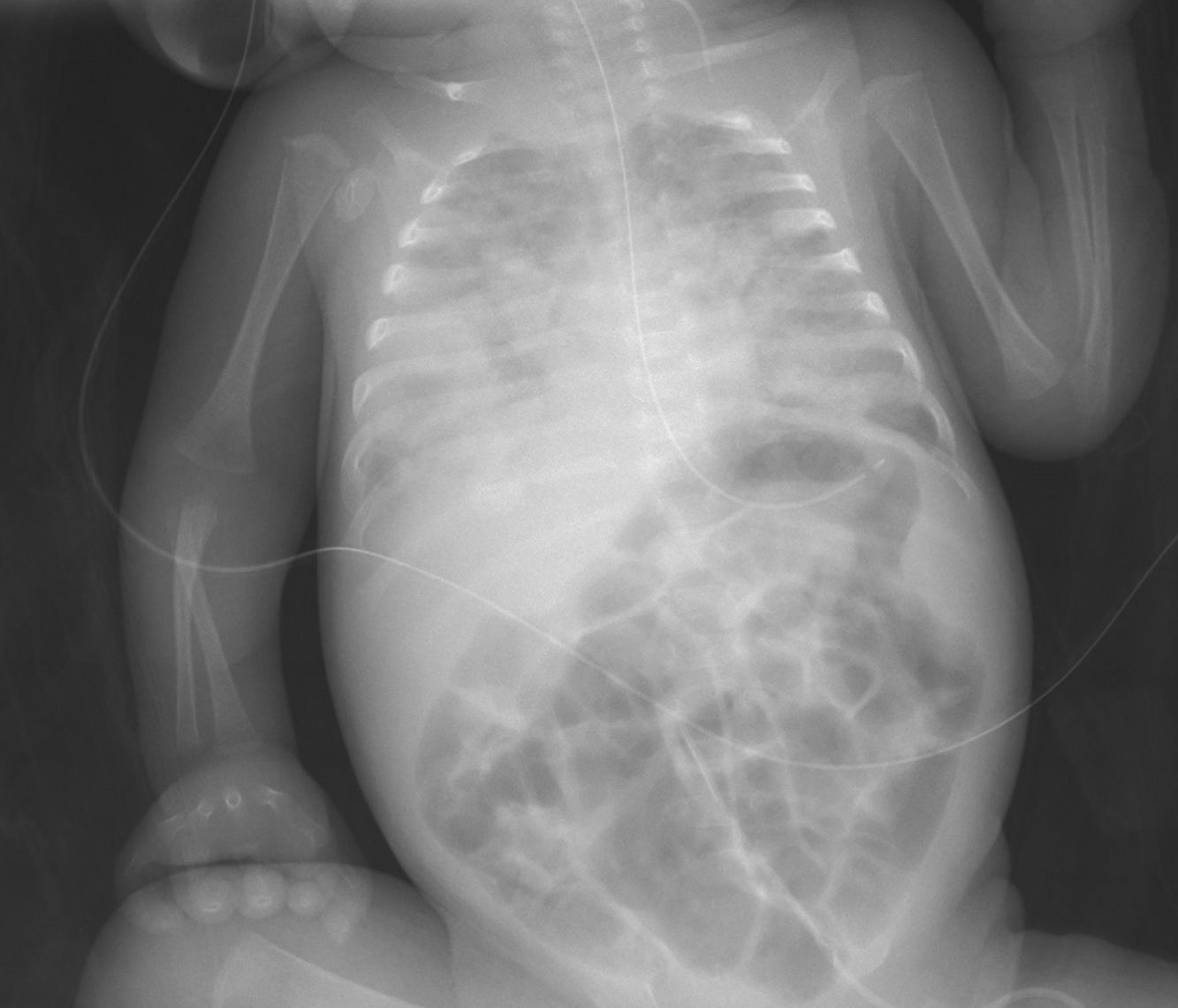
The rarity of some infectious diseases in newborns causes a delay in diagnosis, leading to high morbidity and mortality rates. We present the case of a premature infant of 28 weeks gestational age, with healthy parents of Roma ethnicity, who was admitted to the neonatal unit due to prematurity and had been making good progress. At 57 days of life, close to hospital discharge, the infant developed severe respiratory distress along with fever, hepatosplenomegaly and clotting abnormalities. A diagnosis of nosocomial sepsis-pneumonia was made, but the patient had a poor clinical and radiological response (Fig. 1) to the usual antibiotic therapy, with a finding of extensive bilateral necrotising pneumonia on computed axial tomography. Secondary haemophagocytic syndrome was suspected as six of the eight clinical-analytical criteria were met.1 Fifteen days after the onset of the condition, due to the poor clinical progress and the negative results of the microbiological tests for viruses, fungi and common bacteria, screening was performed for tuberculosis infection. Samples of gastric juices were taken for three consecutive days for polymerase chain reaction (PCR), sputum smear and culture testing, and a tuberculin skin test (TST) was performed. As the TST reading was negative at 48 h, an interferon-gamma release assay (IGRA) test was requested. The sputum smear microscopy and the PCR in gastric juices were positive for Mycobacterium tuberculosis and the IGRA was also positive. Perinatal tuberculosis (TB) was diagnosed, and a positive culture for M. tuberculosis was subsequently obtained. Contact tracing of family members and healthcare personnel revealed bacillary pulmonary tuberculosis in both the mother and the grandmother. Both reported being asymptomatic and denied travelling abroad.
Perinatal TB is that which occurs in children under three months of age, with a low incidence in our setting.2 In a series of cases from La Paz Hospital, it represents 1.4% of TB in the paediatric age group.3 It may be congenital or acquired postnatally, with postnatal transmission being the most common. Diagnosis of TB in infancy is difficult due to the poorer yield of microbiological tests. Even TST produces more false negatives due to poorer cellular immunity and also in disseminated forms of TB, which are common in this population. The IGRA has higher specificity than the TST, but also low sensitivity in infants. Therefore, if there is a high degree of suspicion, TST or IGRA should be performed, and if the result is negative for one of them, it is recommended to perform the other, achieving a sensitivity greater than 90% with the combination of the two.4
With regard to samples for microbiological diagnosis, congenital TB has high bacillary loads in gastric juices and in respiratory samples (bronchoalveolar lavage or bronchial aspiration), being positive in up to 80% of cases,5 unlike postnatal TB, which is barely bacillary. CSF extraction is also necessary to rule out meningitis, requesting PCR, sputum smear microscopy, culture and adenosine deaminase. Other possible samples if TB is highly suspected would be ascitic or pleural fluid, faecal material or urine. Culture is the diagnostic test of reference; it allows the identification of the species and the determination of sensitivity to anti-tuberculosis drugs, although it has the disadvantages of low sensitivity (30–50 %) and the delay of up to four weeks for a definitive result. PCR stands out for its greater speed, high specificity in children and the fact that it also enables the detection of drug resistance. Its sensitivity is 65–70 % compared to culture. Sputum smear microscopy is rapid, but with low sensitivity in children, being higher in congenital TB.
Radiological findings are very common in infants, particularly hilar lymphadenopathy and miliary pattern. Clinical signs vary, ranging from well-appearing patients with mild respiratory distress to severe respiratory distress, pneumonia unresponsive to treatment, pertussis-like cough, fever, hepatosplenomegaly, lymphadenopathy, abdominal distension, ascites, persistent diarrhoea or jaundice.6 The fundus should be examined for chorioretinitis and granulomas. There are rare manifestations of congenital TB, such as skin or middle ear lesions.7 Without treatment, 10–20% will develop disseminated forms (meningitis or miliary dissemination). It is also more common to develop other complications such as septic shock, disseminated intravascular coagulation, multiorgan failure or haemophagocytic syndrome.
Treatment in the intensive phase (first two months) should combine four anti-tuberculosis agents: isoniazid, rifampicin, pyrazinamide and, as a fourth drug, an aminoglycoside or ethambutol,8 withdrawing the fourth drug when sensitivity to the other three first-line drugs is confirmed. The maintenance phase continues with isoniazid and rifampicin for at least nine months, and corticosteroids may be added in severe forms.9 In conclusion, perinatal TB should be suspected in infections that do not respond to standard antibiotic therapy and negative cultures are obtained. Early diagnosis improves the prognosis.
FundingThis study has received no specific funding from public, private or non-profit organisations.