Introduction
In 1985, Cooper et al published the first description of symptoms attributable to an episode of primary human immunodeficiency virus infection (PHI) in 12 homosexual males who presented a clinical picture similar to "infectious mononucleosis" (fever, pharyngitis and exanthema), but with negative Epstein-Barr serology. Seroconversion to HIV was confirmed in these patients and the clinical picture was named "acute retroviral syndrome" 1. According to the World Health Organization, 14,000 new cases of HIV infection occur every day over the world. Most of these cases occur in Africa and Asia, but they are still quite frequent in Europe. Nevertheless, this entity is often underdiagnosed, since clinical symptoms at time of the seroconversion 2-4 are wrongly attributed to non-specific viral infections in most cases.
Early diagnosis of PHI is important both for the patient and to control the epidemic. Notifying the patient can help reduce the risk of transmitting the infection and highly active antiretroviral therapy (HAART) can be considered. In terms of public health, identification of these cases enables us to study contacts, evaluate the efficacy of preventive interventions, ascertain the growth pattern of the epidemic and determine the transmission of resistant viruses 5,6.
This article describes the epidemiological, clinical, immunological and virological characteristics, and the response to HAART of a 75-patient cohort with PHI diagnosed consecutively at a tertiary teaching hospital in the Barcelona area over a seven-year period (1997 to 2003).
Methods
In 1997, the Infectious Disease Department of the Hospital Clínic in Barcelona implemented a program for the diagnosis and follow-up of patients with PHI. The cohort included all patients presenting one of the following criteria during the first evaluation:
1. Negative or undetermined HIV-1 serology (negative enzyme immunoassay [EIA], or positive EIA with a negative or undetermined line immunoassay [LIA]) associated with the detection of virus in blood (HIV-RNA or p24 antigen).
2. Evidence of seroconversion during the last 6 months by EIA and LIA.
3. Syndrome suggestive of PHI during the last three months with a negative HIV-1 serology during the last 12 months.
In all cases, PHI-related epidemiological information, the presence and type of symptoms, and laboratory tests carried out during the first visit and follow-up visits were recorded using a standard computerized system. All patients gave their informed consent for the use of data according to the study protocol approved by the IRB of the Hospital Clínic, Barcelona.
At the first visit, the following were recorded: epidemiological history and clinical data, physical examination, routine blood and biochemistry analysis, HIV-1 RNA viral load in plasma (VL), genotypic resistance test, T-cell sub-populations, baseline serology studies (conventional serological test for hepatitis B [HBV], hepatitis C [HCV], cytomegalovirus [CMV] and Toxoplasma gondii and reagin test for syphilis [VDRL]); tuberculin skin test (PPD) and chest X-ray. The indication and type of HAART used were based on current recommendations, on the available drugs and on the availability of clinical research protocols for the treatment of PHI. Clinical and laboratory follow-up were carried out every three months. Lipodystrophy was considered as the loss or accumulation of body fat as observed by the patient and confirmed by the physician. Dyslipidemia was defined as two consecutive determinations of cholesterol or triglycerides above normal values.
Virological studies
HIV-1 serology was determined by microparticle EIA (MEIA) using the AxSYM system (Abbott Laboratories, North Chicago, IL) and confirmed by LIA (Inno-LIA HIV I/II Score. Innogenetics. Ghent, Belgium). Quantification of VL was by Cobas Amplicor Monitor (Roche Molecular Systems, Branchburg, NJ) with a sensitivity limit of 200 copies/mL. Genotyping of resistance mutations was done in genes coding for reverse transcriptase (RT) and protease enzymes using the ViroSeq HIV Genotyping System v.2 (Abbott Laboratories, North Chicago, IL) and the results were reported according to the consensus document of the International AIDS Society Resistance Testing-USA Panel, 2005 7. Viral subtype was determined using the FASTA sequence from the Stanford database (http://hivdb.stanford.edu).
Immunological parameters
T cell subsets (CD4+, CD8+) were determined by cytofluorometry (FACScalibur, Becton Dickinson).
Statistical analysis
The study closed on 30 July 2004. The date of the infection was set at the day of exposure to HIV (unprotected relations, or syringe exchange with a source of positive or unknown serology). In the case of patients who presented several potential exposures, the date of infection was set at the closest exposure to the 14 days before the onset of symptoms. In the case of asymptomatic patients, this was defined as the halfway point between the last negative serology and the first positive serology. Loss to follow-up was defined as the loss of more than two consecutive visits. Progression events were defined as the appearance of a type C clinical diagnosis according to the 1993 revised CDC classification or a CD4 count of < 350 cells/mm 3 on two occasions after 6 months following exposure.
Descriptive statistics were expressed as proportions and percentages for qualitative variables and as medians and interquartile ranges (IQR) for quantitative variables. The T-test and ANOVA were used for comparisons between two and more than two groups, respectively, in the case of normally distributed continuous variables. In the case of non-normally distributed variables, the Mann-Whitney U and the Kruskal-Wallis tests were used for two and more than two groups, respectively. Categorical variables were compared using the Chi-squared test or Fisher exact test if the expected frequency in more than 25% of the squares was lower than 5. Survival functions were calculated with the Kaplan-Meier method and compared using the log-rank test. A two-sided p value < 0.05 was established as the level of statistical significance for all the tests. The statistical analysis was performed with SPSS version 10.
Results
Demographic characteristics
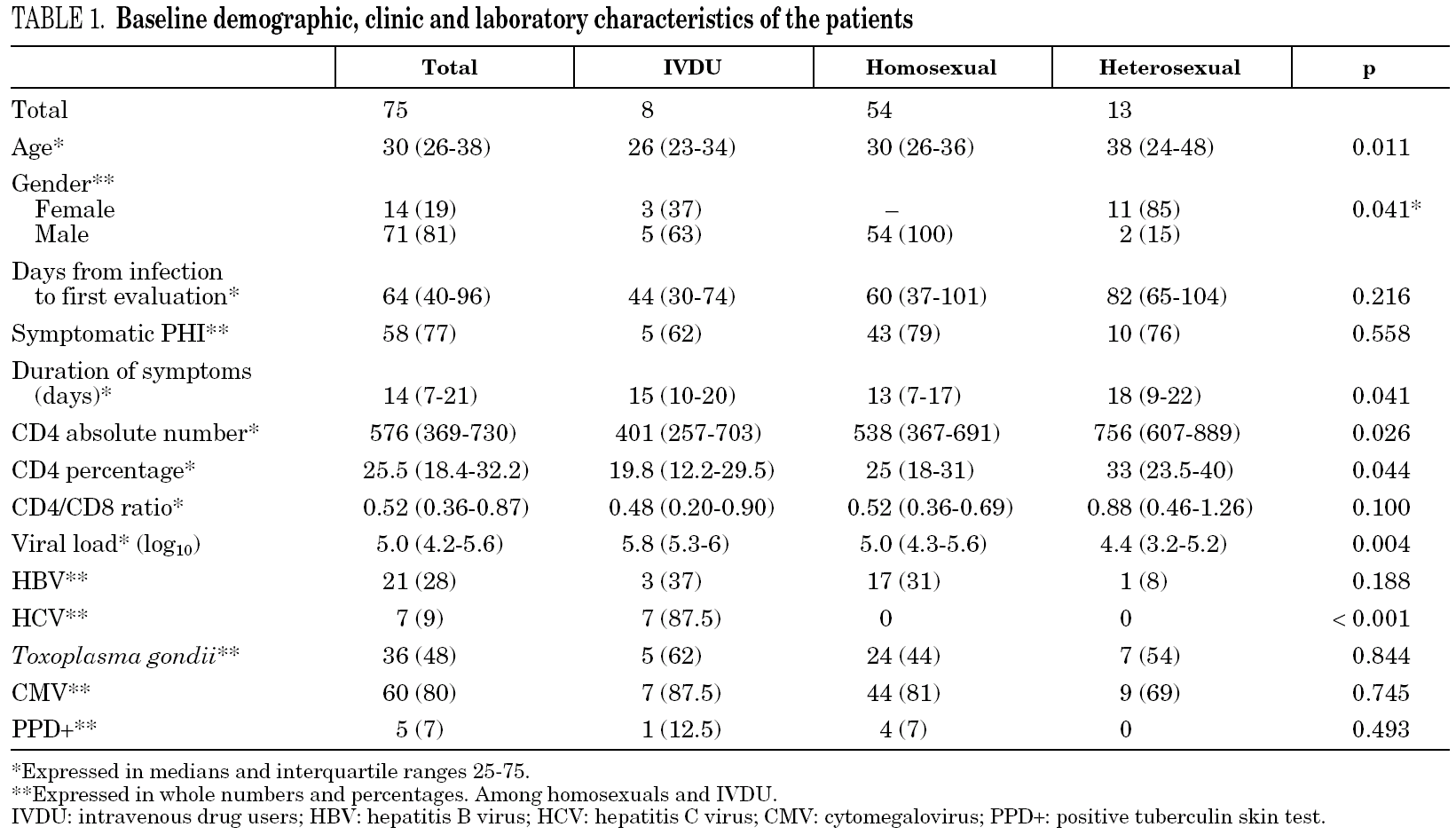
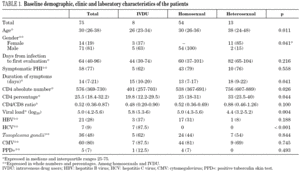
Demographic characteristics are presented in table 1. Between 1997 and 2003, our hospital attended 2,577 new HIV-infected patients, among whom 30% reported homosexual or bisexual (HMS) relationships as the probable route of transmission, 33% reported heterosexual (HTS) relationships and 35% the use of intravenous drugs (IVDU). During the same period, 75 cases of PHI (2.9%) were identified. Sixty-one patients were male (81%) and the median age was 30.5 years (IQR 26-38). The probable routes of transmission were HMS relations in 54 patients (72%), HTS relations in 13 (17%) and IVDU in 8 (11%). Women accounted for 85% of patients who contracted infection by HTS relations. Thirteen patients were immigrants (17%), eight from Europe, four from South America and one from North Africa. At the first evaluation, 39 patients (52%) presented HIV-negative serology with a positive VL, 27 (36%) presented documented seroconversion during the last six months and 9 (12%) presented evidence of seroconversion during the last year. Median time from the date of exposure to HIV until the first clinical visit was 64 days (IQR 40-96): HTS, 82 days; HMS, 60 days and IVDU, 44 days. Median overall follow-up was 37 months (IQR 26-66), which represents a total of 265 patients/year. Only 8 patients (11%) were lost to follow-up.
Baseline immunological, virological and serological parameters
Laboratory parameters are shown in table 1. The first evaluation was carried out a median of 64 days (IQR 40-96) after exposure to HIV-1. Median HIV-1 RNA VL was 5.0 (IQR 4.2-5.6) log10 copies/mL and the absolute and percentage CD4 lymphocyte counts were 576 (IQR 369-730) cells/mm 3 and 25.5% (IQR 18%-32%), respectively, with a CD4/CD8 ratio of 0.52 (IQR 0.36-0.87). Viral subtype and presence of mutations associated with drug resistance were determined in 61 patients (81%). All were subtype B and eight patients (13%) presented mutations associated with resistance to an antiretroviral drug. When VL and CD4 lymphocyte count were compared according to route of transmission, HTS patients presented lower HIV-1 RNA VL values (p = 0.007) and higher CD4 lymphocyte counts (p = 0.026). However, when these parameters were analyzed according to time to the first evaluation, the differences lost statistical value (p = 0.09). The percentage of patients who presented serological evidence of previous infection by Toxoplasma gondii, CMV, HBV, and HCV was 48%, 80%, 28% and 9%, respectively. The tuberculin skin test (PPD) was positive in 7% of cases. One patient had chronic hepatitis B (HbsAg-positive for more than 6 months) and another had positive serology for syphilis of indeterminate duration. The seven HCV-infected patients were IVDU.
Clinical manifestations
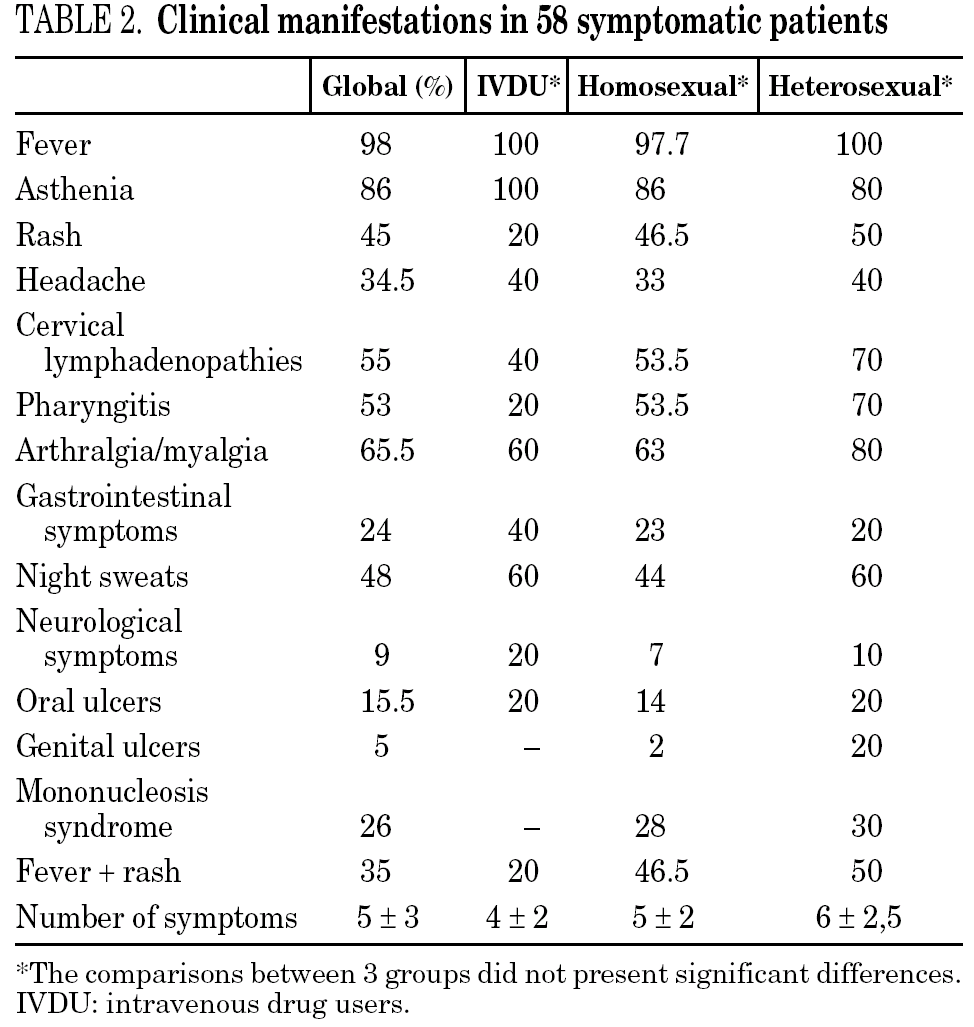
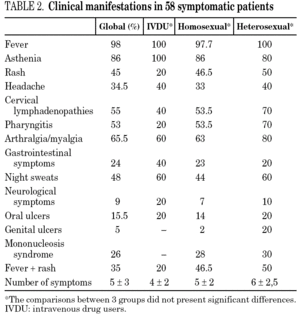
The clinical manifestations of the patients are shown in table 2. PHI was symptomatic in 58 cases (77%). Globally, the most frequent symptoms were fever (76%), asthenia (67%), arthralgia/myalgia (51%), lymphadenopathy (43%), and pharyngitis (41%), with no significant differences between the groups. Only 15 patients (20%) had symptoms consistent with infectious mononucleosis (fever, pharyngitis, and laterocervical adenopathy for more than seven days). A combination of fever and rash was present in 35% of cases. No patients presented C events during HIV-1 seroconversion, although two patients had B events (Candida angular chelosis and oral hairy leukoplakia).
Hospital admission
During the acute episode, 17 (23%) patients were hospitalized. In 11 cases, the reason for admission was fever of unknown origin. In five patients, admission was for neurological symptoms (three episodes of viral meningitis, one accompanied by facial paralysis, one episode of severe paresis of the lower limbs and one patient with convulsions). Another patient was admitted for a study of polyarthritis, which was considered reactive to HIV-1.
Associated infections
An acute infection was present in 13 patients at the same time as PHI. A total of nine patients, all HMS, presented a sexually transmitted disease. One of these patients presented acute HBV hepatitis, seven presented syphilis (one case of primary syphilis and the rest secondary), and another patient had both infections simultaneously.
In four IVDU, PHI was diagnosed during the course of a severe bacterial infection (two cases of tricuspid endocarditis due to Staphylococcus aureus, one case of severe cellulitis in the lower limbs and one episode of pneumococcal pneumonia). In these patients, PHI was suspected due to the persistence of fever despite correct antibiotic therapy.
Highly active antiretroviral therapy
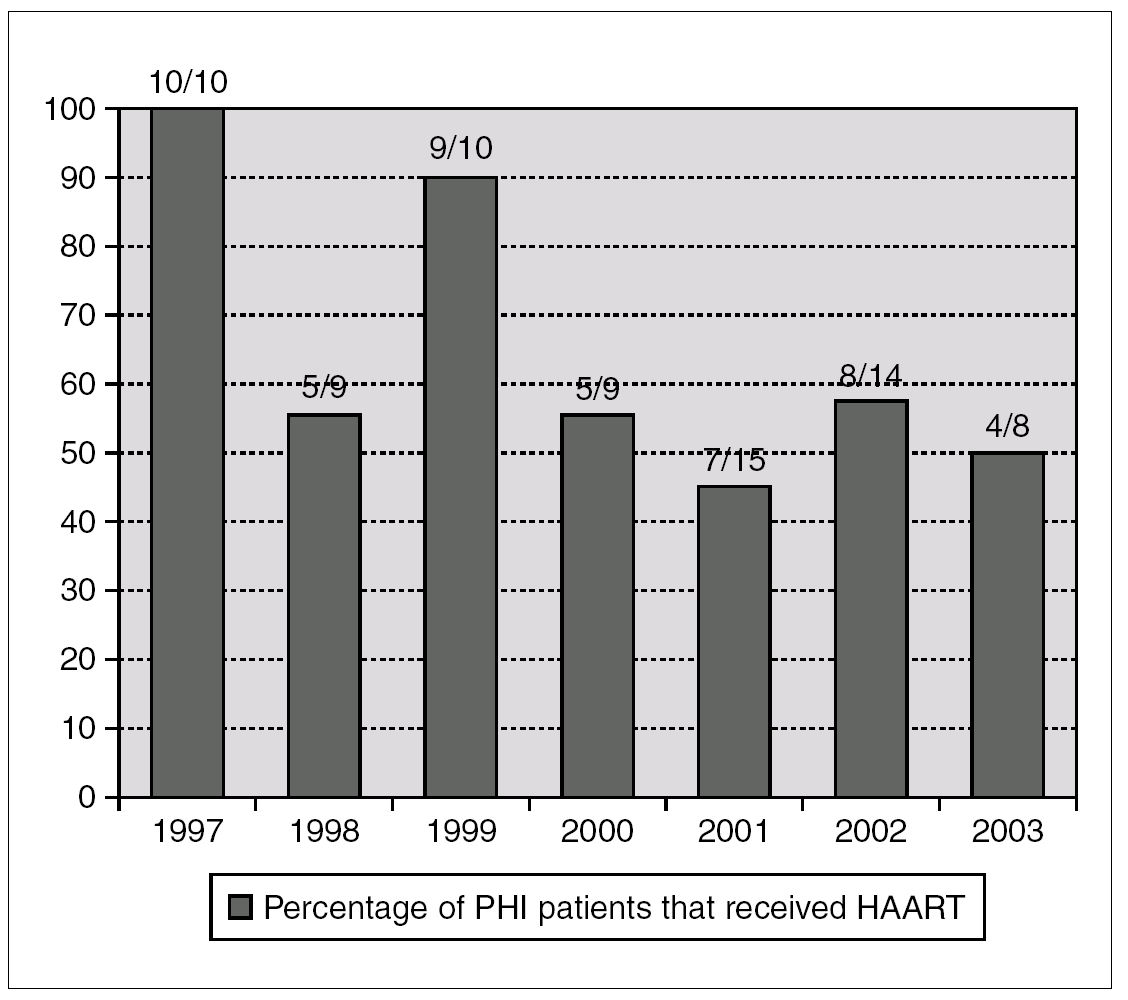
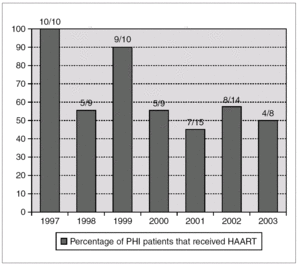
A total of 48 patients (65%) started HAART during the first 180 days after infection, with a median of 69 days (IQR 45-102). The number of patients who received HAART fell over time: 79% in the group of patients evaluated during 1997-2000 and 49% during 2001-2003 (p = 0.01) (fig. 1).
Figure 1. Annual percentage and number of cases of patients with acute HIV infection receiving HAART. Number of treated/diagnoses cases are on the top of boxes.
Outcome of patients receiving HAART
Ninety percent of the patients treated (n = 43) received a combination of two nucleoside analog reverse transcriptase inhibitors (NRTI) and a protease inhibitor (PI), boosted with ritonavir in a third of the cases. The five remaining patients (10%) started therapy with two NRTI and a non-nucleoside analog reverse transcriptase inhibitor (NNRTI). At 12 months of follow-up, 98% had a non-detectable VL (NDVL) and an increase (median, IQR) in CD4 lymphocyte count of 324 (117-537) cells/mm 3.
In half the patients the first HAART regimen was changed due to toxicity (17 cases), simplification (4 cases), boosting with low-dose ritonavir to simplify posology (2 cases), and failure to suppress viral load (1 case). The median duration of the first regimen was 64 weeks (IQR 18-127). The most common toxicity was indinavir-induced renal lithiasis (7 cases), peripheral polyneuritis (3 cases), allergic reactions (2 cases), and gastrointestinal disorders (2 cases). A total of 58% of patients had dyslipidemia and 37.5% had lipodystrophy.
In total, 21 patients stopped HAART after a median of 37 weeks' treatment (IQR 21-46), 14 in the framework of structured treatment interruptions and seven for personal reasons. During follow-up, only six patients presented CD4 lymphocyte counts below 350 cells/mm 3, although none developed opportunistic infections. One patient with PHI-associated myelitis who required corticosteroid therapy died three months after being infected due to disseminated aspergillosis. She received HAART the last six weeks.
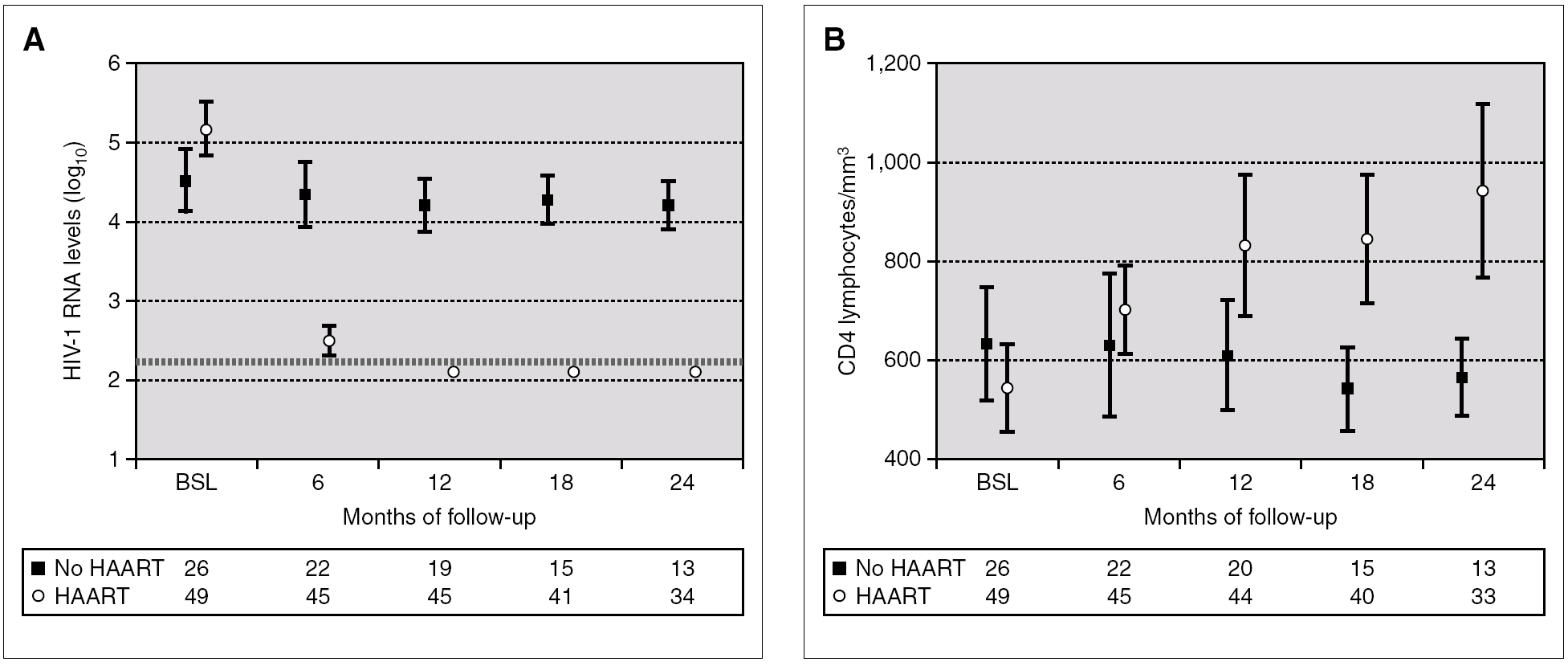
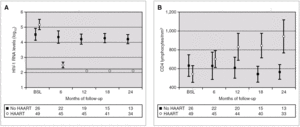
Figure 2 compares HIV-1 RNA VL and CD4 lymphocyte values during the follow-up of these patients with respect to those who did not receive HAART.
Figure 2. HIV-1 RNA levels (A) and CD4 lymphocytes count (B) in patients with and without HAART at baseline, and at 6, 12, 18 and 24 months of follow-up (median and IQR). ¿ , Patients without HAART; ○, patients with HAART; ---, limit of detection 200 copies HIV-1 RNA/mL. HAART: highly active antiretroviral treatment.
Outcome of patients who did not receive HAART
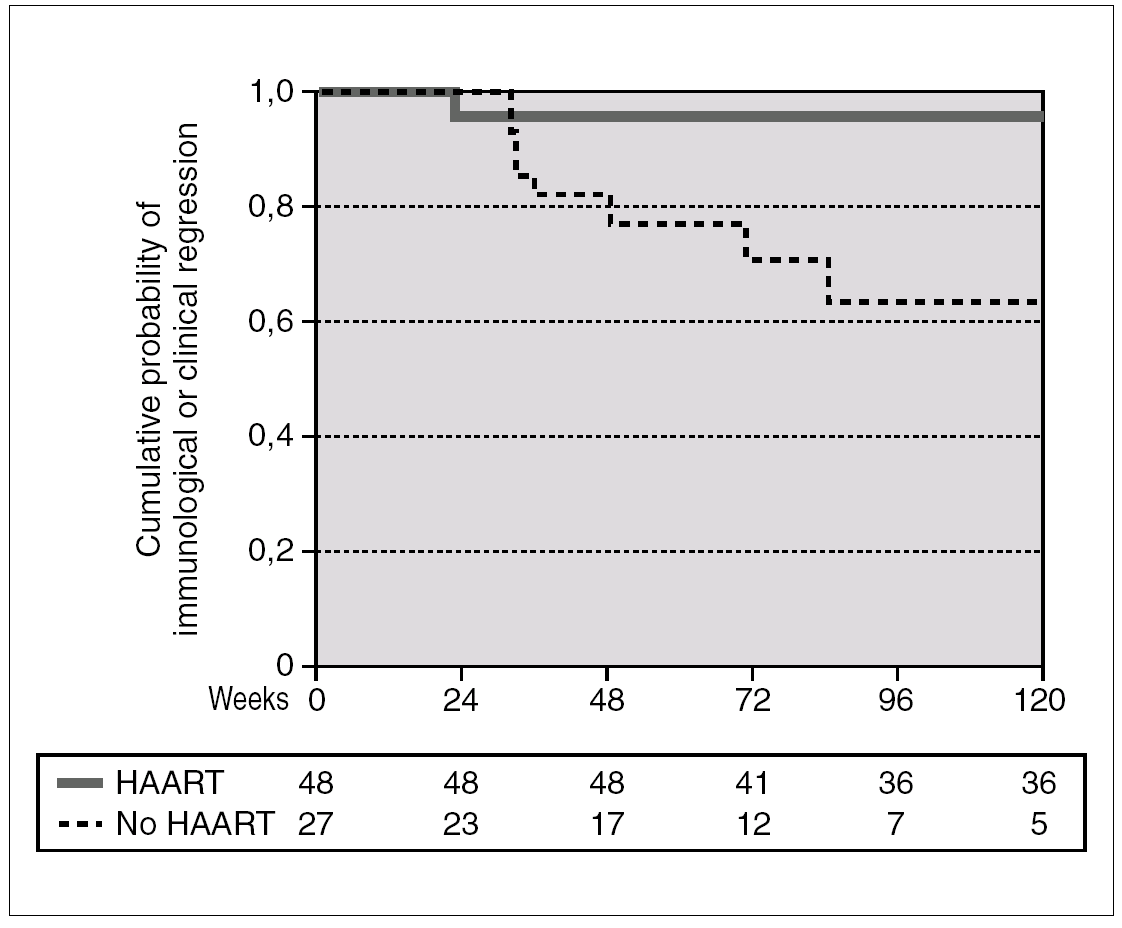
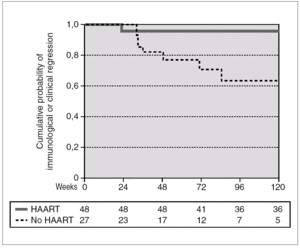
A total of 27 patients did not receive HAART during PHI. In 15 cases, the therapy was offered but the patients preferred not to take it. In the other 12 cases, the physician decided not to offer HAART, as it was impossible to include the patient in therapeutic research protocols (11 cases), or due to non-compensated psychiatric illness (one case). In one of these 12 cases, the patient was receiving lamivudine for chronic hepatitis B. In comparison with the patients who received HAART, this group had a greater interval between PHI and the first evaluation, a median of 91 days (IQR 64-131) vs. 46 (IQR 33-78), p < 0.001; a lower frequency of symptomatic seroconversion (54% vs. 92%, p < 0.014); and a lower median VL (4.6 [IQR 3.7-5.3] vs. 5.1 [IQR 4.5-5.9] log10 copies/mL; p < 0.001). However, this group presented faster immunological progression to CD4 counts below 350 cells/mm 3 (fig. 2). During follow-up, 11 patients had CD4 lymphocyte figures below 350 cells/mm 3. One patient developed Kaposi sarcoma and multidermatomal herpes zoster, and another recurrent oral candidiasis. This group of patients presented a greater likelihood of immune deterioration and/or clinical progression during follow-up than the HAART group (42.3% versus 12.3%; p < 0.00001) (fig. 3).
Figure 3. Accumulated probability of immunological (i.e. CD4 lymphocytes < 350 cells/mm 3) or clinical progression in patients with PHI according to receive or not HAART. The difference was statistically significant (p < 0.001).
Discussion
PHI is defined as the 30- to 45-day period from the date of infection until the appearance of a full humoral anti-HIV response, generally detected by Western-Blot. The period from this point up to 180 days after the infection is known as recent infection and the next phase as chronic infection. The prevalence of PHI in countries with a high rate of infection is almost 1.8% in sexually transmitted disease clinics 8. In the West, it is detected in up to 0.5/1000 serology tests 9 and, when the serology is performed in patients with consistent symptoms, in up to 1% of cases 10. In our series, PHI represented 2.9% of all newly diagnosed cases of HIV infection attended in a Barcelona teaching hospital during the seven years of the study.
Most of the patients in the cohort were homosexual males. This positive selection may have been influenced by characteristics that are specific to this population (greater frequency of serological testing, greater access to information on HIV and, in particular, the symptoms of PHI). Moreover, this group was more frequently exposed to CMV, an indirect marker of sexual activity, and had a high frequency of sexually transmitted diseases (syphilis and acute HBV hepatitis), which may also have favored the consultation. The low number of patients infected after heterosexual relations, despite the fact that this is currently a common route of transmission 11, suggests that delays in the diagnosis of HIV infection are much more notable in this population, since PHI infection is not usually suspected. The group least represented in our series was IVDU. The diagnosis of PHI was made in half the cases during admission to hospital for severe bacterial infections, when the drug user continued with fever despite correct antimicrobial therapy. These data taken as a whole suggest that PHI should be actively investigated in patients with fever and a history of high-risk sexual exposure in the past month, especially if they have a sexually transmitted disease, and in all IVDU with a febrile illness.
As in previous series 2,12, most patients presented symptoms associated with seroconversion. It has been suggested that the progression to clinical or immunological deterioration is faster in patients with symptomatic primary infection, and even worse in those with a long symptomatic phase (more than 15 days) or with a short incubation time 13,14. The presence of symptoms and faster progression may represent epiphenomena of a higher VL since the VL level is the most widely recognized risk factor for HIV progression 15. This relationship has even been described as "dose-dependent", owing to the fact that, for each symptom present, there is an increase in the VL of 0.4 log10 copies/mL 16. The most common symptoms during PHI include fever, rash, oral and/or genital ulcers, lymphadenopathy, marked asthenia, arthralgia/myalgia and aseptic meningitis 17,18. The frequency of presentation of these symptoms in our series was no different from previously published series. The typical mononucleosis syndrome was present in only 20% of patients; one study has recommended that this combination of symptoms should not be used as a PHI case definition criterion due to its low frequency 19. Historically, it has been suggested that the most severe manifestations are the neurological symptoms. There have been reports of patients with aseptic meningitis, meningoencephalitis, myelitis, peripheral neuropathy, Guillain Barré syndrome, facial paralysis, convulsions and psychotic disorders. The presence of neurological symptoms has also been associated with the level of viral load in cerebrospinal fluid and with a greater rate of clinical progression 20,21. A quarter of the patients presented headache in our series, although aseptic meningitis was only diagnosed in three cases. The only patient with severe neurological manifestations (transverse myelitis) died after a few weeks from disseminated aspergillosis.
With regard to the differences in clinical presentation between patient groups with different types of exposure to HIV, we observed a lower frequency of symptomatic PHI in IVDU than in the other groups. Although one study had established a similar frequency of symptoms in all the risk groups 22, the same authors later reported a lower frequency of symptoms in IVDU 23. From the practical viewpoint, it is very difficult to establish whether intravenous transmission leads to a different clinical presentation or whether active consumption of drugs can mask the symptoms. Conversely, heterosexual patients presented a higher number and longer duration of symptoms, and a greater frequency of pharyngitis, laterocervical lymphadenopathy, arthralgia/myalgia and genital ulcers. We cannot establish whether this clinical picture is representative of these patients or, and this seems more likely, whether it involves a bias in which the most highly affected patients consulted a physician. Some authors have reported the simultaneous appearance of opportunistic infections at the time of PHI, such as viral infections (e.g. herpes simplex or zoster virus), esophageal candidiasis 24 and, less frequently, other AIDS-defining events 25-27. We did not observe any C event in our cohort during PHI.
With regard to HAART in PHI, no randomized studies have shown a clinical benefit from starting this therapy during acute or recent HIV infection as compared to treating patients during the chronic phase of infection 28. Nevertheless, some studies assessing the repercussion of HAART on the immune system have shown that HAART may be beneficial during PHI as it preserves the immune system and maintains/restores the specific cell response against HIV 29,30. Other studies have shown a greater rate of viral suppression and immune recovery than when HAART is started during chronic infection 31,32, or the possibility of transient control of HIV-1 RNA VL after stopping HAART 33,34. Finally, some cohort studies have suggested that clinical progression and the development of AIDS is greater in patients who did not received HAART during the acute phase when compared with patients who received HAART 35,36. Furthermore, the disadvantages of HAART (cost, adverse effects due to longer exposure to drugs, possible non-adherence and resistance), the lack of evidence of a clear clinical benefit, and the impossibility of eradicating HIV infection has meant that physicians are currently less likely to prescribe HAART. This fact has been observed in the French PRIMO cohort 37 and in our center since 2001. In our experience, HAART was effective, since almost all patients had an undetectable VL and, after a median follow-up of three years, the possibility of clinical or immunological progression fell in comparison with untreated patients. Nevertheless, the high frequency of adverse effects was a limiting factor. The small number of patients in this series and the descriptive nature of the study prevent definitive conclusions from being established. This question must be answered in the coming years. The availability of new diagnostic methods that will enable us to detect more cases of acute or recent infection and new HAART regimens that are more patient-friendly, more potent and less toxic, would justify multicenter and randomized clinical trials to ascertain whether HAART started during this phase of infection, either alone or in combination with immune-mediated therapies, is beneficial to PHI patients from a clinical viewpoint.
This study was presented in part at the XI Congress of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). Bilbao, 16-19 May 2004. Abstract 255. This study has been funded in part by research grants FIPSE 3128/00 and FIS PI 04/0363. Drs. J.M. Miró and F. García received a research grant from IDIBAPS.
Correspondence: José M. Miró, MD.
Servicio de Enfermedades Infecciosas. Hospital Clínic.
Villarroel, 170. 08036 Barcelona. Spain.
E-mail: jmmiro@ub.edu
Manuscrito recibido el 15-5-205; aceptado el 24-10-2005.