Factors associated with a poorer prognosis of COVID-19 are advanced age, male sex, comorbidities, and laboratory parameters such as lymphopenia and elevated inflammatory markers. Bacterial coinfections have also been associated with a worse prognosis.1
Coinfection with Legionella pneumophila has been scarcely reported,2–5 and to the best of our knowledge no cases have been described in Spain. We present a case of community-acquired coinfection by L. pneumophila and SARS-CoV-2 admitted to our hospital.
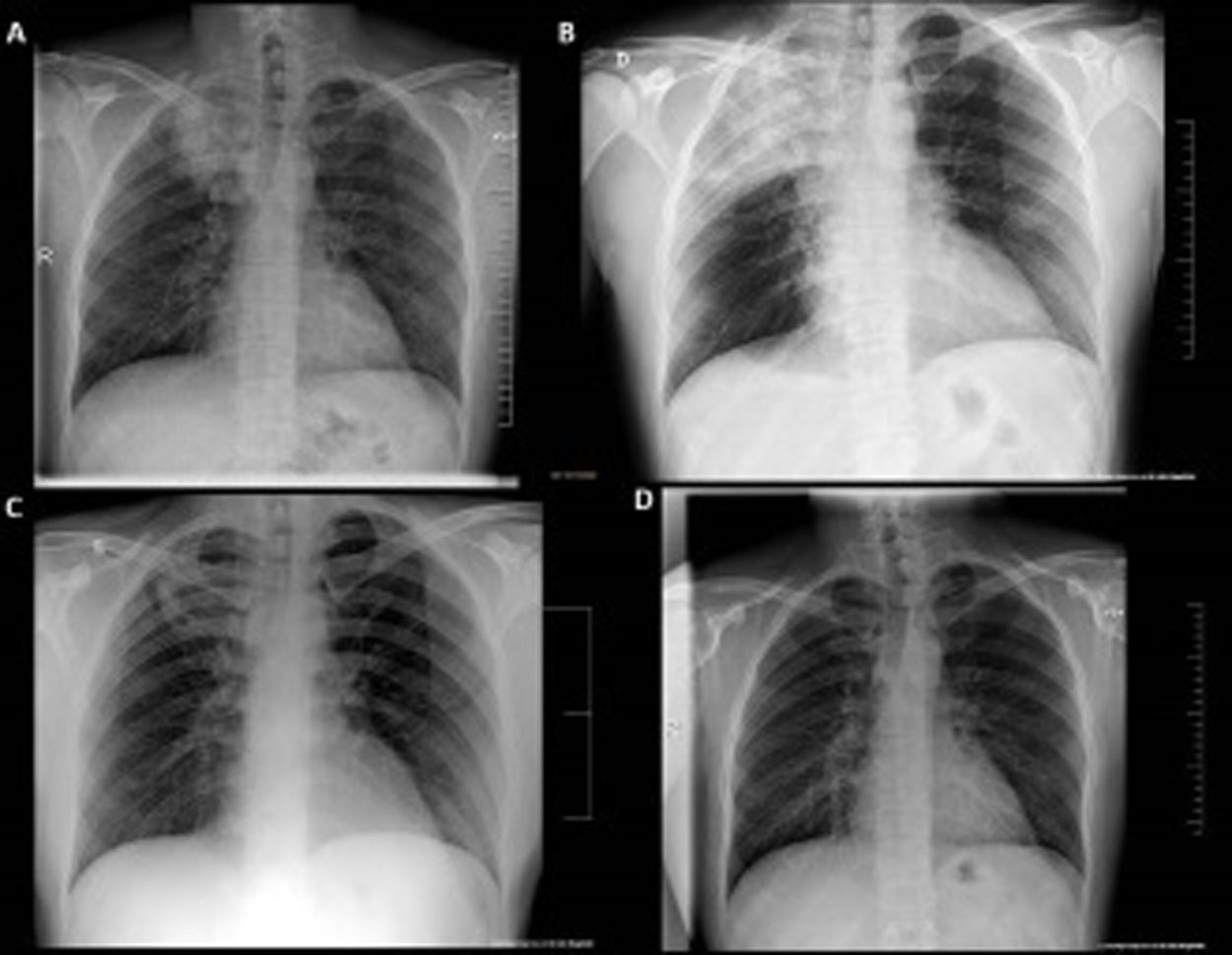
This is a 35-year-old male patient, non-smoker and with no medical history of interest, who works as a butane cylinder deliverer. He arrived to the emergency room on November 15, 2020 with a 10-day history of fever, myalgia, nausea, diarrhea, anosmia and dysgeusia. A dry cough without dyspnea appeared later. A PCR for SARS-CoV-2 performed in his primary care center two days before admission was positive. Vital signs on admission were as follows: blood pressure 130/93mmHg, heart rate116bpm, axillary temperature 39°C, respiratory rate 26 breaths per minute. Respiratory and cardiac auscultations were normal. The chest X-ray showed a condensation in the right upper lobe (RUL) with air bronchogram (Fig. 1A). Blood tests showed the following results: CRP 306mg/L, ferritin 1303ng/mL, procalcitonin 2.81μg/L, sodium 128mmol/L, alanine aminotransferase 26.4U/L, LDH 262U/L, leukocytes 10,510×109/L (85.9% neutrophils), lymphocytes 890×109/L, ferritin 2353ng/ml, and D-dimer 638ng/mL. The baseline arterial blood gas showed: pH 7.56, pCO2 21.0mmHg, pO2 69.0mmHg, and bicarbonate 18.8mmol/L. The electrocardiogram was normal.
The patient was admitted with a diagnosis of community-acquired bacterial pneumonia and SARS-CoV-2 coinfection. Treatment was started with low-flow oxygen therapy and empirical antibiotic therapy with ceftriaxone and azithromycin. Culture of sputum, pneumococcal urinary antigen test and HIV serology were negative, and the urinary antigen test for Legionella (STANDARD F Legionella Ag FIA, SD BIOSENSOR, Republic of Korea) was positive. At this point, ceftriaxone was withdrawn.
The patient was still febrile and presented minimal effort cough 48h after admission. The chest X-ray showed an increase in RUL condensation and bilateral peripheral patchy infiltrates, which was interpreted as progression to inflammatory pneumonia due to SARS-CoV-2 (Fig. 1B). Treatment with dexamethasone (6mg/24h) was added. At this point the fever subsided, the tachypnea decreased and low oxygen flow achieved good saturations. The inflammatory parameters progressively decreased and the RUL condensation improved, while the bilateral peripheral infiltrates persisted (Fig. 1C). The patient was discharged on the ninth day after admission, continuing with oral azithromycin until completing 2 weeks of treatment. Follow up was satisfactory, with resolution of the pulmonary infiltrates (Fig. 1D).
Bacterial coinfection in patients with COVID-19 has been less frequently reported than in other viral infections, such as influenza.1,6,7 Coinfection of SARS-CoV-2 with L. pneumophila has been reported in 4 cases, two diagnosed by urinary antigen test and two by culture of respiratory secretions.2–5 Another 7 cases8,9 of possible coinfection with L. pneumophila have been reported, but this diagnosis was based solely on positive IgM, which does not constitute a diagnostic criterion for legionellosis. Three studies that investigated coinfection in more than 1900 patients admitted for COVID-19 did not identify any case of coinfection with L. pneumophila.6,7,10 Our patient was diagnosed of Legionnaires’ disease by a qualitative analysis in a urine sample, using an immunochromatographic assay with a specificity>95% in detecting the disease caused by L. pneumophila serogroup 1.11
Respiratory and digestive symptoms can coexist in patients with COVID-19, mimicking other diseases such as legionellosis. In SARS-CoV-2 pneumonia, the most common radiological findings are peripheral bilateral infiltrates, while consolidation occurs more rarely. In general, unilobar consolidation suggests bacterial infection and can be found in about 70% of cases of legionellosis. Although pneumonia due to L. pneumophila can progress to bilateral infiltrates, in this case the confirmation of SARS-CoV-2 infection in the previous days and the peripheral distribution of the infiltrates, without signs of airspace consolidation, were highly suggestive of the development of an inflammatory pneumonia due to SARS-CoV-2.
The diagnosis of coinfection in these patients is hampered by the lack of specific symptoms. Consequently, coinfection might be underdiagnosed, and broad-spectrum antibiotics overprescribed.10
In Catalonia the reported incidence of Legionnaires’ disease is higher than in other Spanish autonomous communities. Specifically, in our Vallès Occidental area, the incidence rate in 2019 was 9.94 cases/100,000 persons (95%CI 8.11–12.19). In addition, in the two weeks prior to the patient's admission, two other cases of legionellosis were diagnosed in the city where the patient resided, although no common source was identified for the three cases (data obtained from the Health Department, Government of Catalonia). On the other hand, it must be taken into account that, due to his work, the patient entered many homes and commercial premises. These data, together with the season of the year and the clinical picture of a male with a lobar condensation, hyponatremia, diarrhea, and a negative determination of pneumococcal antigen in urine, made it necessary to consider the diagnosis of Legionnaires’ disease. Once the diagnosis was established, treatment with azithromycin was continued, since there is no difference in the observed outcomes for patients with Legionella spp. pneumonia treated with azithromycin vs. levofloxacin.12
In conclusion, this case demonstrates the importance of making an etiological diagnosis of bacterial coinfection in patients with COVID-19 when there are suggestive clinical and/or radiological findings. We consider that in areas with a relatively high incidence of legionellosis, the urinary antigen test for Legionella should be performed in cases of community-acquired pneumonia with epidemiological suspicion, or with criteria for hospital admission and a negative pneumococcal urinary antigen test. With a specific diagnosis the antibiotic treatment can be targeted, thus reducing the threat of bacterial resistance.
FundingThis research did not receive funding from the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors declare no conflict of interests.