In this study, the potential of matrix-assisted laser desorption/ionization time-of-flight intact cell mass spectrometry (MALDI-TOF ICMS) was investigated for the identification of clinical isolates. The isolates were analyzed at the species and strain level.
MethodsSpectral identification by MALDI-TOF ICMS was performed for all strains, and compared with the results of sequencing of the internal transcribed spacers (ITS1 and ITS2), and the 5.8S rDNA region. PCR fingerprinting analysis using primers M13, (GACA)4, and (AC)10 was performed in order to assess the intra-specific variability of Trichophyton rubrum strains.
ResultsThe identification of strains at species level by MALDI-TOF ICMS was in agreement with the previously performed morphological and biochemical analysis. Sequence data confirmed spectral mass identification at species level. Intra-specific variability was assessed. Within the T. rubrum cluster, strains were distributed into smaller highly related sub-groups with a similarity values above 85%.
ConclusionsMALDI-TOF ICMS was shown to be a rapid, low-cost and accurate alternative tool for the identification and strain typing of T. rubrum.
En este estudio se investigó el potencial de matrix-assisted laser desorption/ionization time-of-flight intact cell mass (MALDI-TOF ICMS) para la identificación de aislados clínicos. Los aislados fueron analizados al nivel de especie y de cepa.
MétodosSe realizó una identificación espectral mediante MALDI-TOF ICMS de todas las cepas que se comparó con los resultados de secuenciación de la región espaciadores transcritos internos (ITS1 e ITS2) y el ADNr 5,8S. Los análisis PCR fingerprinting usando los primers M13, (GACA)4 y (AC)10 se realizaron con la intención de evaluar la variabilidad intraespecífica de las cepas de Trichophyton rubrum.
ResultadosLa identificación de las cepas al nivel de especie mediante MALDI-TOF ICMS concordó con la ID realizada retrospectivamente en el análisis morfológico y bioquímico. La secuencia de datos confirmó la identificación por espectro de masas a nivel de especie. Se evaluó la variabilidad intraespecífica. Dentro del clúster de T. rubrum, las cepas se distribuyeron en subgrupos menores y muy relacionados con valores de similaridad superiores a 85%.
ConclusionesSe demuestra que MALDI-TOF ICMS es una herramienta alternativa rápida, de bajo coste y precisa para la identificación y tipificación de cepas de T. rubrum.
Dermatophytes are the etiological agents of fungal infections of the skin, hair, and nails, globally known as tinea. Despite dermatophytes belong to a homogenous group of fungi closely related to each other phylogenetically, the identification of species, as well as strain level, remains an important issue for epidemiological and taxonomic purposes.1–4 Phenotypic characters and ecological features have been for a long time the basis for phylogenetic studies and taxonomic classification. Additionally, biochemical and physiological tests can be used as complementary tools.5
Although morphology-based identification has been the main technique for the identification of dermatophytes, some limitations of the procedure could be pointed out. Firstly, it is based on the recognition of specific morphological features in macroscopic and microscopic observations; however, morphological similarity, intra-specific variability and polymorphism of dermatophytes are difficult for the identification procedure. Furthermore, confusion is caused by the atypical growth forms that occur frequently, and the requirement of a long incubation period (2–4 weeks) could be a significant drawback for rapid diagnosis and treatment. In addition, these approaches are time-and labour-consuming, expensive and requiring highly skilled-specific staff,3,6 which normally delays the diagnosis.
PCR technology is a powerful method that enables the development of new techniques in molecular biology for accurate organism identification.7–9 Molecular biology-based techniques are useful to reveal intra-specific DNA polymorphism that enables defining clusters within the same species.2,9,10 Furthermore, they have the advantage, over traditional methods, of being fast, simple, sensitive, and reproducible.3 Nevertheless, these methods remain time/labour consuming and relatively expensive.11
Along the past decade matrix-assisted laser desorption/ionization time of flight intact cell mass spectrometry (MALDI-TOF ICMS) technique has been established as a valuable system for microorganisms identification to be introduced in laboratory routine.12,13 Potentialities of MALDI-TOF ICMS have been revised for filamentous fungi.14 Some procedures for the identification of dermatophyte species, including Trichophyton rubrum, have been recently established. MALDI-TOF ICMS system was evaluated in comparison with sequencing the internal transcribed spacer 1 (ITS1), ITS2 and the 5.8S rDNA regions,15 D2 28S rRNA,16 and ITS region of the ribosomal DNA with V9D and LSU 266 primers.17 High confidence level (99.9%) for identification was found. Authors found similar or even better specificity of MALDI-TOF ICMS than an ITS/PCR-based approach for species identification purposes. MALDI-TOF ICMS proved to be a rapid, low cost, accurate, and reliable alternative to morphological and PCR analysis for the identification of dermatophytes.15–19 Although MALDI-TOF ICMS technique for species identification is a widely investigated theme, only a few recent studies focalize on the potential capacity for intra-specific discrimination in fungi.13,20–23
The aim of this work was to examine the potential of MALDI-TOF ICMS for the identification of clinical isolates of T. rubrum at the species and strain level. Molecular typing using three different primers was compared with spectrometric analysis.
Materials and methodsStrains and culture conditionsA set of 24 clinical isolates of T. rubrum from the fungal collection of Micoteca da Universidade do Minho (MUM), Portugal, were used for analysis. Two T. rubrum isolates were collected in Spain from human patients with tinea pedis (MUM 09.26) and onychomycosis (MUM 09.20). The remaining clinical isolates MUM 08.05, 08.09, 08.11, 08.12, 08.13, 08.15, 09.08, 09.09, 09.10, 09.12, 09.18, 09.29, 10.128, 10.133, 12.01, 12.02, 12.03, 12.04, 12.06, 12.08, 12. 09 and 12.10 were collected in Portugal from human patients with onychomycosis. Clinical isolates of Trichophyton interdigitale (MUM 10.136 and MUM 12.07) were also used as out-group. Two reference strains were obtained from the American Type Culture Collection (ATCC) and included in this study. Strain T. rubrum ATCC MYA-4438 was used as positive control and strain Trichophyton mentagrophytes ATCC MYA-4439 was used as out-group in MALDI-TOF analysis.
Potato dextrose agar (PDA, OXOID, UK) was used to maintain the cultures and to grow the fungi for molecular and mass spectral analyses.
Morphological identificationAll isolates were tested by conventional method, according to the standard procedures of morphological identification (macro-and micromorphology). Urease test was used as complementary biochemical test.
Matrix-assisted laser desorption/ionization time-of-flight intact cell mass spectrometry identificationSamples preparation: In a first attempt several experimental conditions were tested on four strains (ATCC-MYA 4438, MUM 09.08; MUM 08.11; MUM 10.133) and spectra were compared in order to select the best method. Two matrices were used: 2,5-dihydroxybenzoic acid (DHB) and α-cyano-4-hydroxycinnamic acid (CHCA). Different extraction protocols (including formic acid and sonication), as well as previous suspension of cells in water or solvent mixtures did not result in an improvement compared to the direct application of cells to the MALDI target. As no clear improvement in spectrum quality was observed in the preliminary study we followed the one described by Santos et al.14 for the clinical isolates. A growing time of 5 days at 30°C was set for all cultures before MALDI-TOF analysis to avoid age-dependent variability. Each sample was spotted in quadruplicate to test reproducibility.
MALDI-TOF analysis: The analysis was performed on an Axima LNR system (Kratos Analytical, Shimadzu, UK) equipped with a nitrogen laser (337nm), where the laser intensity was set just above the threshold for ion production. The mass range from 2000 to 20000Da was recorded using the linear mode with a delay of 104ns and an acceleration voltage of +20kV. Final spectra were generated by summing 20 laser shots accumulated per profile and 50 profiles produced per sample, leading to 1000 laser shots per summed spectrum.
Spectral Identification and software analysis: Resulting peak lists were exported to the SARAMIS™ software package (Spectral Archiving and Microbial Identification System, AnagnosTec, Germany, www.anagnostec.eu) where the final microbial identification was achieved. Peak lists of individual samples were compared on SARAMIS™ database generating a ranked list of matching spectra. This software uses a point system based on peak list with mass signals weighted according to their specificity. A dendrogram and matrix of spectral similarity between isolates were created using SARAMIS™ package. A minimum of 100 peaks, and at least 90% of mass similarity with species SuperSpectra was defined as the acceptance criteria to validate the result. The similarity between individual spectra was expressed as the relative or absolute number of matching mass signals after subjecting the data to a single link agglomerative clustering algorithm.
Molecular identificationDNA isolation: Spores and mycelia from 5 days old culture grown on PDA medium were transferred into a sterile 1.5ml tube and 1ml of extraction buffer (1% SDS, 25mmol/l EDTA, 250mmol/l NaCl and 200mmol/l Tris–HCl, pH 8) was added. A sterile pestle was used to ground the sample. The content was then incubated for 1h at 65°C. After incubation samples were centrifuged at 14000rpm for 5min and the supernatant was aliquoted in 800μl volumes into 1.5ml tubes. Tubes were then cooled on ice, and protein extraction was performed with 800μl of phenol following with equal volumes of phenol–chloroform (1:1) and, finally, with 800μl chloroform. Purified nucleic acids were precipitated with 0.6 volumes of ice-cold 2-propanol. DNA was washed twice with 150μl of ice-cold 70% ethanol and air dried, suspended in 50μl of ultra-pure water, and stored at −20°C.
ITS sequencing: The analysis was carried out using primer pair ITS1 5′-TCCGTAGGTGAACCTTGCGG-3′ and ITS4 5′-TCCTCCGCTTATTGATATGC-3′.24 The PCR reactions were performed in a total volume of 50μl, containing the 1× PCR buffer (Promega, USA), 2.5mmol/l MgCl2 (Promega, USA), 0.2mmol/l of deoxynucleoside triphosphates (dNTPs) (Promega, USA), 0.5mmol/l of each primer, 1U of GoTaq® Hot Start Polymerase (Promega, USA) and 50ng of DNA. PCR was run in a MyCycler™ Thermal Cycler (Bio-Rad Laboratories, USA) using the following conditions: 2min, 95°C; 35 cycles of 1min, 95°C, 1min, 55°C, 1min, 72°C; and final extension, 5min, 72°C. Amplification products were separated by electrophoresis in 1.0% agarose gel, stained with SafeView DNA Stain (NBS Biologicals, UK), at 80V for 30min. The PCR products were purified using QIAquick PCR Purification Kit (Qiagen, Germany) and then sequenced. The sequences were compared using the GenBank database (http://www.ncbi.nlm.nih.gov/BLAST/). GenBank access numbers for the isolates MUM 08.11 (JQ663974), MUM 10.128 (JQ663986), MUM 08.05 (JQ663971), MUM 09.26 (JQ663984), MUM 09.20 (JQ663983), MUM 08.12 (JQ663975), MUM 09.09 (JQ663979), MUM 08.13 (JQ663976), MUM 08.15 (JQ663977) and MUM 09.29 (JQ663985) with the reference strain T. rubrum ATCC MYA-4438 (FJ746657).
Typing: For typing analysis oligonucleotides M13 5′-GAGGGTGGCGGTTCT-3′,25 (GACA)426 and (AC)1027 were used. PCR reactions were performed in a total volume of 50μl, containing the 1× PCR buffer, 2.5mmol/l MgCl2 0.2mmol/l of dNTPs, 0.5μmol/l of each primer, 2U of GoTaq® Hot Start Polymerase and 50ng of DNA. PCRs were run in a MyCycler™ Thermal Cycler using the following conditions: 2min, 95°C; 35 cycles of 1min, 95°C, 1min, 50°C, 2min, 72°C; and a final extension cycle, 5min, 72°C. Amplification products were separated by electrophoresis in 1.5% agarose gel, stained with SafeView DNA Stain, at 60V for 120min.
ResultsTwenty six dermatophyte clinical isolates (24 T. rubrum and 2 T. interdigitale) and two ATCC reference strains were analyzed in quadruplicate, and the spectra were chosen according to the greatest number of peaks (data count) and the highest percentage of identity. Differences in the confidence level of identification between the replicates never exceeded 5%. A percentage identity over 99% was found in all strains, except MUM 08.11 (80.2%), MUM 09.10 (89.8%), MUM 10.128 (93.1%), MUM 12.10 (96.4%), MUM 08.05 (96.2%), and MUM 09.18 (97.1%). The identification of clinical isolates at the species level by MALDI-TOF ICMS was in agreement with the ID performed by the conventional method in 95.8% (23/24), of the T. rubrum isolates. Isolate MUM 12.01 was identified by conventional methods as Trichophyton spp., and as T. rubrum by MALDI-TOF ICMS. An agreement of 100% was found between conventional and MALDI-TOF ICMS results for T. interdigitale (2/2). Comparison of spectral analysis with molecular “gold standard” sequence of ITS region was performed, in ten T. rubrum strains, to confirm MALDI-TOF ICMS identification. Results of both methods agreed in 90% (9/10). Isolate MUM 08.11 was identified as Trichophyton megnini by sequence analysis of the ITS of the ribosomal DNA. Urease test was positive for this strain.
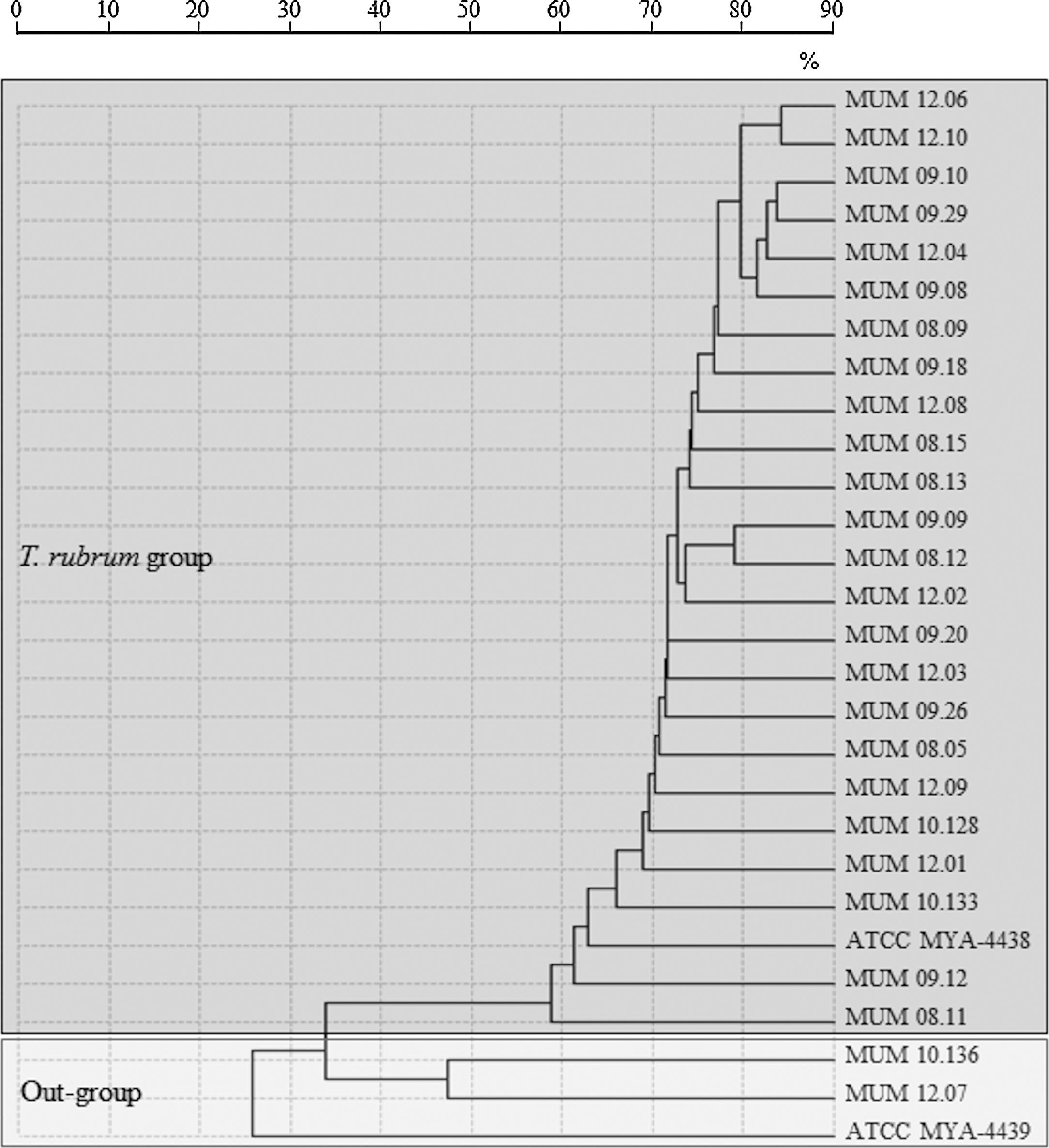
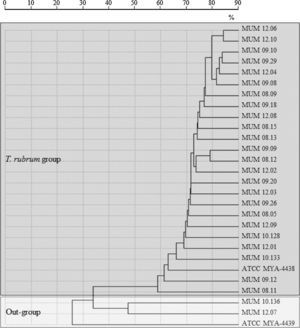
A spectra-based dendrogram obtained by conventional identification algorithm of SARAMIS software had shown an evident separation between out-group cluster T. mentagrophytes ATCC MYA-4439 and T. interdigitale (MUM 10.136 and MUM 12.07) with T. rubrum cluster (Fig. 1). A low percentage of mass similarity (40%) was obtained between T. rubrum cluster and out-group cluster. Within the T. rubrum cluster, strains were distributed into highly related sub-groups, with similarity values above 85%.
Dendrogram resulting from single-linkage cluster analysis mass spectra of T. rubrum strains and out-group strains T. interdigitale (MUM 10.136 and MUM 12.07) and reference strain T. mentagrophytes (ATCC MYA-4439) obtained by MALDI-TOF ICMS analysis. Distances were measured as percentage of mass similarity.
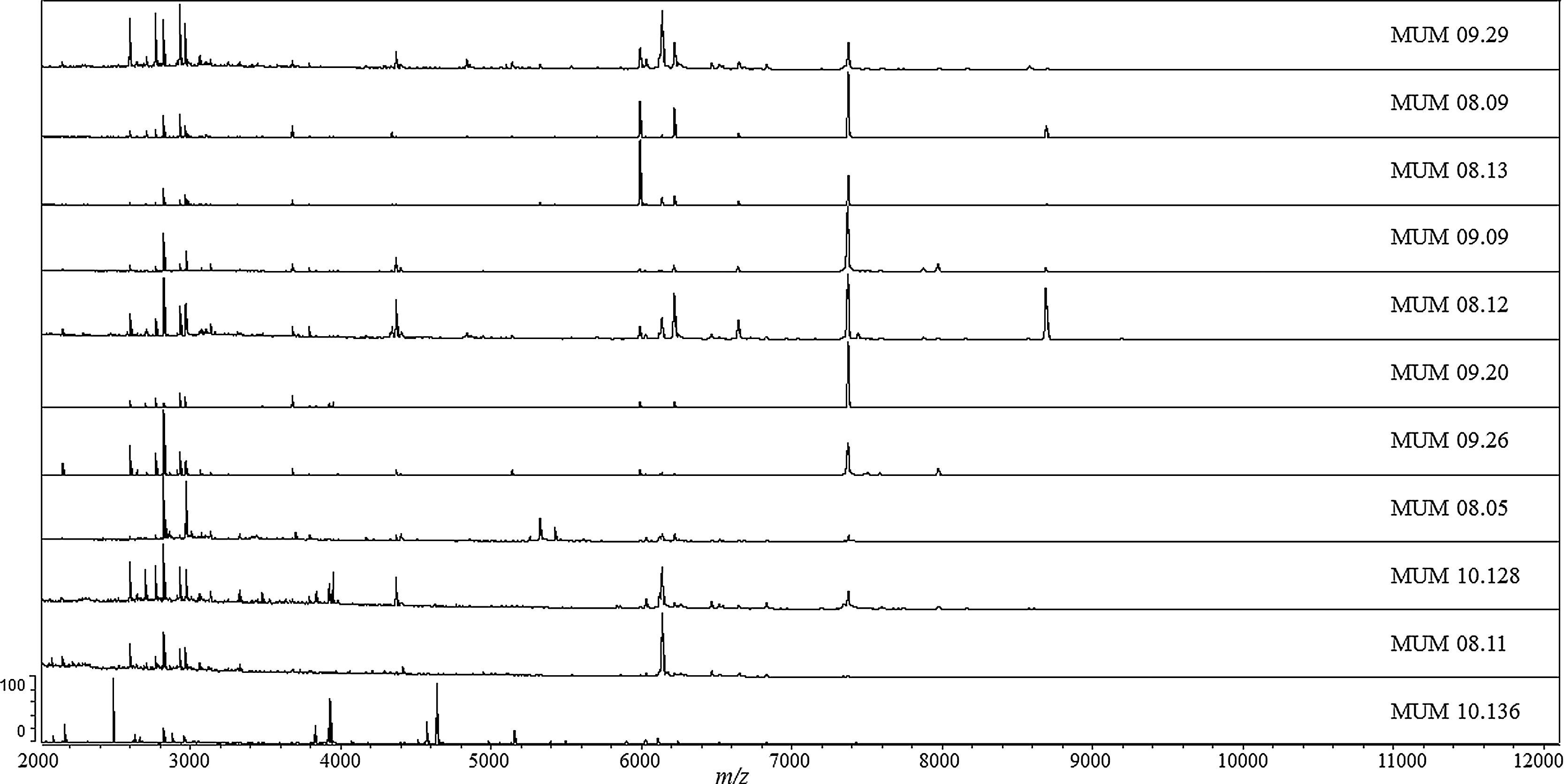
In order to understand the intra-specific differences inside T. rubrum cluster, the mass spectra profiles (MSP) were analyzed and compared. To perform this analysis, ten profiles of T. rubrum strains (MUM 09.29, MUM 08.09, MUM 08.13, MUM 09.09, MUM 08.12, MUM 09.20, MUM 09.26, MUM 08.05, MUM 10.128 and MUM 08.11) were selected randomly from different localizations of the dendrogram. Strain T. interdigitale MUM 10.136 was selected from out-group cluster. The spectra obtained for each selected strain were graphically reported highlighting specific and intra-specific variability (Fig. 2). Very similar mass patterns were generated, although slight variations were observed. The most relevant peaks were between 2000 and 11000 m/z, especially in the region encompassing 2500 to 9000 m/z, where the highest peaks were observed. A single peak with m/z 7380 stood out, due to its range of intensity in profile spectra along the strains. This peak was absent in out-group strain MUM 10.136 and was nearly present in T. rubrum strain MUM 08.11. Moreover, the percentage of identification of this strain reached 90% in only one spectrum.
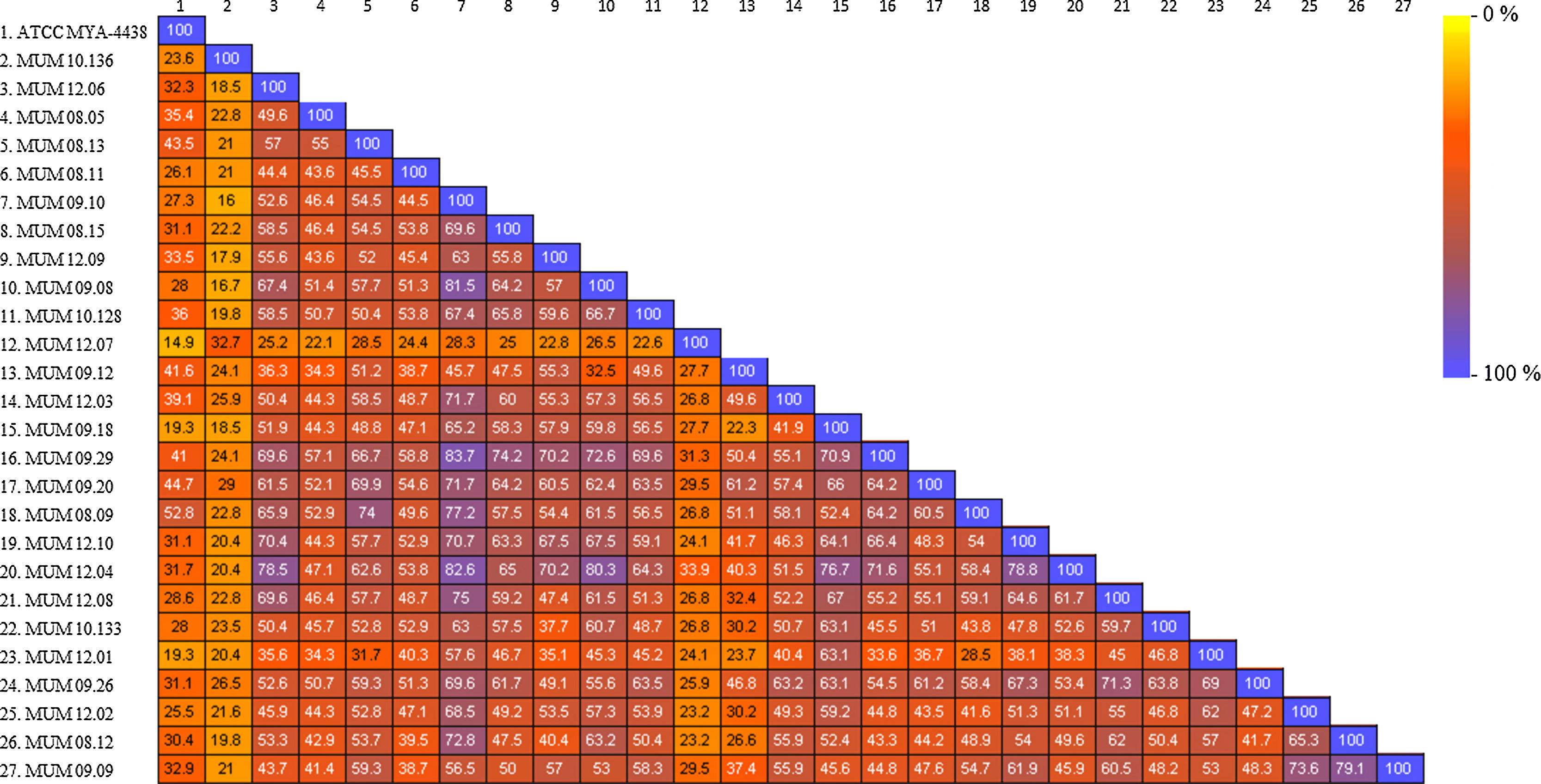
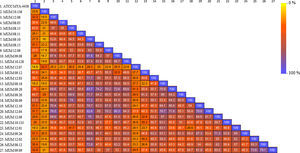
All the spectra datasets were correlated in a similarity matrix, obtained from SARAMIS™ software (Fig. 3). The matrix displayed a relation between all the strains based on the percentage of similarity by a range colour scale from 0% (yellow) to 100% (blue). The out-group T. interdigitale (MUM 10.136 and MUM 12.07) obtained a maximum percentage of similarity of 32.7% and 33.9%, respectively, among all the strains analyzed. In T. rubrum cluster, it was possible to observe intra-specific variability between isolates. Comparing with the other isolates, the strain ATCC MYA-4438 presented a global low range of mass similarity percentage of 42.2%. Other strains such as MUM 08.05, MUM 08.11, and MUM 12.01 had also shown low mass similarity percentage. The remaining strains revealed an average mass similarity percentage ranging from 40 to 60% and a global average of 52.52%, excluding out-group strains.
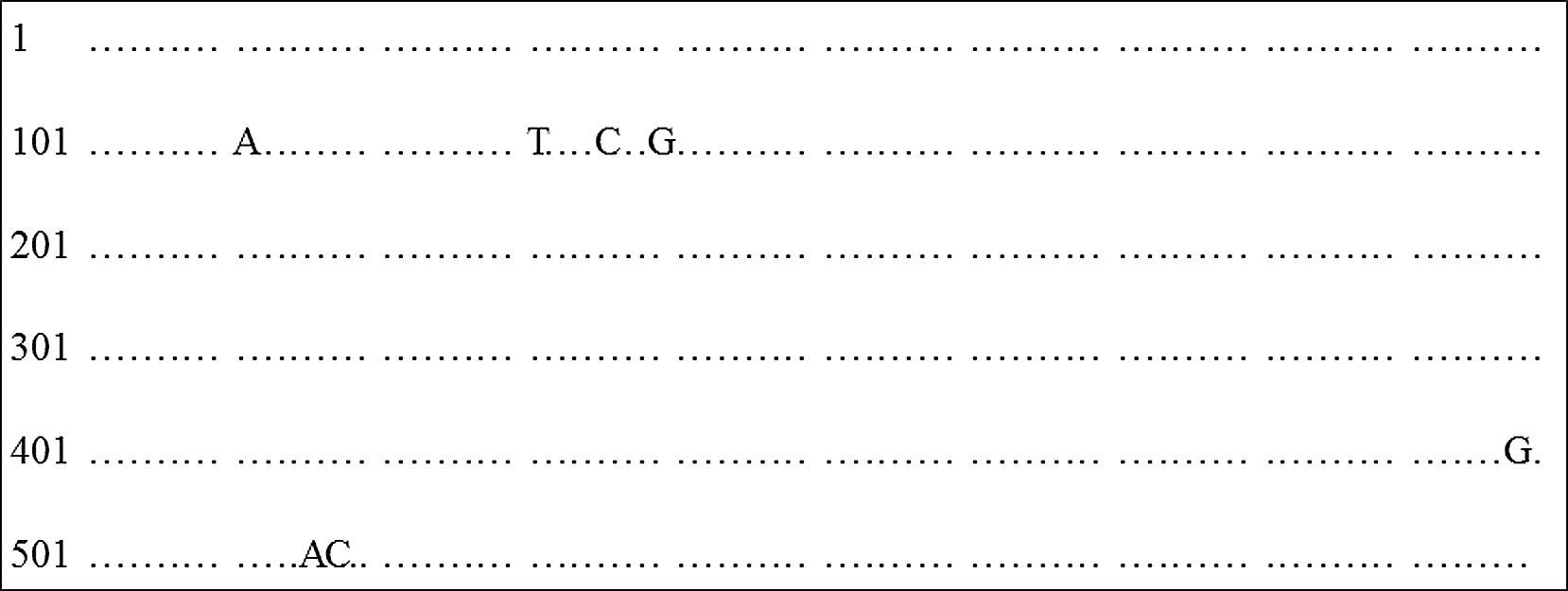
ITS sequence analysis: Analysis of sequence data in GenBank database (http://www.ncbi.nlm.nih.gov/BLAST/) confirmed T. rubrum identification for the ten strains that profile spectra was analyzed and compared. The DNA sequence of ITS region was aligned and compared with T. rubrum reference strain ATCC MYA-4438. We found 100% nucleotide identity between clinical strains and reference strain, except for MUM 08.11 (99%), which presented 7 nucleotide differences (Fig. 4).
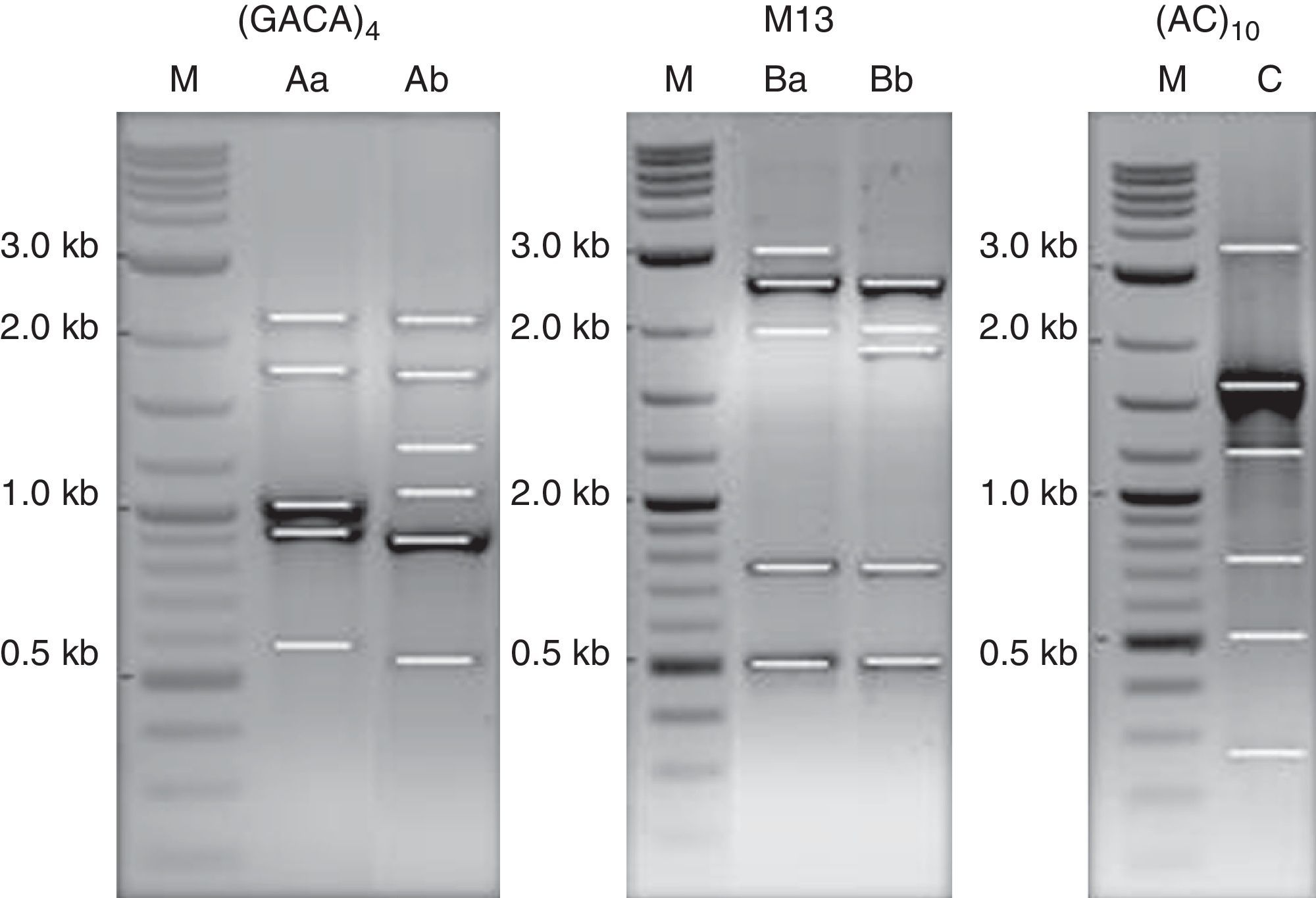
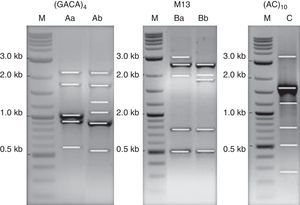
DNA strain typing: PCR amplification of single primer M13, and repetitive DNA sequences (GACA)4 and (AC)10 were performed in order to clarify if the results obtained by MALDI-TOF ICMS spectral analysis had a correspondence to molecular typing. Two profiles of DNA fragments were obtained for the primers M13 and (GACA)4. A single partner was obtained using the primer (AC)10 (Fig. 5). The (GACA)4 profiles varied between 0.6 and 2.3kb; the band-pattern of PCR using M13 varied between 0.5 and 3.0kb, whereas the banding pattern of the (AC)10 was from 0.3 to 3.5kb. Profiles of DNA fragments Ab and Ba were found for isolate MUM 08.11 while patterns Aa and Bb were observed for the other nine strains. A unique profile of 6 DNA fragments (lane C) was obtained for single repeat primer (AC)10 amplicons for all isolates.
PCR pattern for primers M13, (GACA)4, and (AC)10. Lane M, 0.1–10kb ladder; lanes Aa and Bb as DNA profiles for reference strain, MUM 09.09, MUM 08.05, MUM 08.12, MUM 08.13, MUM 08.15, MUM 10.128, MUM 09.20, MUM 09.26 and MUM 09.29. Lanes Ab and Ba as DNA profile for isolates MUM 08.11. Lane C, DNA profile for all isolate.
Fungi species can be easily identified using MALDI-TOF ICMS by detecting a narrow number of specific biomarker peaks. Erhard et al.15 stated that a minimum of 17 and 25 peaks matching the superspectrum of each species was enough to discriminate T. interdigitale and T. rubrum, respectively, with a confidence level of 99.9%. Different sample preparation methods were used to obtain reproducible and informative fungal spectra. Several methods have been reported to improve the protein extraction, and consequently the spectra obtained from the samples.28–30 In the present study, some of these sample preparation methods were evaluated on a small scale (data not shown), but no clear improvement in spectrum quality was observed.
Analysis of mass spectra-based dendrogram obtained by MALDI-TOF ICMS revealed an intra-specific variability within T. rubrum cluster, where clinical strains MUM 09.12, MUM 08.11, MUM 10.133 and reference strain ATCC MYA-4438 were more distant from the remaining strains. Nevertheless, high percentage of similarity (60%) was observed revealing a very high phylogenetic proximity. Molecular strain typing profiles confirmed the lack of genetic variability and DNA polymorphism of T. rubrum although the distribution of strains on clusters was different from MALDI-TOF ICMS, showing less discriminative power than spectral analysis. Among the ten selected strains for this study, MUM 08.11 had shown a different fingerprinting pattern using primers M13 and (GACA)4. Mass spectral data suggest the existence of intra-specific variability between different strains of T. rubrum. These results agreed with clonal mode of reproduction of T. rubrum stated by other authors.6,10,31,32 In fact, it is referred that recent adaptation to a highly specialized ecological niche, the skin of the human host, combined with exclusively asexual reproduction of T. rubrum could explain genetic uniformity within the species.
Spectra profiles of ten T. rubrum strains were obtained showing a similar pattern of mass peaks, and at least one peak with m/z 7380 was consistently present in all T. rubrum being absent in T. interdigitale. Strain MUM 08.11 lack mass signal at m/z 7380, and was identified with a percentage of identity of 80.2%. The result suggested that the presence/absence of only one mass signal is critical for the accurate identification of the strain. Interestingly, this peak has been previously identified as a marker of T. rubrum species.15
The mutual relation of 27 strains analyzed by SARAMIS™ software was represented by the colour similarity matrix. Values corresponded to the percentage of similar peaks between strains. A maximum value of 100% was found as the highest intrinsic mass relation (each strain with itself) and 19.3% was the lowest intrinsic mass relation. It suggested that a maximum of 20 peaks was enough to identify T. rubrum specie, and at least a part of the remaining peaks might be associated with intra-specific variability. A global average of similarity of 52.52% suggested that about 50% of the peaks was associated with strain variability.
Molecular identification using sequence data from the ITS region is shown to be accurate but unable to clearly discriminate species that exhibited slight genetic variation such as T. rubrum.10 It is worthy to refer that molecular biology-based methods such as sequencing and typing are time-consuming and expensive.
MALDI-TOF ICMS has herein proved to be sensitive and accurate for the discrimination between species of dermatophytes.15–19 Thus, it can be regarded as an extra step to the polyphasic scheme of identification33 being an objective and fast analytical methodology. Furthermore, relating to labour and consumables, it is cost-effective when compared to other biological techniques.
This study provided new insights into mass spectra analysis and the potentialities of strain typing for MALDI-TOF ICMS. Analysis of the mass spectral profiles and comparison with molecular typing profiles provide new insights in the proteomic approach, namely the determination of phenotypic similarity and variability of strains for T. rubrum. Moreover, results suggested that MALDI-TOF ICMS is an alternative discriminating tool for intraspecific variability determination of T. rubrum at the strain level than strain DNA typing. MALDI-TOF ICMS allows a fast, reliable and low-cost identification over molecular biology approach.
FundingL. Pereira was granted by the EMbaRC project. The research leading to these results has received partial funding from the European Community's Seventh Framework Programme (FP7, 2007–2013), Research Infrastructures action, under the grant agreement No. FP7-228310 (EMbaRC project).
Conflicts of interestThe authors report no conflicts of interest.