Quantiferon-TB-Gold in Tube® test (QFT-G-IT) may have advantages if combined with TST when screening for Latent Tuberculosis Infection (LTBI) prior to initiating anti-TNF therapy in an area of intermediate tuberculosis incidence such as Spain. In a small-scale prospective study, we evaluate the use of QFT-G-IT in combination with the screening recommended in Spain (Tuberculin-Skin Test, TST retest, clinical data, and Chest X-Ray (CXR)) for LTBI in patients considered as candidates for anti-TNFα treatment.
ResultsFrom June 2008 to October 2010, 123 patients from a 300-bed hospital in Palma de Mallorca (Spain) were included in the study. The majority of patients were under immunosuppressive therapy. A positive TST and TST booster were found in 22 and 17 patients, respectively. Thus 39 (31.7%) of the 123 patients had a positive TST. QFT-G-IT was positive in 16 patients (13.6%), indeterminate in 4 (3.2%), and negative in 103 (83.7%). One of the two tests was positive and LTBI was diagnosed in 34.1% of patients. The agreement between TST and QFT-G-IT among vaccinated patients was low and not statistically significant (Kappa=0.15) and was almost perfect among non-BCG vaccinated patients (K=0.81). TST positive responses were significantly related to BCG-vaccination (p<0.05) and QFT-G-IT positive response rates were related to older age (p<0.05).
ConclusionQFT-G-IT may have advantages when combined with TST in immunosuppressed patients especially in older patients with a negative TST; in BCG vaccinated patients with a positive TST, QFT-G-IT could avoid unnecessary treatments and toxicities related to a false-positive TST result.
Quantiferon-TB-Gold in Tube® (QFT-G-IT) en combinación con la Prueba de la tuberculina (PT) puede ser útil para el diagnóstico de infección tuberculosa latente (ITL) en pacientes candidatos a tratamiento con anti-TNF en un país de incidencia intermedia de tuberculosis como España. Se evalúa en un estudio piloto prospectivo QFT-G-IT en combinación con las pruebas recomendadas en España (PT, PT-booster, datos clínicos y radiografía de tórax) para el diagnóstico de ITL en pacientes con enfermedades inmunológicas candidatos a tratamiento con fármacos anti-TNFα.
ResultadosSe incluyeron 123 pacientes desde junio de 2008 a octubre de 2010 en el hospital Son Llàtzer de Palma de Mallorca. La PT inicial y la PT booster fueron positivas en 22 y 17 pacientes, respectivamente, el 31,6% tuvo una PT positiva, QFT-G-IT fue positivo en 16 (13,6%), indeterminado en 4 (3,2%) y negativo en 103 pacientes (83,7%). En 34,1% al menos uno de los dos tests fue positivo y se diagnosticó ITL. La concordancia entre PT y QFT-G-IT fue baja en pacientes vacunados con BCG (Kappa=0,15) y excelente en no vacunados con BCG (K=0,81). La positividad de la PT se relacionó con la vacunación con BCG (p<0.05) y la de QFT-G-IT con una mayor edad (p<0.05).
ConclusiónEl uso de QFT-G-IT puede optimizar el diagnóstico de ITL en estos pacientes especialmente en los más añosos con una PT negativa. En pacientes vacunados de BCG con una PT positiva, QFT-G-IT podría evitar tratamientos innecesarios de ITL relacionados con un falso positivo.
The use of biological agents interfering with TNF α is an established tool in the treatment of an increasing number of immune-mediated inflammatory chronic diseases (IMIDs). However anti-TNFs cause immunosuppression and have been associated with the reactivation of latent tuberculosis infections (LTBI).1 The Food and Drug Administration2 and the European Medicines Agency3 recommend screening for LTBI prior to the use of anti-TNF agents. Patients with IMIDs are already at high risk of progression to active tuberculosis disease (TB) as a result of their treatments with glucocorticoids and other immunosuppressive therapies,4 and because of the inflammatory disease itself.5 Treatment with anti-TNF-α agents notably intensifies this threat.6
Spain, where the World Health Organization estimates the occurrence of 30 TB cases/100,000 population/year, is one of the Western European countries with the highest incidence of TB.7 An early report from the FDA in 2001 described 70 cases of TB among 147,000 patients treated with infliximab, of which 10 were reported in Spain.1
Interferon-γ Release Assays (IGRAs) have been introduced as important diagnostic tests, complementing or replacing the Tuberculin Skin-Test (TST) for the diagnosis of LTBI. Two ex vivo assay formats, ELISA and ELISpot, are used to detect the interferon-gamma (IFN-γ) response to specific-Mycobacterium tuberculosis (MTB) secreted proteins such as ESAT6, CFP-10, and TB7.7 (this additional antigen is only present in the last generation ELISA test). Both formats have commercially available tests: Quantiferon-TB-Gold in Tube® (QFT-G-IT)8 (Cellestis Ltd., Carnegie, Australia) and T-SPOT.TB® (Oxford Immunotec, UK).9 Both have shown a higher specificity compared with TST especially in BCG-vaccinated populations and a higher sensitivity in immunodepressed patients, and positive IGRA responses are closely associated with risk factors for MTB infection.10 In some countries, IGRAs are already recommended for clinical use in immunodepressed patients who are candidates for anti-TNF α treatment.11,12 In Spain, guidelines recommend their use in clinical studies, always in combination with TST.13,14 For patients undergoing immunosuppressive treatment and considered as candidates for anti-TNFα treatment, the recommendations of the Spanish Health Authorities regarding LTBI screening include: obtaining a history of previous exposure, performing a positive initial TST or positive retest (booster), or a CXR finding suggestive of old TB. There are no specific recommendations regarding IGRAs in this setting.15–17
The aim of this study was to evaluate the usefulness of QFT-G-IT in combination with TST, booster TST, and CXR for the diagnosis of LTBI in patients considered as candidates for anti-TNFα treatment for different IMIDs in routine clinical practice in a hospital in Palma de Mallorca from 2008 to 2010.
Patients and methodsDesignThis is an observational, small-scale pilot study.
PatientsAll consecutive patients considered as candidates for anti-TNFα treatment were evaluated at the Tuberculosis Unit of Hospital Son Llàtzer, a 300-bed hospital in Palma de Mallorca (Spain), from June 2008 to October 2010. All patients provided informed consent before enrolment and the study protocol was approved by the local Ethics Committee. Patients who had previously been under any anti-TNF treatment in other hospitals and who were referred to our centre were also included and evaluated before restarting treatment.
The following data were recorded: underlying diseases; comorbidities considered as risk factors for TB disease (HIV infection, alcohol consumption, intravenous drug abuse, leukopenia, chronic renal disease, diabetes, chronic liver disease); concomitant medications (steroids, disease-modifying antirheumatic drugs-DMARD-anti-TNFα); clinical symptoms; past history of tuberculosis and previous exposure to a known TB patient. Bacillus of Calmette and Guérin (BCG) vaccination was recorded taking into account the absence of a BCG-vaccination certificate in most of our patients: the presence of a typical BCG scar was accepted as an indicator of past vaccination; patients born in Spain after 1980 as well as patients without a typical BCG scar who stated they had not been vaccinated were considered as non-vaccinated patients. All other patients were considered as having an “unknown vaccination” status.
LTBI screening- -
A TST was performed in all patients: Tuberculin PPD 2TU/0.1ml was injected intracutaneously into the volar side of the forearm and the degree of induration was measured 72h after the injection. The test was considered as being positive if the induration was 5mm or larger in diameter.18 All patients with an induration <5mm underwent a second TST one week later to evaluate the booster effect.
- -
QFT-G-IT: peripheral blood samples were taken at the visit during which the TST was performed: 1ml of each blood sample collected was injected into each of the pre-coated tubes and tested with the QFT-G-IT following the manufacturer's instructions. First, we directly collected peripheral venous blood in three 1-ml heparin containing tubes. One tube contained the M. tuberculosis specific peptides EAST-6, CFP-10, and TB7.7 antigens; a positive control tube contained the T cell mitogen phytohemagglutinin; and a negative control tube contained only heparin. The test tubes were incubated at 37°C in a carbon dioxide incubator for 16–24h. After overnight incubation, 200μl plasma was removed from each tube and the IFN-γ concentration was measured using the assay kit according to manufacturer's instructions. A positive result was defined as an IFN-γ concentration ≥0.35IU/ml and results were considered indeterminate when a low mitogen and antigen response occurred or in case of a high response (>8.0IU/ml) of the negative control.
- -
A CXR was taken and reviewed by a staff radiologist to detect radiographic abnormalities suggestive of past TB.
Patients were considered as having LTBI if any of the QFT-G-IT or TST tests were positive.
Statistical analysisData were entered in a SPSS 16 database. Descriptive statistics included median and range for continuous variables and frequencies and proportions for categorical variables. Quantitative variables were compared by using a t-Student test and qualitative variables by using a Chi square test, with Fisher test applied when required. Statistical significance was deemed to be at p<0.05. The correlation between QFT-G-IT and TST was evaluated using kappa statistics. This method enables the measurement of the agreement above and beyond that expected by chance. This agreement is expressed in degrees and is considered as being poor or slight when it ranges from 0 to 0.2, as fair when it ranges from 0.2 to 0.4, as moderate when it ranges from 0.4 to 0.6, as substantial when it ranges from 0.6 to 0.8 and as almost perfect when it ranges from 0.8 to 1.19 The Mantel–Haenszel test was used to assess the degree of relationship between TST and QFT-G-IT, taking into account the BCG vaccination status. A standard bivariate and a multivariate analysis were performed to analyse the association of TST and QFT-G-IT results to age, BCG vaccination and type of immunosuppressive therapy.
ResultsOne hundred and twenty-three patients were recruited and all consented to the study. There were two withdrawals during follow-up, both due to unexpected deaths which were unrelated to any infectious disease.
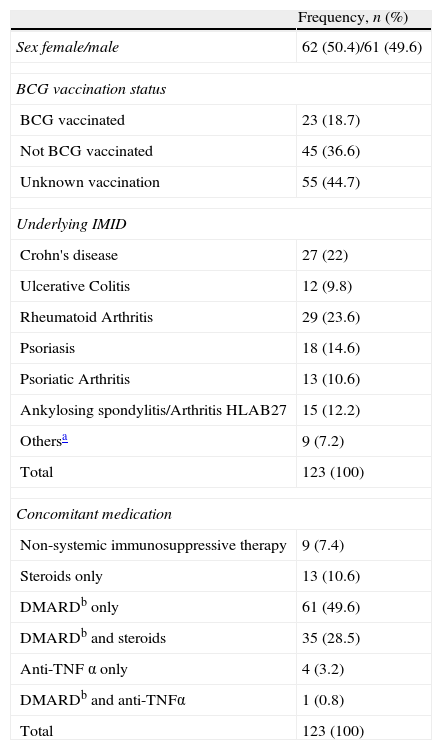
Median age was 45 years [range 18–79years]. There were 105 (84.7%) patients born in Spain, 9 (7.32%) patients born in Western Europe, and 9 patients born in high TB-incidence countries (8 South America, 1 India). There were no patients with known HIV infection. Seventeen patients had at least one comorbidity considered as risk factor for TB disease: 4 (3.3%) had leukopenia, 4 (3.3%) had chronic renal disease, 9 (7.3%) had diabetes and 1 (0.8%) had chronic liver disease. There were neither intravenous drug abusers nor heavy alcohol consumers. Underlying IMIDs and BCG vaccination status are described in Table 1. The majority of patients (92.7%) were under immunosuppressive therapy (Table 1). One patient had a prior history of treated LTBI. Residual TB findings were described in CXR in 7 cases (5.9%).
Characteristics of the 123 patients. Sex, BCG-vaccination status, underlying immune-mediated inflammatory chronic diseases (IMIDs) and concomitant medication. PM 2008–2010.
| Frequency, n (%) | |
| Sex female/male | 62 (50.4)/61 (49.6) |
| BCG vaccination status | |
| BCG vaccinated | 23 (18.7) |
| Not BCG vaccinated | 45 (36.6) |
| Unknown vaccination | 55 (44.7) |
| Underlying IMID | |
| Crohn's disease | 27 (22) |
| Ulcerative Colitis | 12 (9.8) |
| Rheumatoid Arthritis | 29 (23.6) |
| Psoriasis | 18 (14.6) |
| Psoriatic Arthritis | 13 (10.6) |
| Ankylosing spondylitis/Arthritis HLAB27 | 15 (12.2) |
| Othersa | 9 (7.2) |
| Total | 123 (100) |
| Concomitant medication | |
| Non-systemic immunosuppressive therapy | 9 (7.4) |
| Steroids only | 13 (10.6) |
| DMARDb only | 61 (49.6) |
| DMARDb and steroids | 35 (28.5) |
| Anti-TNF α only | 4 (3.2) |
| DMARDb and anti-TNFα | 1 (0.8) |
| Total | 123 (100) |
IMIDs include: uveitis (2 cases) and keratoderma, scleroderma, penphigus, Behçet¿s syndrome, Lupus Erythematosus, other vasculitis, epidermolysis acquisita, one case each.
b DMARD (Disease-modifying antirheumatic drugs) therapy includes: cyclosporine, azatioprine, hydroxychloroquine, leflunomide, methotrexate, sulfasalazine.
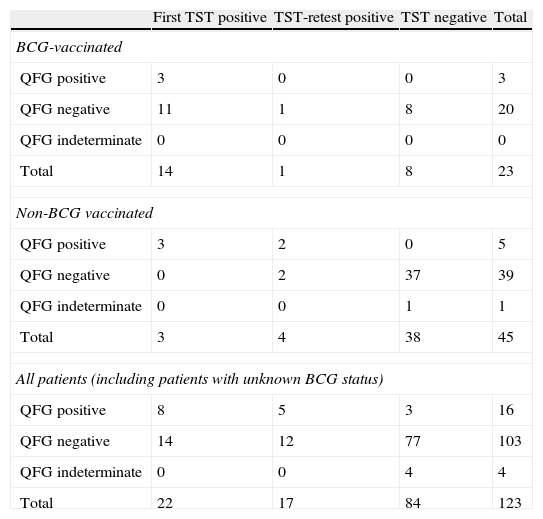
A positive TST was found in 22 patients, and the TST-booster was positive in 17 patients who previously had a negative TST. Thus 39 (31.7%) of the 123 patients had a positive TST. QFT-G-IT was positive in 16 patients (13.6%), indeterminate in 4 (3.2%), and negative in 103 (83.7%). One of the two tests was positive and LTBI was diagnosed in 42/123 (34.1%) patients. LTBI was diagnosed only through QFT-G-IT in 3 4/123 (2.4%) patients. Agreement among both tests was fair, either when the first TST (Kappa (K)=0.31; CI 95%: 0.009–0.53; p<0.05) or TST and TST retest were considered (K=0.35; CI 95%: 0.18–0.52, p<0.05) (Table 2).
Results of TST and QFT-G-IT among different groups: BCG vaccinated, non-BCG-vaccinated patients and all patients (independent of the BCG-status). PM 2008–2010.
| First TST positive | TST-retest positive | TST negative | Total | |
| BCG-vaccinated | ||||
| QFG positive | 3 | 0 | 0 | 3 |
| QFG negative | 11 | 1 | 8 | 20 |
| QFG indeterminate | 0 | 0 | 0 | 0 |
| Total | 14 | 1 | 8 | 23 |
| Non-BCG vaccinated | ||||
| QFG positive | 3 | 2 | 0 | 5 |
| QFG negative | 0 | 2 | 37 | 39 |
| QFG indeterminate | 0 | 0 | 1 | 1 |
| Total | 3 | 4 | 38 | 45 |
| All patients (including patients with unknown BCG status) | ||||
| QFG positive | 8 | 5 | 3 | 16 |
| QFG negative | 14 | 12 | 77 | 103 |
| QFG indeterminate | 0 | 0 | 4 | 4 |
| Total | 22 | 17 | 84 | 123 |
The 4 patients who had an indeterminate QFT-G-IT result had a negative TST.
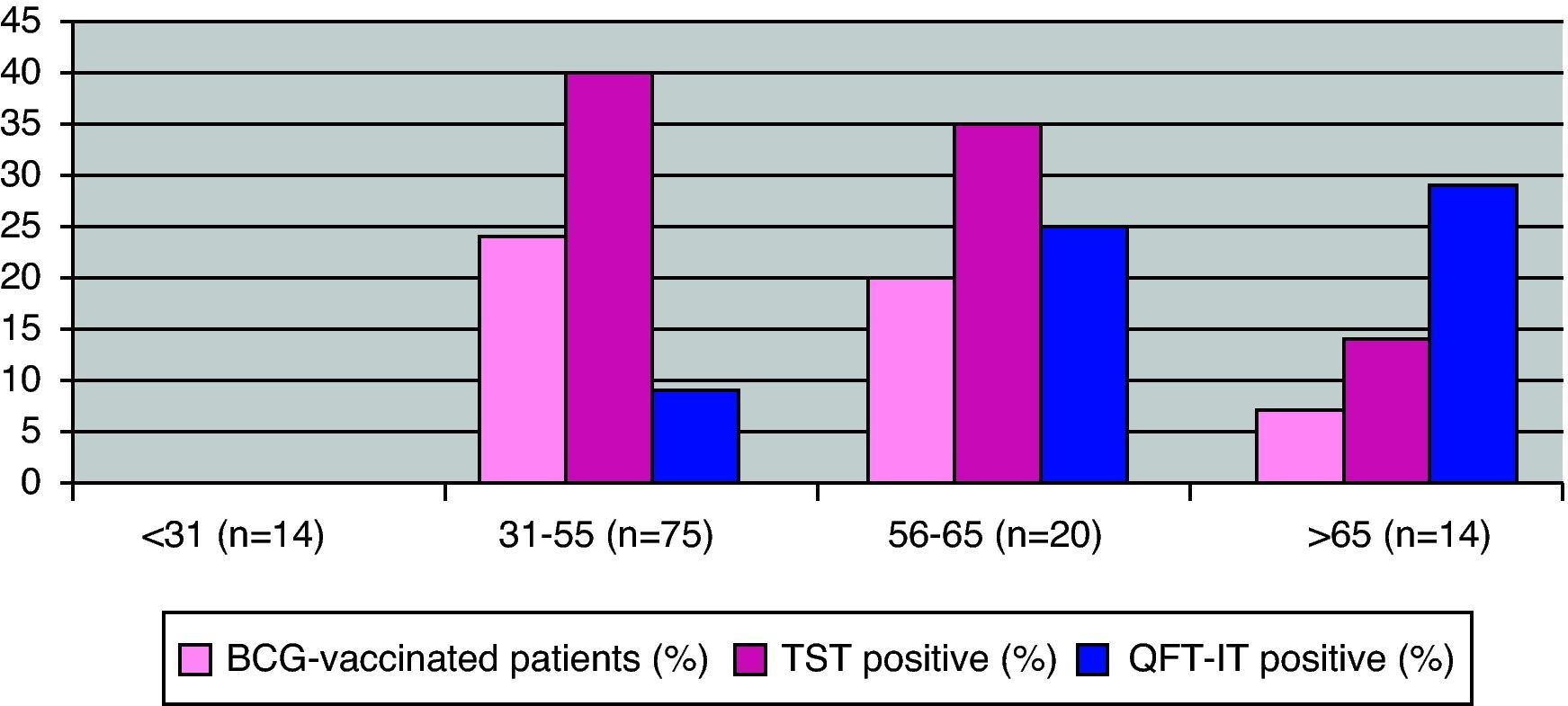
Effect of age, risk factors and immunomodulatory treatments for LTBI on TST and QFT-G-IT resultsIn the bivariate analysis, QFT-G-IT positive response rates were related to older age [QFT-G-IT positive: mean age: 54.81 (SD: 12.97); QFT-G-IT negative: mean age: 45.22 (SD: 14.39); p<0.05, CI 95%: 2.0–17.2]. This relationship with age was not observed for the TST (Fig. 1). Patients above 55 years old were at a higher risk for a QF-G-IT positive test than patients with 55 years old or younger (OR: 4.2, CI 95%: 1.5–12.2, p<0.005). The multivariate analysis did not improve the bivariate analysis.
Among the 17 patients with comorbid conditions, 8 (47.1%) were diagnosed of LTBI, TST was positive in 8 (47.1%) cases, and QFT-G-IT was positive in 2 (11.8%) cases. We could not find any relationship between comorbidities and a negative TST or QFT-G-IT.
None of the immunosuppressive treatments could be related to a negative TST or negative QFT-G-IT. Five patients had already received anti-TNF therapy when they were included in the study, all of them but one had negative TST and QFT-G-IT tests; a patient with both TST positive and QFT-G-IT positive tests was under anti-TNF therapy and had previously been diagnosed and treated for LTBI in another hospital. Four patients had an indeterminate QFT-G-IT result, two of them were receiving DMARDs whereas one was on anti-TNFα treatment; CXR was normal in all cases.
Effect of BCG vaccination on TST and QFT-G-IT resultsTwenty-three patients had received previous BCG vaccination. The agreement between TST and QFT-G-IT among vaccinated patients was low and was not statistically significant, neither when considering only the first TST, K=0.18 (CI 95%: −0.23 to 0.37, p>0.05) nor when considering both, K=0.15 (CI 95%: −0.30 to 0.32 0, p>0.05) (Table 2).
There were 45 non-BCG vaccinated patients. The agreement between TST and QFT-G-IT among non-BCG vaccinated patients was substantial when the first TST was considered, K=0.73 (CI 95%: 0.37–1, p<0.05) and almost perfect when both TST and TST retest were taken into account, K=0.81 (CI 95%: 0.55–1, p<0.05) (Table 2). TST positive responses were significantly related to BCG-vaccination (p<0.05) and this relationship was not observed for the QFT-G-IT.
BCG-vaccination status significantly affects the relationship between TST and QFT-G-IT (Mantel–Haenszel test, p<0.05).
Correlation between abnormal CXR and QFT-G-ITSeven patients had a CXR suggestive of past TB, 3 had a positive TST, and 2 had a positive QFT-G-IT. The two patients with a positive QFT-G-IT had negative TST and booster TST. Three sputum samples were collected from each of these patients for Acid-Fast Bacilli examination and for mycobacterium culture, and all were negative. When available, a previous CXR was evaluated by a radiologist to ensure the stability of the radiological findings.
Treatment and follow-upAll patients with either a positive QFT-G-IT or a positive TST were advised to receive treatment; 39/42 patients received 9-month isoniazid therapy and the treatment was changed to 4-month rifampicin therapy in 4 patients because of toxicity. One patient had already been treated for LTBI and two patients rejected treatment but they were not treated with anti-TNF therapy. In addition, two patients with an abnormal CXR and negative TST and QFT-G-IT had not started receiving anti-TNFα therapy yet and had not received LTBI treatment either. The median follow-up period was 31 months [range 1–47]. Fifty-two (42.3%) patients had already initiated an anti-TNFα therapy whereas 71 (57.7%) had not. TB was not reactivated in any of these cases during follow-up.
DiscussionIn the current study, LTBI was diagnosed in 34.1% of patients considered as candidates for anti-TNFα treatment. This rate is higher than previously reported in studies conducted in Southern Europe which have found rates ranging from 12 to 22%.17,20 TST positive rates were higher among BCG-vaccinated patients than in non BCG-vaccinated patients and this difference with regard to BCG vaccination was not seen in QFT-G-IT results. Furthermore, QFT-G-IT positive response rates were related to older age. In 3.2% of patients, LTI was diagnosed only through QFT-G-IT.
This high prevalence of LTI may be explained in various ways. First, in our study, a higher proportion of positive QFT-G-IT tests were detected in older patients. Patients over 65 years old were most likely exposed to MTB infection at a young age, when Spain was a very high incidence TB country in the 1950s and had a tuberculosis-related mortality above 125 deaths/100,000persons/year.21 Therefore age is also a predictor of LTBI. We observed discordant results (negative TST/positive QFT-G-IT) among older patients. Our findings may be related to decreased immunity due to age resulting from a reduced mobility of the T lymphocytes that have to migrate to the forearm where the TST is applied, as has been previously described.22,23 The hypothesis that TST might be more sensitive to remote infections while IGRA mainly detects recent infections is not supported by our data.24 In addition, the higher rates of positive TSTs observed in the 31–55-year-old group could be related to BCG-vaccination. Despite a high proportion of patients with an unknown vaccination status, we have found that TST positivity is influenced by BCG-vaccination. It is generally agreed that BCG vaccination interferes with TST25,26 and 25% of individuals vaccinated at primary school may have persistent tuberculin reactions.25 The two-step TST may cause a booster phenomenon mostly related to the BCG-vaccine27 but in our study only 1/15 BCG-vaccinated patients were with a positive TST. This result could be related to the booster phenomenon. False positive results in TST induced by previous non-tuberculous mycobacteria sensitisation could be another possibility to be considered.28
In high and low TB incidence countries, a lower TST sensitivity has been demonstrated in immunosuppressed patients compared with immunocompetent patients.29,30 In our study, 4/123 (3.2%) patients had discordant negative TST/positive QFT-G-IT results but an association with immunosuppressive therapy could not be demonstrated. Similar data have been described by other investigators,20,31 and only one study conducted in Peru in patients with rheumatoid arthritis has found a higher rate (23.8%) of discordant negative TST/positive QFT-G-IT.32 A small Spanish study in patients with inflammatory rheumatologic diseases found that positive TST, T-SPOT. TB and QFN-G-IT results were not affected by the immunosuppressive therapies.33 In our study, the rate of indeterminate results was low (3.4%), as previously described20,34 and we did not find any association between steroids or other immunosuppressive treatment and indeterminate results, as it has been suggested.35,36
We have observed a good agreement between TST and QFT-G-IT in non-vaccinated patients and a poor agreement in vaccinated patients. BCG-vaccination status significantly affects the relationship between TST and QFT-G-IT. Studies in Italy20 and Turkey31 have found a similar rate of positive QFT-G-IT in immunosuppressed patients. In countries with a high BCG-vaccination rate, the percentage of discordant results (positive TST/negative IGRA) varies from 25.4% in a low TB incidence country such as Switzerland34 to 47.5% in a high TB incidence country such as Turkey.31 In two studies conducted in Germany35 and Italy20 which have a low percentage of BCG-vaccinated patients, the proportion of discordant results (positive TST/negative flow-cytometric-interferon-γ assay or IGRA test) was low (7.2% and 6%, respectively). However, a recently published Spanish study found discordant positive TST/negative QFT-G-IT results in 8.9% but the authors were unable to demonstrate an association between these discordant results and BCG vaccination.37
Clinical guidelines differ between countries. In Switzerland11 and in the UK,38 TST is not recommended in patients under immunosuppressive treatment, and LTBI treatment is recommended if an IGRA test is positive or if there are risk factors for TB infection. In Germany12 TST is recommended only if the IGRA test is negative and if there is any evidence of prior TB exposure. In France39 TST is recommended for all patients and there is no guidance regarding the IGRA test. Spanish guidelines recommend an IGRA test for LTBI in controlled studies in immunodepressed patients with a negative TST and in BCG-vaccinated patients with a positive TST, but no specific recommendation about IGRAs are made for patients on anti-TNF therapies.13–16 Recently, the European Tuberculosis Network Trials Group (TBNET)6 has provided consensus recommendations regarding TB and anti-TNF treatment: a TST and an IGRA test should be done to all patients except BCG-vaccinated patients for whom a TST is not required; treatment for LTBI is recommended if any of the tests are positive. Moreover, it should be considered that among anti-TNF therapies, infliximab and adalimumab carry a higher TB risk than etanercept.40
In our study, we decided to treat all patients with either a positive TST or a positive QFT-G-IT. There were patients with a probable false positive TST related to BCG vaccination, who had received unnecessary preventive TB treatment but most of the available information about IGRA in patients with IMID comes from small studies and false-negative results are not uncommon. We still do not have national recommendations about the use of IGRAs in patients with IMID based on large-prospective randomized and controlled studies.
Our study is a small-scale pilot study and therefore shows some limitations. Underlying immune-mediated diseases and immunosuppressive treatments were heterogeneous, some patients had already been treated with anti-TNFα agents, and BCG-vaccination status could not be assessed in all patients, but this situation reflects what happens in the daily clinical practice. Furthermore, another limitation is the lack of a gold-standard method for the diagnosis of LTBI. Despite these limitations, our results suggest that QFT-G-IT may have advantages compared to TST when screening for LTBI before initiating anti-TNFα therapy in our setting, especially in old age and in BGG-vaccinated patients. In our opinion, QFT-G-IT should be used in immunosuppressed patients if the TST is negative and probably in BCG vaccinated patients with a positive TST to avoid unnecessary treatments and toxicities related to false-positive TST result. Large longitudinal studies are required in this setting to define the role of IGRA testing in the diagnosis of LTBI in patients considered as candidates for anti-TNFα therapy.
FundingThe study was supported by a grant from the Ministerio de Sanidad y Consumo Instituto de Salud Carlos III, Madrid, Spain, FIS 06/801.
Conflict of interestThe authors declare that they have no conflict of interest.
We would like to thank Claire Graham for translation assistance and Dr. Antonio Pareja for statistical assistance.
This study was presented in part at the XIVth Congress of the Spanish Society of Infectious Diseases and Clinical Microbiology, 19–22 May 2010, Barcelona, Spain.