In order to assess the relationship between the concentrations of airborne fungi and particles, particle counting was combined with fungal air sampling in several rooms of a hospital.
MethodsConcentrations of ≥0.5μm particles (P05) and ≥1μm particles (P1) were measured using a particle counter; fungal air sampling was performed with volumetric air samplers, which impacted air on Rodac plates with Sabouraud chloramphenicol agar. Particle counts were categorised according to ISO 14644-1 standard cut-off points; their association with fungal detection was assessed with Fisher's exact test.
ResultsForty-two simultaneous samplings were carried out: 24 in operating rooms, 13 in rooms for burns or haematology patients, 3 in pharmacy clean rooms, and two in other procedure rooms. Filamentous fungi were recovered in 5 samples, which also had higher particle counts. No fungi were detected in 12 samplings with both P05 and P1 concentrations below the maximum for class 6 clean rooms; 4 of 7 samplings with both concentrations within the range for class 8 clean rooms were positive for fungi. The association between fungal detection and higher particle counts was statistically significant, both for P05 (p=.004) and P1 (p=.003). There was a partial overlap between the concentrations of particles of samplings which were positive or negative for fungi.
ConclusionsThere is a relationship between the concentrations of P05 and P1 and airborne fungi in hospital rooms. When both P05 and P1 concentrations are below the maximum for class 6 clean rooms, a negative fungal detection can be predicted.
Para evaluar la relación entre las concentraciones de esporas de hongos y de partículas vehiculados por aire, el recuento de partículas se añadió al estudio microbiológico del aire de varias salas de un hospital.
MétodosLas concentraciones de partículas ≥0,5μm (P05) y ≥1μm (P1) se midieron con contador de partículas; el muestreo para estudio microbiológico se efectuó con aspiradores volumétricos que impactaban aire sobre placas Rodac con agar Sabouraud cloranfenicol. Los recuentos de partículas se categorizaron según puntos de corte de norma ISO 14644-1; su asociación con la detección de hongos se evaluó con la prueba exacta de Fisher.
ResultadosSe realizaron 42 muestreos simultáneos: 24 en quirófanos, 13 en habitaciones para pacientes quemados o hematológicos, 3 en salas blancas de farmacia y 2 en salas para otros procedimientos. Se aislaron hongos filamentosos en 5 muestreos, cuyas concentraciones de partículas fueron superiores. No se detectaron hongos en 12 muestreos con concentraciones de clase 6 de P05 y P1; sí se detectaron en 4 de 7 muestreos con concentraciones de ambas partículas de clase 8. La asociación entre detección de hongos y recuentos elevados de partículas fue estadísticamente significativa para P05 (p=0,004) y P1 (p=0,003). Hubo una superposición parcial de las concentraciones de partículas de los muestreos con y sin detección de hongos.
ConclusionesEn salas hospitalarias hay una asociación entre concentraciones de P05, P1 y hongos en aire. Concentraciones de P05 y P1 inferiores al máximo para salas de clase 6 pueden predecir ausencia de detección de hongos.
Fungal air sampling in health-care facilities is controversial due to unresolved technical limitations, and the lack of standards linking fungal spore levels with infection rates.1 Furthermore, there are no standardized methods for fungal air sampling or for its frequency, and the variety of procedures used impedes comparisons.2 Despite these limitations, Vonberg et al.3 suggested that during construction work, fungal air sampling may be useful to detect increases in the concentrations of fungal spores, which could precede subsequent nosocomial aspergillosis outbreaks. Microbiological sampling has also been recommended by several infection control organizations,4 particularly during construction work.5
There has been a tendency to measure the concentrations of airborne particles greater than 0.5μm, 1.0μm and 5.0μm in operating theatres and in protective environment rooms.6 Since technology standards for cleanrooms with HEPA filters are based on these concentrations, their measurement has been recommended, instead of fungal air sampling,2 as a routine procedure in operating theatres. According to some experts, this could be a technique to complement microbiological air sampling, requiring further scientific literature.7
As the relationship between the concentrations of airborne fungal spores and nonviable particles is not fully understood,1 the aim of our study was to assess this relationship in different hospital areas.
MethodsThe study was undertaken at the Hospital Universitari Vall d’Hebron, in Barcelona, Spain, a referral university hospital with more than 1100 beds, where routine fungal air sampling was performed in rooms for burn or haematology patients, in operating rooms, and in rooms for minimally invasive procedures (cystoscopy, epidural blockade, etc.). Fungal air sampling is also carried out after construction work or other incidents, in accordance with recommendations from the Sociedad Española de Medicina Preventiva, Salud Pública e Higiene, and INSALUD.4 Any fungi detection in operating theatres or in protective environment rooms is managed according to these recommendations.4 Between December 2007 and April 2008, measurement of the concentrations of airborne particles was added to several scheduled fungal air samplings, which were selected in order to obtain the broadest spectrum of environments; fungal samplings and particle counts were performed consecutively, in any order. In operating rooms and in rooms for minimally invasive procedures, both sampling procedures were performed “at rest” (i.e., the condition where the installation is complete, with equipment installed and operating, but with no personnel present), before any surgical activity; in rooms for burn or haematology patients samplings might be performed while patients were in their rooms (but without any other healthcare personnel).
Microbiological studyMicrobiological air sampling was performed with an impactor volumetric air sampler (Microflow; Aquaria srl; Lacchiarella, MI, Italy): 1000l (1m3) of air were impacted at a flow rate of 1.5l/s on Rodac plates with Sabouraud chloramphenicol agar. The sampler was placed above the main table (in operating and other procedure rooms), or over a table placed near the bed (in protective environment rooms). The impacted plates were then incubated at 28–30°C in an aerobic atmosphere for five to seven days. Colony-forming units (CFU) of filamentous and/or levaduriform fungi were identified and enumerated by standard techniques. Fungal counts were expressed as CFU/m3.
Particle countingThe concentrations of ≥0.5μm particles (P05), and ≥1μm particles (P1) were measured using a particle counter (Met One 237B; Pacific Scientific Instruments, Grants Pass, OR, USA), for 1min at a flow rate of 0.1 cubic feet/min (2.8l/min), according to the manufacturer's instructions. In each room, several sampling points were chosen, either around the main table (in operating or procedure rooms) or around the bed (in patient rooms); the number of sampling points was equal to or greater than the square root of the room surface area in square meters; two consecutive samples of 0.1 cubic feet were obtained at each point; samples were obtained at 0.75–1m above floor level. In most operating theatres particle counting lasted about 15min (two consecutive countings lasting 1min in six or seven sampling points).
The pilot phase of the study coincided with the training in particle counting methodology. Five combined samplings performed during this phase could not be included in the study because the sampling points had not been specified correctly in the particle counter (two samplings in rooms for haematologic patients, and three in operating theatres). The remaining combined samplings performed in operating theatres during this phase have been included in the study, although in two of them particle counting had been performed in ten sampling points (instead of just the square root of theatre surface in meters), and in another three operating theatres, two consecutive samples of 0.25 cubic feet (instead of 0.1 cubic feet) were obtained from each sampling point.
Statistical analysisTo estimate the concordance between the two particle counts in each sampling point, the intraclass correlation coefficient was calculated. For each room, the mean concentration of particles with a diameter equal to or greater than 0.5 and 1.0μm was estimated using PortAll Version 2 Software (Hach Ultra Analytics; Grants Pass, OR, USA). The option of assessing conformity with Class 6 according to the European ISO 14644 Standards8 was used, which only calculates the mean of particle concentrations when the number of samples and their volume are in accordance with the specifications of these standards (in order to avoid excessive variability of results).
Particle concentrations were expressed as the number of particles per cubic meter. Although particle measurements were not intended to classify rooms according to ISO 14644-1 standard (rooms for burn or haematology patients had not been measured “at rest” – in fact, the patient might be present during sampling), the concentrations of P05 and P1 were categorised according to the superior limits allowed in cleanrooms classified as Class 6, 7 or 8 according to this standard.2 Fisher's exact was used to assess the association between particle concentrations and fungal detection; exact confidence intervals (CI) for proportions were calculated according to the binomial distribution; the chi-squared test was used to assess the association between P05 and P1 counts; the Rho Spearman rank correlation was used to assess the degree of association between fungal colony counts and particle counts; the statistical package R9 was used.
ResultsForty-two simultaneous samplings were carried out: 24 (57%) in 22 operating rooms (two of which were studied twice), 13 (31%) in eight rooms for burn or haematology patients (three rooms were sampled twice, and another one three times), 3 (7%) in pharmacy cleanrooms, and 2 (5%) in other procedure rooms without HEPA filtration. Two combined samplings (performed in operating rooms) could not be included because the number of sampling points for particle counting was lower than the square root of room surface in meters. The 24 samplings in operating rooms included 15 scheduled routine controls (both fungal air sampling and particle counting were performed consecutively before any surgical activity, between 7:00 and 8:30 a.m.) and 9 controls after renovation work (both sampling procedures performed consecutively the same day, before resuming surgical activity, although the interval between them might be larger). All samplings in patient rooms were scheduled routine controls: in rooms for haematology patients both sampling procedures were performed consecutively the same day in the morning (although the interval between them might be slightly larger than for operating rooms), whereas in rooms for burn patients, fungal air sampling was done in the morning and particle counting in the afternoon. In pharmacy cleanrooms, both sampling procedures were performed consecutively in a two-hour interval, during their commissioning and before any activity. As the 2 samplings in rooms for minimally invasive procedures were scheduled routine controls, both sampling procedures were performed consecutively between 7:00 and 8:30 a.m., before any activity.
Microbiological analysis was positive in 6 of 42 bioaerosol samples (14%); filamentous fungi (Ascomycota, Dematiaceous) were recovered in 5 samples, 2 of which were also positive for levaduriform fungi (Basidiomycota). Fungal counts ranged from 2 to 5CFU/m3 (median=2). The five bioaerosol samples that were positive for fungi were obtained in settings which had been studied only once.
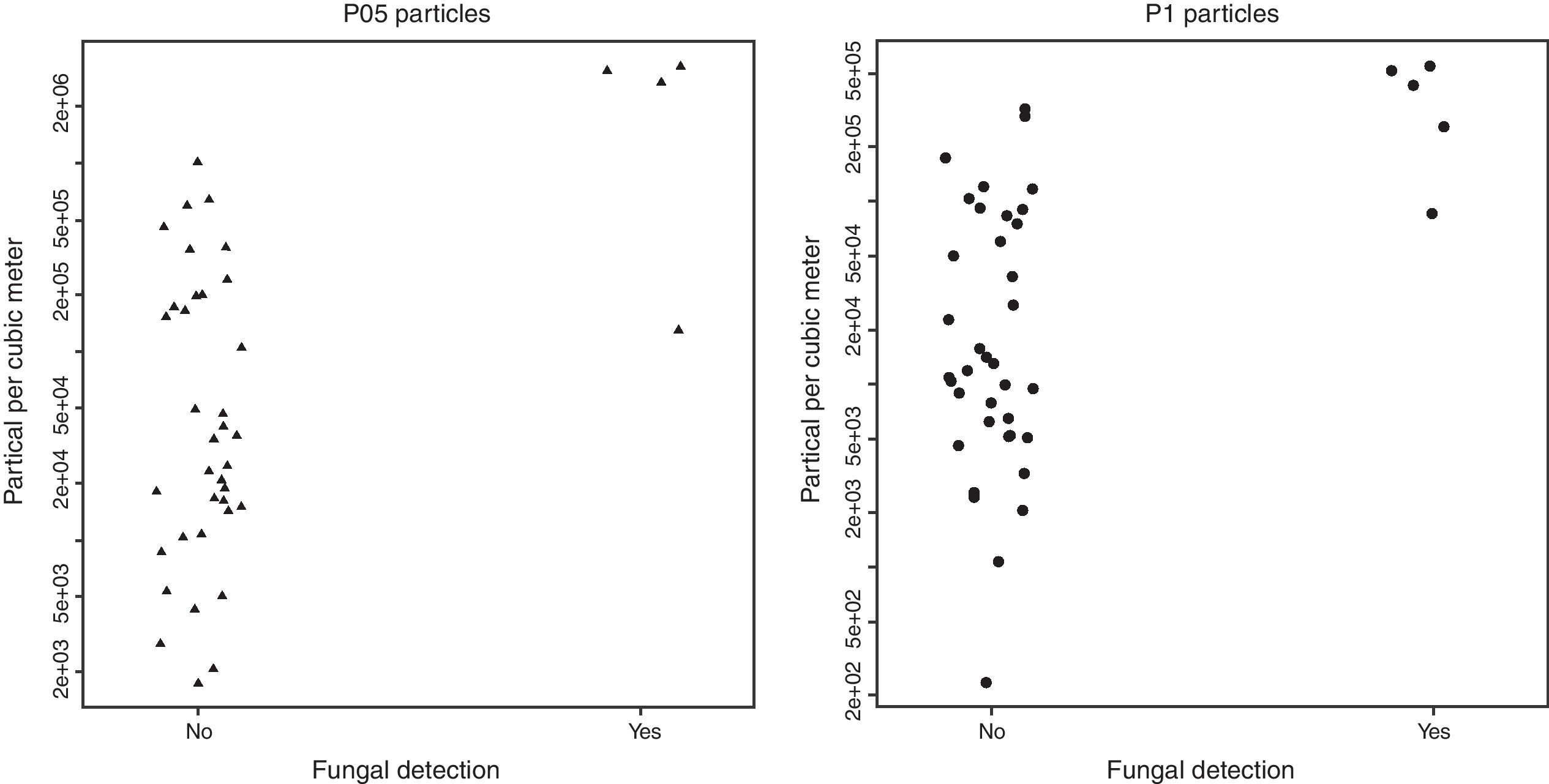
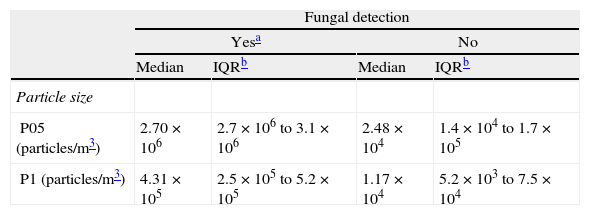
The intraclass correlation coefficients for P05 and P1 counts were 0.992 and 0.949, respectively. The concentrations of particles were higher when fungi were detected in bioaerosol samples (Table 1).
Distribution of concentrations of particles in 42 samplings, according to the results of the simultaneous fungal air sampling.
| Fungal detection | ||||
| Yesa | No | |||
| Median | IQRb | Median | IQRb | |
| Particle size | ||||
| P05 (particles/m3) | 2.70×106 | 2.7×106 to 3.1×106 | 2.48×104 | 1.4×104 to 1.7×105 |
| P1 (particles/m3) | 4.31×105 | 2.5×105 to 5.2×105 | 1.17×104 | 5.2×103 to 7.5×104 |
P05: particles ≥0.5μm; P1: particles ≥1μm.
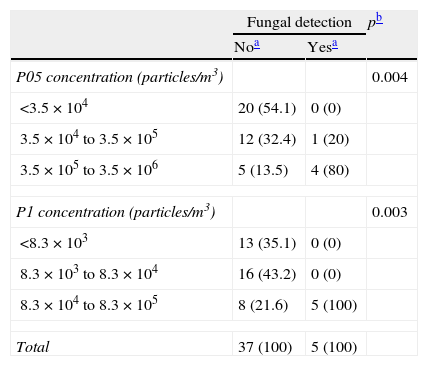
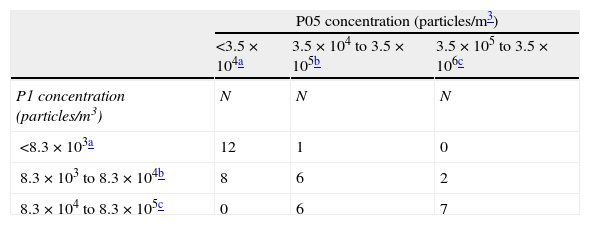
As shown in Table 2, the concentrations of P05 particles were below the maximum for Class 6 cleanrooms (3.5×104 P05/m3) in 20 samplings, whose associated fungal samplings were negative. Among the 22 samplings with P05 counts above this cutpoint, fungi were detected in 5 (22.7%). The concentrations of P1 particles were below the maximum for Class 7 cleanrooms (8.3×104 P1/m3) in 29 samplings, whose associated fungal samplings were negative; among the 13 samplings with P1 counts above this cutpoint, fungal samplings were positive in 5 (38.5%). The association between fungal detection and higher particle counts was statistically significant, both for P05 (p=0.004) and P1 (p=0.003).
Distribution of samplings according to concentrations of particles and results of the simultaneous fungal air sampling.
| Fungal detection | pb | ||
| Noa | Yesa | ||
| P05 concentration (particles/m3) | 0.004 | ||
| <3.5×104 | 20 (54.1) | 0 (0) | |
| 3.5×104 to 3.5×105 | 12 (32.4) | 1 (20) | |
| 3.5×105 to 3.5×106 | 5 (13.5) | 4 (80) | |
| P1 concentration (particles/m3) | 0.003 | ||
| <8.3×103 | 13 (35.1) | 0 (0) | |
| 8.3×103 to 8.3×104 | 16 (43.2) | 0 (0) | |
| 8.3×104 to 8.3×105 | 8 (21.6) | 5 (100) | |
| Total | 37 (100) | 5 (100) | |
P05: particles ≥0.5μm; P1: particles ≥1μm.
Twelve samplings had both P05 and P1 concentrations below the maximum for class 6 cleanrooms (Table 3), all of which were negative for fungi, and both concentrations were above these cutpoints in 21 samplings. There were no samplings with P1 concentrations below the class 6 maximum and P05 concentrations within the range for class 8, and vice versa. Among the 13 samplings with P1 concentrations within the range for class 8 cleanrooms, P05 concentrations were within this range in 7, 4 of which (57%, 95% CI: 18–90%) were positive for fungi. The association between P05 and P1 concentrations was statistically significant (Chi-squared test=24.88, p-value=0.0005).
Distribution of samplings according to the concentrations of both P05 and P1 particles.
| P05 concentration (particles/m3) | |||
| <3.5×104a | 3.5×104 to 3.5×105b | 3.5×105 to 3.5×106c | |
| P1 concentration (particles/m3) | N | N | N |
| <8.3×103a | 12 | 1 | 0 |
| 8.3×103 to 8.3×104b | 8 | 6 | 2 |
| 8.3×104 to 8.3×105c | 0 | 6 | 7 |
P05: particles ≥0.5μm; P1: particles ≥1μm.
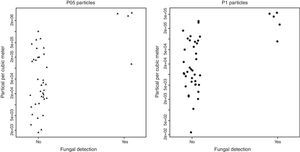
As can be seen in Table 2 and Fig. 1, there was a considerable overlap between the concentrations of particles of samplings positive and negative for fungi: the lowest P05 concentration among positive samplings was 1.29×105 particles/m3, whereas the highest P05 concentration among negative samplings was 106 particles/m3; the lowest P1 concentration among positive samplings was 8.6×104 particles/m3, whereas the highest among negative samplings was 3.2×105 particles/m3.
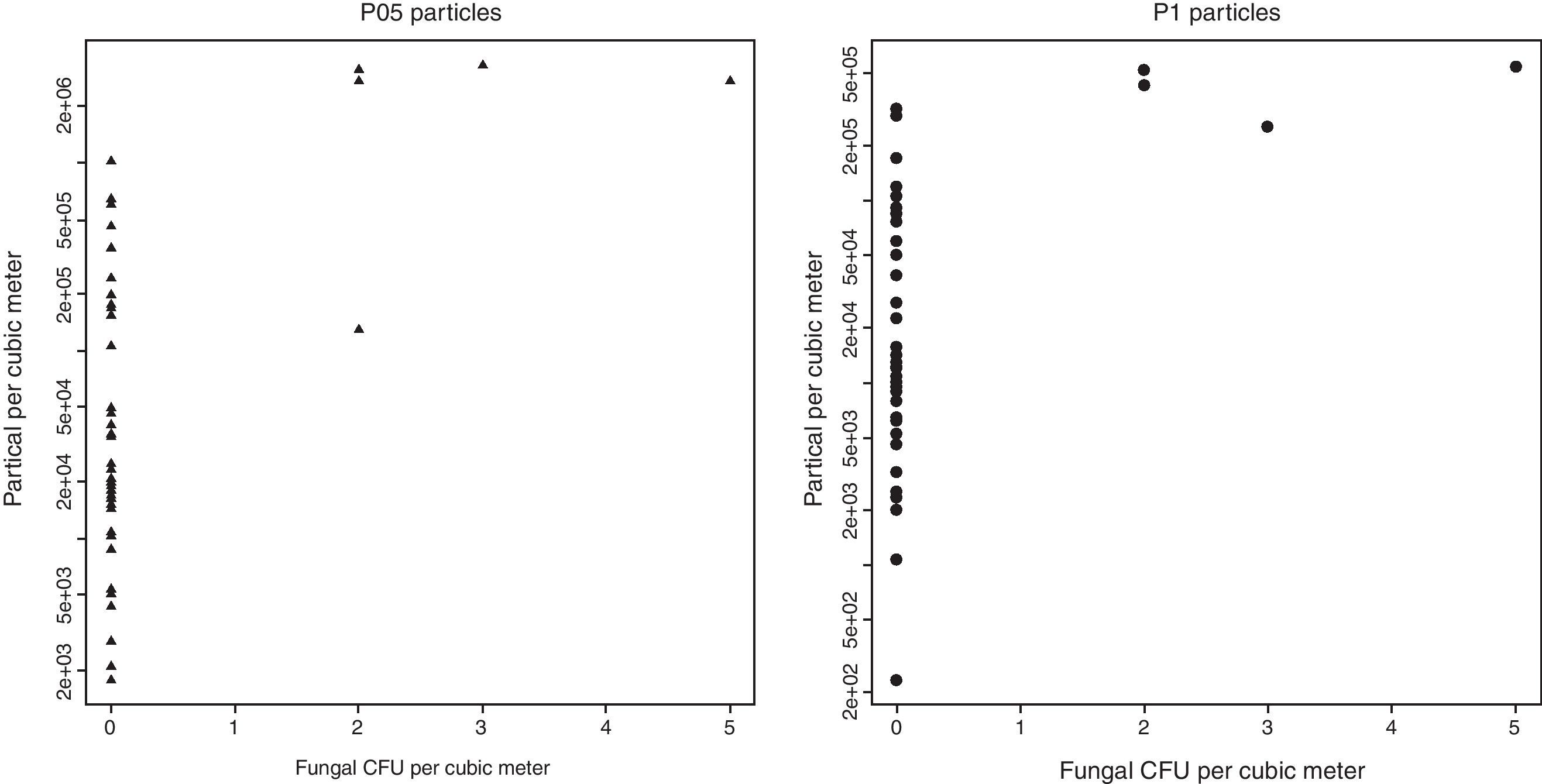
The correlation coefficients between air fungal counts and particle concentrations were statistically significant, both for P1 (Rho=0.502; p=0.0007) and P05 (Rho=0.491; p=0.001) concentrations (Fig. 2).
DiscussionThis study performed 42 combined air samplings for fungal detection and particle counting in various types of hospital rooms. Our results indicate a positive relationship between the concentrations of P05 and P1 particles and airborne fungi. Particle concentrations were categorised according to ISO 14644-1 cutpoints, due to the widespread use of this standard, which also includes the area of health care.7 Fungal detection has been associated with higher particle counts (higher than class 6 maximum for P05, higher than class 7 maximum for P1). The positive relationship between the concentrations of particles and airborne fungi in the present study is in accordance with other studies: Li et al.10 observed a weak correlation between cleanroom class level, particle concentrations and bioaerosol levels. In an operating room, under simulated conditions, Bergeron et al.11 observed that a 2-log decrease in the concentration of P05 particles in air (obtained with an additional air-treatment unit) was associated with a reduction in the concentrations of fungal species to undetectable levels.
In our study, there were several samples negative for fungi whose associated particle concentrations were as high as those observed in samplings positive for fungi. Furthermore, the correlation coefficient between particulate and fungal concentrations was quite low. These discrepancies may be related to not taking into account the concentrations of bacteria in air and the variability of microbiological air sampling.
The relationship between particle concentrations and bacteria can be significant in certain hospital environments, such as operating theatres in which surgery is being performed, where many particles in air are derived from skin scales from healthcare personnel who might be carrying bacteria, especially gram-positive cocci1,6; therefore the correlation between particles and bacteria might have been higher than that observed in this study, particularly among samplings from occupied rooms of burn or haematology patients. In the study performed by Bergeron et al.,11 the decrease in the concentrations of P05 particles in air was also associated with a reduction in the concentrations of total mesophilic flora; however, Landrin et al.12 detected no relationship between P05 concentrations and microbiological counts (including bacteria), in HEPA-filtered operating theatres (although their conclusions may be related to a narrower spectrum of settings, because they only included operating theatres, in fact, their range of P05 concentrations was lower). Instead, our study focused on the relationship between particle counting and environmental fungi because, according to Streifel6 and current Spanish guidelines,4 air sampling should be considered only for the evaluation of the presence of airborne fungi.
The observation of negative fungal results in rooms with a high particle count could also be related to the considerable variability of fungal air sampling1: in the study performed by Bergeron et al.11 in an empty operating theatre with particle concentrations of the order of 2.56×104 P05/m3, the results of 12 air samples ranged between 0 and 4UFC/m3 of fungi. Samples negative for fungi in the zones with relatively uncontrolled air would be frequent (although they would be expected to be detected): Li and Hou10 detected fungi concentrations of between 0 and 51UFC/m3 in operating theatres with a class equivalent to ISO 7, and between 0 and 319UFC/m3 in rooms with a class equivalent to ISO 8. Falvey et al.13 also observed a wide range of fungal counts (including zero fungal detection) in the air of rooms without HEPA filters. In monitoring concentrations of filamentous fungi in operating theatre air, Robles García et al.14 observed a median of 0UFC/m3, with sporadic high counts being detected (in most cases the cause could not be identified). According to Hernández-Calleja,15 the lower the concentration of fungi or the volume of air sampled, the lower the probability of capturing fungi, and hence the sensitivity of fungal air sampling: this author considers that if a sample detects less than 10UFC, the variability among consecutive samples could be considerable. Due to the low precision and accuracy of fungal air samplings, it has been recommended to rule out a false-positive result when their results are over the desired limits.16
According to the Spanish guidelines on the prevention of fungal infections in high risk patients,4 when pathogenic fungi with airborne transmission are detected in protective environment rooms, corrective actions must be taken and high risk patients cannot be admitted until the result of a new fungal air sampling is negative. During the five days necessary to obtain a definitive negative result4 a decision must be made between not using the room, or using it with a provisional negative result.
As the results of particle counting are immediate,2 this procedure could be used to predict fungal air sampling results. In order to prevent exposure to fungi in patients with a high risk of infection, a cutpoint for particle counting as a predictor of fungal air sampling must have high sensitivity and negative predictive value. Although in the present study the number of simultaneous samplings and fungal detections were not large enough, no fungi were detected when P05 concentrations were within the range for class 6 cleanrooms (according to ISO 14644-1 standard) and P1 concentrations were lower than maximum for class 7 cleanrooms. According to our results, if both P05 and P1 concentrations are within the range for class 6 cleanrooms, a negative fungal detection can be predicted, whereas P05 and P1 concentrations within the class 8 range would indicate a risk of fungal detection. However, to better assess the accuracy of particle counting as a predictor of negative fungal detection,1 the simultaneous use of both procedures must be assessed in more rooms where fungi might be detected (mainly in class 7 or 8 cleanrooms).
FundingFinancial support: Grant from Patient Safety Alliance in Catalonia, Health Department of Catalonia.
Conflict of interestThe authors declare no conflicts of interest.
We would like to thank Inmaculda Albero Andrés and Mercé Delsors Beguer, nurses of the Preventive Medicine and Epidemiology Service at the Hospital Universitari Vall d’Hebron, for performing the microbiological air samplings.