactam (MRV), imipenem/relebactam (IPR) and ceftolozane/tazobactam (CZT). Results were interpreted following EUCAST criteria.6
Carbapenemase detectionThe presence of carbapenemases was detected by the immunochromatographic test NG-Test®/Carba 5 (NG Biotech®) following the manufacturer's recommendations. The presence of carbapenemase genes in the isolates was confirmed by high-resolution melting-PCR (HRM-qPCR), targeting the genes: blaIMP, blaVIM, blaNDM-1, blaKPC, blaGES, and blaOXA-48.7
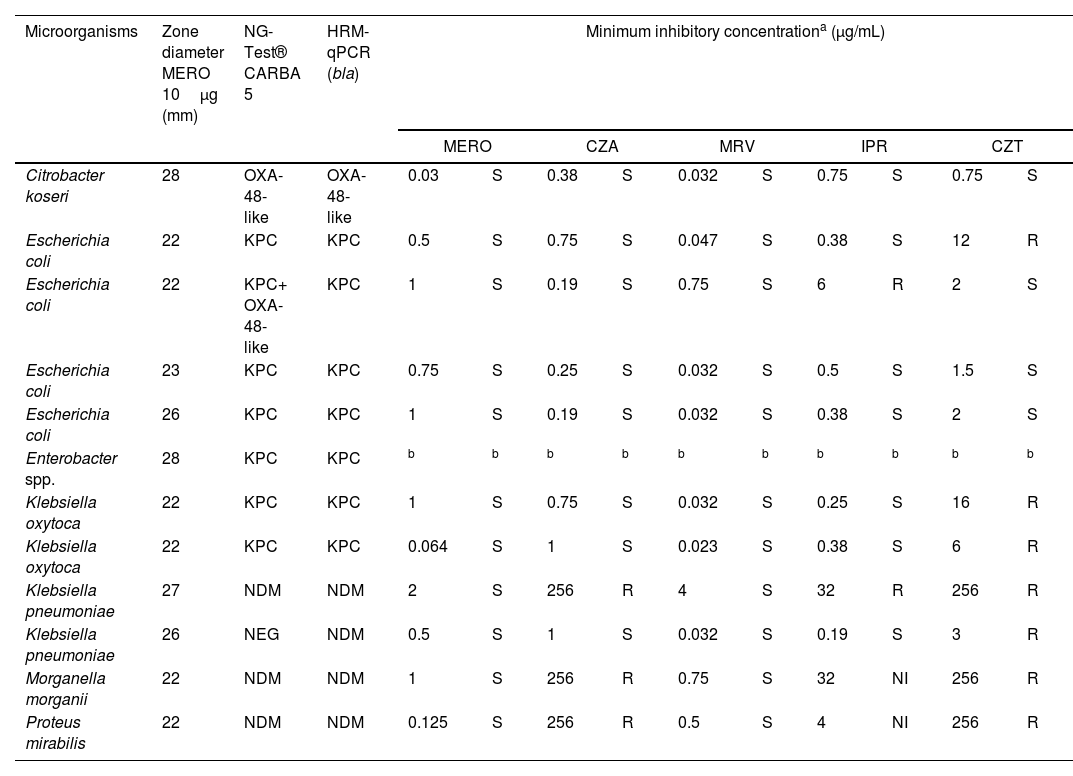
ResultsResults of immunochromatography, HRM-qPCR, and susceptibility profile of carbapenemase-producing isolates are presented in Table 1.
Results of immunochromatographic test, HRM-qPCR, and susceptibility profile of carbapenemase-producing isolates.
| Microorganisms | Zone diameter MERO 10μg (mm) | NG-Test® CARBA 5 | HRM-qPCR (bla) | Minimum inhibitory concentrationa (μg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MERO | CZA | MRV | IPR | CZT | |||||||||
| Citrobacter koseri | 28 | OXA-48-like | OXA-48-like | 0.03 | S | 0.38 | S | 0.032 | S | 0.75 | S | 0.75 | S |
| Escherichia coli | 22 | KPC | KPC | 0.5 | S | 0.75 | S | 0.047 | S | 0.38 | S | 12 | R |
| Escherichia coli | 22 | KPC+ OXA-48-like | KPC | 1 | S | 0.19 | S | 0.75 | S | 6 | R | 2 | S |
| Escherichia coli | 23 | KPC | KPC | 0.75 | S | 0.25 | S | 0.032 | S | 0.5 | S | 1.5 | S |
| Escherichia coli | 26 | KPC | KPC | 1 | S | 0.19 | S | 0.032 | S | 0.38 | S | 2 | S |
| Enterobacter spp. | 28 | KPC | KPC | b | b | b | b | b | b | b | b | b | b |
| Klebsiella oxytoca | 22 | KPC | KPC | 1 | S | 0.75 | S | 0.032 | S | 0.25 | S | 16 | R |
| Klebsiella oxytoca | 22 | KPC | KPC | 0.064 | S | 1 | S | 0.023 | S | 0.38 | S | 6 | R |
| Klebsiella pneumoniae | 27 | NDM | NDM | 2 | S | 256 | R | 4 | S | 32 | R | 256 | R |
| Klebsiella pneumoniae | 26 | NEG | NDM | 0.5 | S | 1 | S | 0.032 | S | 0.19 | S | 3 | R |
| Morganella morganii | 22 | NDM | NDM | 1 | S | 256 | R | 0.75 | S | 32 | NI | 256 | R |
| Proteus mirabilis | 22 | NDM | NDM | 0.125 | S | 256 | R | 0.5 | S | 4 | NI | 256 | R |
MERO, meropenem; CZA, ceftazidime–avibactam; MRV, meropenem–vaborbactam; IPR, imipenem–relebactam; CZT, ceftolozane–tazobactam (CZT); S, susceptible; R, resistant; NI, non-interpretable; NEG, negative.
Over the nine months covered by this study, 257 consecutive Enterobacterales isolates were included. Of these, 12 (4.7%) produced carbapenemases as follows: seven (58.3%) produced KPC (Klebsiella pneumoniae carbapenemase), four (33.3%) produced NDM (New Delhi metallo-β-lactamase), and one (8.4%) produced OXA-48-like enzymes. The minimum inhibitory concentration (MIC) values for meropenem ranged from 0.032 to 2μg/mL, confirming the susceptibility inferred by disk diffusion. The susceptibility rates of eleven isolates to the other antimicrobials evaluated were: 72.7% (8/11) for CZA; 100% (11/11) for MRV; 63.6% (7/11) for IPR; and 36.4% (4/11) for CZT.
DiscussionThe increasing occurrence of CPE has compromised the use of antimicrobials such as β-lactams.8 CPE detection is important not only for therapeutic strategies, when the effectiveness of new therapeutic options depends on the carbapenemase involved in the resistance mechanism, but also for infection control.9 Therefore, several tests have been developed for the detection of carbapenemases, however, these are often only used in cases of reduced susceptibility to carbapenems.8,10
As demonstrated by other authors, some carbapenemase-producing isolates can be categorized as susceptible to carbapenems when using the established EUCAST clinical breakpoints. In the study of Fattouh et al., among isolates categorized as susceptible to meropenem, 14% were carbapenemase-producers.11 In our study, more than half of CPE susceptible to meropenem produced KPC (58.3%), followed by NDM (33.3%), percentages that are similar to the distribution of carbapenemases among isolates categorized as resistant to meropenem in our institution, where 63.8% are KPC producers and 33.1% are NDM producers (unpublished internal data).
Our results are consistent with those of other studies indicating that the production of class A carbapenemases (i.e., KPC) is the most prevalent mechanism of resistance to carbapenenems in many regions of the world.9,12,13 In addition, we also found two OXA-48-like-producing isolates, Citrobacter koseri and Escherichia coli, in co-production with KPC. However, for the E. coli isolate, the detection of OXA-48-like was only observed in the immunochromatographic test but was not confirmed by HRM-qPCR. Despite this, the EUCAST established strategy for CPE screening among meropenem-susceptible isolates proved to be an important tool for the detection carbapenemase-producing isolates with known low hydrolytic activity toward carbapenems.14,15 Noteworthy, this was the only discrepancy found when comparing the results generated by NG-CARBA and HRM-qPCR, resulting in an agreement of 83.3% between both methodologies.
To our knowledge, this is the first Brazilian study evaluating the prevalence of carbapenemase production among meropenem-susceptible isolates, which is of great importance as the detection of infected patients and carriers are the two main approaches for preventing their spread. As indicated by EUCAST, the screening of carbapenemase-producing Enterobacterales is of high epidemiological importance, particularly when they confer decreased susceptibility to carbapenems, i.e. when the inhibition zones are below the epidemiological cut-off (ECOFF) values or the MICs are above the ECOFF. Clinical microbiology laboratories must perform these screening tests with the aim of understanding the profile of CPE in their institutions and to help clinicians in therapeutic guidance. This study highlights the importance of carbapenemases screening for epidemiological control and therapeutic guidance, pointing out that 5% of the meropenem-susceptible tested strains showed carbapenemases. Implementing this screening routine in hospital laboratories would help to reduce the risk of horizontal transmission.
We believe that new studies should be carried out addressing the detection of other enzymes. In this study we evaluated the presence of five types of carbapenemases that could not have been detected if this screening testing had not been performed. Increasing sample sizes to analyze enzymatic variability could confirm the trend of CRE epidemiology and the increased dissemination of metallo-β-lactamases.
FundingThis work was supported by Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul, Financiamento e Incentivo à Pesquisa (FIPE/HCPA), and a master's degree scholarship from Coordination of Superior Level Staff Improvement.
Conflict of interestsAll authors report no conflicts of interest relevant to this article.
The authors would like to thank the collaboration of the funding from Financiamento e Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE/HCPA).