Mycoplasma genitalium is a major cause of urethritis and other genital syndromes. Antibiotic resistance, especially to macrolides, is increasing at an alarming rate worldwide. The aim of this study was to estimate the rate of macrolide resistance in M. genitalium among a 2016–2017 cohort of patients in Barcelona, Spain; and to compare this estimate with previous data from 2013 to 2014 in this region.
MethodsThe study was conducted retrospectively with M. genitalium-positive samples collected between December 2016 and February 2017 at the Hospital Vall d’Hebron Microbiology Department. Genotypic markers of macrolide resistance were primarily detected using the ResistancePlus® MG molecular assay (SpeeDx). Mutations were then confirmed by sequencing.
ResultsMacrolide resistance-mediating mutations were detected in 30/83 infections (36.1% [95% CI, 25.9%–47.4%]). This resistance was more frequent among men who have sex with men (55.0% [95% CI, 38.5%–70.7%]) compared to heterosexual men (27.3% [95% CI, 10.7%–50.2%]) and women (9.5% [95% CI, 1.3%–30.4%]), p<0.001. Additionally, macrolide resistance did not significantly increase in this cohort when compared with previous investigations.
ConclusionDespite the current notable rate of macrolide resistance in M. genitalium, resistance did not significantly increase between 2013–2014 and 2016–2017 in our region. Nevertheless, strict local surveillance and the implementation of rapid diagnostic tests that combine the detection of the bacterium and resistance-mediating mutations may facilitate the optimization of antibiotic administration and reduce the transmission of resistance in M. genitalium.
Mycoplasma genitalium es causa de uretritis y otras enfermedades genitales. Las resistencias antibióticas, especialmente a macrólidos, están aumentando de forma alarmante a nivel mundial. El objetivo del estudio fue estimar la tasa de resistencia a macrólidos en M. genitalium sobre una cohorte de pacientes entre los años 2016-2017 en Barcelona, España; y comparar esta estimación con datos previos de 2013-2014 en esta región.
MétodosEl estudio se realizó de forma retrospectiva sobre muestras positivas para M. genitalium recogidas entre diciembre 2016 y febrero 2017 en el Departamento de Microbiología del Hospital Vall d’Hebron. Los marcadores genotípicos de resistencia a macrólidos se detectaron en primer lugar con el ensayo molecular ResistancePlus® MG (SpeeDx). Las mutaciones se confirmaron posteriormente por secuenciación.
ResultadosSe detectaron mutaciones asociadas a resistencia a macrólidos en 30/83 (36,1% [IC 95%: 25,9-47,4%]) infecciones. Esta resistencia fue más frecuente en hombres que tienen sexo con hombres (55,0% [IC 95%: 38,5-70,7%]) comparada con la tasa en hombres heterosexuales (27,3% [IC 95%: 10,7-50,2%]) y mujeres (9,5% [IC 95%: 1,3-30,4%]), p<0,001. Además, la resistencia a macrólidos no aumentó significativamente en esta serie en comparación con investigaciones previas.
ConclusiónA pesar de la tasa notable de resistencia a macrólidos en M. genitalium, esta no aumentó significativamente entre los años 2013-14 y 2016-17 en nuestro entorno. No obstante, una estricta vigilancia a nivel local junto con la implementación de pruebas diagnósticas rápidas que combinan la detección de la bacteria y las mutaciones de resistencia puede facilitar la optimización de la administración antibiótica y reducir la transmisión de resistencias en M. genitalium.
Mycoplasma genitalium is a major cause of urethritis, accounting for 15–20% of non-gonococcal urethritis (NGU) in men.1 Additionally, although its pathogenicity in women is less well established, this sexually transmissible infection (STI) is associated with cervicitis, pelvic inflammatory disease (PID), preterm birth and miscarriage.2
Since doxycycline demonstrated poor efficacy eradicating the infection,3,4 the macrolide azithromycin (AZM), given as an extended regimen with 500mg day one followed by 250mg days 2–5, is the recommended first-line treatment against M. genitalium.5 However, the widespread use of this antibiotic, particularly as a single 1g dose, has enhanced the emergence of macrolide resistance worldwide.6–10 Single-nucleotide mutations (SNPs) in domain V of the 23S ribosomal RNA (rRNA) gene, predominantly at positions 2058 and 2059 (Escherichia coli numbering), are consistently associated with this resistance.11,12 Thus, high levels of macrolide resistance in M. genitalium have been reported in European countries,8,13–17 in the USA and the Asia-Pacific region.9,10,18 Moxifloxacin (MXF) is currently recommended as second-line antibiotic.5 Nevertheless, MXF resistance is appearing in many countries,19–22 including Spain (<10%),8,23 associated with point mutations in the quinolone resistance determining region (QRDR) of the parC and gyrA genes.
In 2016, as a response to the increasing macrolide resistance in M. genitalium and the lack of therapeutic alternatives, international treatment guidelines have recommended AZM to be replaced as the initial treatment for NGU with doxycycline (DOX) 100mg twice daily for one week.24 Furthermore, commercial assays for simultaneous detection of M. genitalium and macrolide resistance have recently been developed.25 These facts are providing an ideal scenario for the gradual implementation of “resistance-guided sequential therapy” for M. genitalium that has already demonstrated good efficacy, and that may potentially reduce the selection and transmission of resistance.26
To date, limited data has been published regarding AZM resistance in M. genitalium in Spain.8,23,27,28 Thus, the aim of this retrospective study was to estimate the rate of macrolide resistance in M. genitalium among a 2016–2017 cohort of patients in Barcelona, Spain. Additionally, evolution of macrolide resistance in M. genitalium was evaluated over time by comparing these series with a previous cohort (2013–2014), studied with similar methodology in the same region.8
MethodsPatients and specimensBetween December 2016 and February 2017, a total of 1191 specimens from 1109 patients were tested for M. genitalium at the Microbiology Department of the Vall d’Hebron University Hospital. The samples were collected from four clinical settings as follows: 398 were from subjects attending to the STI Reference Unit Vall d’Hebron-Drassanes, 356 specimens came from different wards at the Vall d’Hebron University Hospital, 356 came from primary care centers in Barcelona and 81 from private clinical centers. Specimens consisted of 613 urethral swabs/first-void urines, 288 vaginal/endocervical swabs, 26 rectal swabs, 4 pharyngeal swabs, 253 semen samples and, regarding adverse pregnancy outcomes, 3 endometrial samples and 4 amniotic fluid samples.
M. genitalium testing was usually performed in samples from patients with urethritis, cervicitis, in sexual contacts of infected partners and when PID or chronic prostatitis were suspected. Its detection among other clinical presentations was performed only if requested by the clinician. Sociodemographic and clinical characteristics of the patients were collected through a comprehensive review of the medical record. Treatment outcomes were determined with a microbiological test of cure (TOC), usually performed three weeks after antibiotic therapy according to clinical guidelines.5 Cure was defined when M. genitalium DNA was not detected.
Ethical approval for the study was obtained from the HUVH Ethics Committee (351/2018).
Laboratory analysesFirstly, clinical samples were tested for M. genitalium by real-time polymerase chain reaction (qPCR) using the AllplexTM STI Essential Assay (Seegene, South Korea). During the study period, positive primary samples were stored at −20°C for subsequent analyses. These specimens were retrospectively tested for M. genitalium and macrolide resistance genotypic markers with the ResistancePlus® MG Assay (SpeeDx, Australia). This is a multiplex qPCR for detection of M. genitalium and five mutations associated with macrolide resistance (A2058G, A2059G, A2058C, A2059C and A2058T; Escherichia coli numbering).25 Briefly, 400μl of sample was extracted using the NucliSENS® easyMag® platform (bioMérieux, France) and eluted in 100μl of elution buffer. M. genitalium and macrolide resistance was tested with the ResistancePlus® MG Assay on a LightCycler® 480 Instrument II (Roche Diagnostics, Germany). Analysis of the results was performed using the supplied software. Finally, mutations were confirmed by Sanger sequencing of domain V of the 23S rRNA gene using a previously described methodology.11
Statistical analysesSince some patients had several specimens taken over time, only first-test-positive samples of each M. genitalium infection episode were selected and studied in order to estimate the rate of antimicrobial resistance.
Statistical analyses were performed using Stata (StataCorp, USA). Distributions of categorical variables were compared by Chi-squared (χ2) or Fisher exact tests, and quantitative variables were evaluated by Mann–Whitney U test. The 95% confidence intervals (CI) of the proportions were calculated by exact methods. Differences with p<0.05 were considered statistically significant.
The original anonymized database from the study conducted by Barberá et al.8 was kindly given by the corresponding author Antonia Andreu so comparison between cohorts could be performed.
ResultsOf the initial 1191 samples tested trough the AllplexTM STI Essential Assay during the study period, 122 specimens (10.2% [95% CI, 8.9%–12.1%]) from 106 patients (9.6% [95% CI, 7.9%–11.4%]) resulted positive for M. genitalium. Positivity by specimen was: 63 were urethral swabs/first-void urines (10.3% [95% CI, 8.0%–13.0%]), 31 were vaginal/endocervical swabs (10.8% [95% CI, 7.4%–14.9%]), 22 were rectal swabs (84.6% [95% CI, 65.1%–95.6%]), one was a pharyngeal swab (25.0% [95% CI, 0.6%–80.6%]) and five were semen samples (2.0% [95% CI, 0.6%–4.6%]). Due to insufficient sample volume, of the 122 M. genitalium positive specimens, only 89 were suitable for subsequent analysis of macrolide resistance genotypic markers. Therefore, 89 samples collected from 86 patients with a M. genitalium infection were finally included in the study.
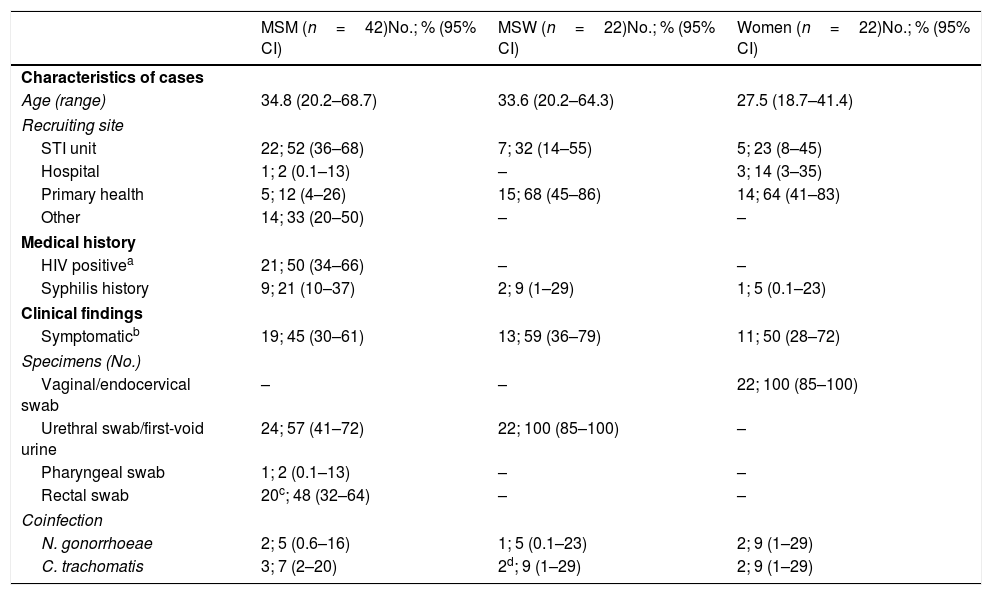
Characterization of study individualsThe demographic and clinical characteristics of the 86 patients studied are described in Table 1. Among them, 42 were men who had sex with men (MSM) (48.8% [95% CI, 37.9%–59.9%]) and most of them were attended at the STI Reference Unit Vall d’Hebron-Drassanes. In contrast, the majority of men who have sex with women (MSW) and women came from primary health centers and the Vall d’Hebron University Hospital wards.
Characteristics of the 86 M. genitalium infection episodes.
| MSM (n=42)No.; % (95% CI) | MSW (n=22)No.; % (95% CI) | Women (n=22)No.; % (95% CI) | |
|---|---|---|---|
| Characteristics of cases | |||
| Age (range) | 34.8 (20.2–68.7) | 33.6 (20.2–64.3) | 27.5 (18.7–41.4) |
| Recruiting site | |||
| STI unit | 22; 52 (36–68) | 7; 32 (14–55) | 5; 23 (8–45) |
| Hospital | 1; 2 (0.1–13) | – | 3; 14 (3–35) |
| Primary health | 5; 12 (4–26) | 15; 68 (45–86) | 14; 64 (41–83) |
| Other | 14; 33 (20–50) | – | – |
| Medical history | |||
| HIV positivea | 21; 50 (34–66) | – | – |
| Syphilis history | 9; 21 (10–37) | 2; 9 (1–29) | 1; 5 (0.1–23) |
| Clinical findings | |||
| Symptomaticb | 19; 45 (30–61) | 13; 59 (36–79) | 11; 50 (28–72) |
| Specimens (No.) | |||
| Vaginal/endocervical swab | – | – | 22; 100 (85–100) |
| Urethral swab/first-void urine | 24; 57 (41–72) | 22; 100 (85–100) | – |
| Pharyngeal swab | 1; 2 (0.1–13) | – | – |
| Rectal swab | 20c; 48 (32–64) | – | – |
| Coinfection | |||
| N. gonorrhoeae | 2; 5 (0.6–16) | 1; 5 (0.1–23) | 2; 9 (1–29) |
| C. trachomatis | 3; 7 (2–20) | 2d; 9 (1–29) | 2; 9 (1–29) |
MSM indicates men who have sex with men; MSW, men who have sex with women; CI, confidence intervals; HIV, human immunodeficiency virus.
Considering coinfection as a concurrent infection taking place at the same location, overall 11 M. genitalium cases (12.8% [95% CI, 6.6%–21.7%]) were coinfected with either Chlamydia trachomatis or Neisseria gonorrhoeae. Concretely, C. trachomatis was more frequent (8.1% [95% CI, 3.3%–16.1%]) than N. gonorrhoeae (5.8% [95% CI, 1.1%–13.0%]), p=0.549. Among the 22 women studied, 13 (59.1% [95% CI, 36.4%–79.3%]) were investigated for aberrant vaginal flora using culture and gram staining. Only one case (7.7% [95% CI, 0.2%–36.0%]) was described as normal flora (predominance of Lactobacillus spp.) while 12 (92.3% [95% CI, 64.0%–99.8%]) presented some vaginal dysbiosis (bacterial vaginosis, candidiasis or aerobic vaginitis).
Rate of macrolide resistance mediated mutationsOf the 86 M. genitalium infection episodes studied, macrolide resistance could not be determined in three samples using the ResistancePlus® MG Assay: one vaginal swab, one pharyngeal swab and one rectal swab. Ct values from the AllplexTM STI Essential Assay in these specific cases were 36, 38, and 37, respectively.
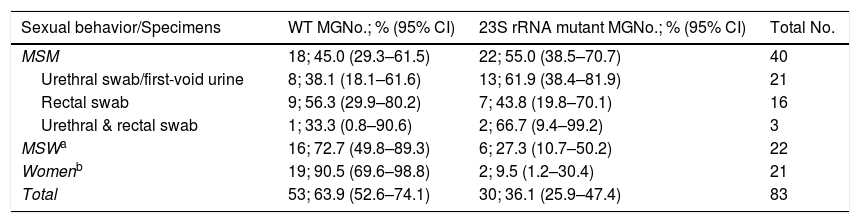
Therefore, of the remaining 83 M. genitalium infections, macrolide resistance-mediating mutations were detected in 30 episodes (36.1% [95% CI, 25.9%–47.4%]) while 53 (63.9% [95% CI, 52.6%–74.1%]) were classified as “wild type”/non-mutated (WT). Results stratified by sexual behavior are described in Table 2. Twelve HIV positive patients had a WT infection while eight had a macrolide resistant infection, p=0.859. Additionally, five patients harboring resistant infections suffered from a previous M. genitalium infection episode during the last 12 months but only three patients reported so among the WT cohort, p=0.100. Macrolide resistance in M. genitalium was significantly more frequent among MSM compared to MSW and women, p<0.001. Furthermore, no significant differences in macrolide resistance rates were found by sexual behavior regarding whether they were attended in an STI-Unit or in other health centers (MSM: 14/21 (66.6%) vs 8/19 (42.1%), p=0.119; MSW: 2/7 (28.6%) vs 4/15 (26.7%), p=0.926; Women: 1/4 (25.0%) vs 1/17 (5.9%), p=0.241).
Rate of macrolide resistance in M. genitalium stratified by sexual behavior.
| Sexual behavior/Specimens | WT MGNo.; % (95% CI) | 23S rRNA mutant MGNo.; % (95% CI) | Total No. |
|---|---|---|---|
| MSM | 18; 45.0 (29.3–61.5) | 22; 55.0 (38.5–70.7) | 40 |
| Urethral swab/first-void urine | 8; 38.1 (18.1–61.6) | 13; 61.9 (38.4–81.9) | 21 |
| Rectal swab | 9; 56.3 (29.9–80.2) | 7; 43.8 (19.8–70.1) | 16 |
| Urethral & rectal swab | 1; 33.3 (0.8–90.6) | 2; 66.7 (9.4–99.2) | 3 |
| MSWa | 16; 72.7 (49.8–89.3) | 6; 27.3 (10.7–50.2) | 22 |
| Womenb | 19; 90.5 (69.6–98.8) | 2; 9.5 (1.2–30.4) | 21 |
| Total | 53; 63.9 (52.6–74.1) | 30; 36.1 (25.9–47.4) | 83 |
MSM indicates men who have sex with men; MSW, men who have sex with women; MG, Mycoplasma genitalium; WT, “wild type”/non-mutated; CI, confidence intervals.
AZM resistance associated mutations in the 23S rRNA gene were confirmed by Sanger sequencing in 29/30 M. genitalium infection episodes. Thus, 12 (41.4% [95% CI, 23.5%–61.1%]) harbored a SNP at position A2058G (A2071G, M. genitalium numbering), 15 (51.7% [95% CI, 32.5%–70.6%]) had mutations at position A2059G (A2072G), one (3.4% [95% CI, 0.1%–17.8%]) at A2058T (A2071T) and one (3.4% [95% CI, 0.1%–17.8%]) at A2059C (A2072C). The remaining infection that tested positive for a resistant M. genitalium was unable to be amplified and sequenced.
Treatment outcomesSince the mutational analysis for macrolide resistance was performed retrospectively, antibiotic therapy was established without resistance information.
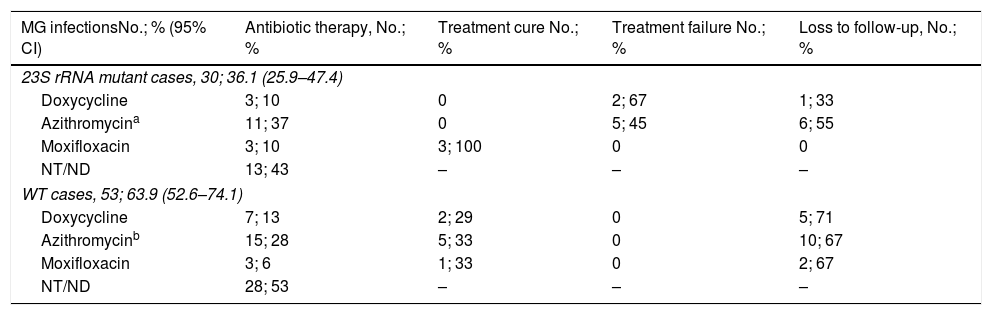
Among the 83 M. genitalium infection episodes which macrolide resistance status was determined, 12 did not received antibiotic therapy. Furthermore, in 29 cases information regarding therapy established could not be collected. Thereby, treatment outcomes were evaluated in 42 M. genitalium infections following the registrations in the medical record (Table 3). Overall, AZM was used in 26 cases (61.9% [95% CI, 45.6%-76.4%]), DOX in 10 (23.8% [95% CI, 12.1%–39.5%]) and MXF was used in six cases (14.3% [95% CI, 5.4%–28.5%]). Among the macrolide resistant cases, only MXF was able to eradicate M. genitalium while no treatment cure was reported using DOX neither AZM. However, no treatment failure was detected using MXF, DOX or AZM among the WT infections.
Treatment outcomes of the M. genitalium infections with determined macrolide resistance status.
| MG infectionsNo.; % (95% CI) | Antibiotic therapy, No.; % | Treatment cure No.; % | Treatment failure No.; % | Loss to follow-up, No.; % |
|---|---|---|---|---|
| 23S rRNA mutant cases, 30; 36.1 (25.9–47.4) | ||||
| Doxycycline | 3; 10 | 0 | 2; 67 | 1; 33 |
| Azithromycina | 11; 37 | 0 | 5; 45 | 6; 55 |
| Moxifloxacin | 3; 10 | 3; 100 | 0 | 0 |
| NT/ND | 13; 43 | – | – | – |
| WT cases, 53; 63.9 (52.6–74.1) | ||||
| Doxycycline | 7; 13 | 2; 29 | 0 | 5; 71 |
| Azithromycinb | 15; 28 | 5; 33 | 0 | 10; 67 |
| Moxifloxacin | 3; 6 | 1; 33 | 0 | 2; 67 |
| NT/ND | 28; 53 | – | – | – |
MG indicates Mycoplasma genitalium; CI, confidence intervals; NT/NF, no treatment or information not found; WT, “wild type”/non-mutated.
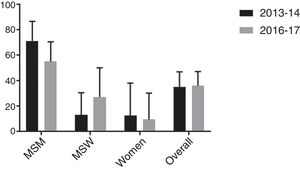
Macrolide resistance over time was evaluated in our setting, Barcelona (Spain), by comparing the current cohort with a previous one from 2013 to 2014, studied with a similar methodology.8
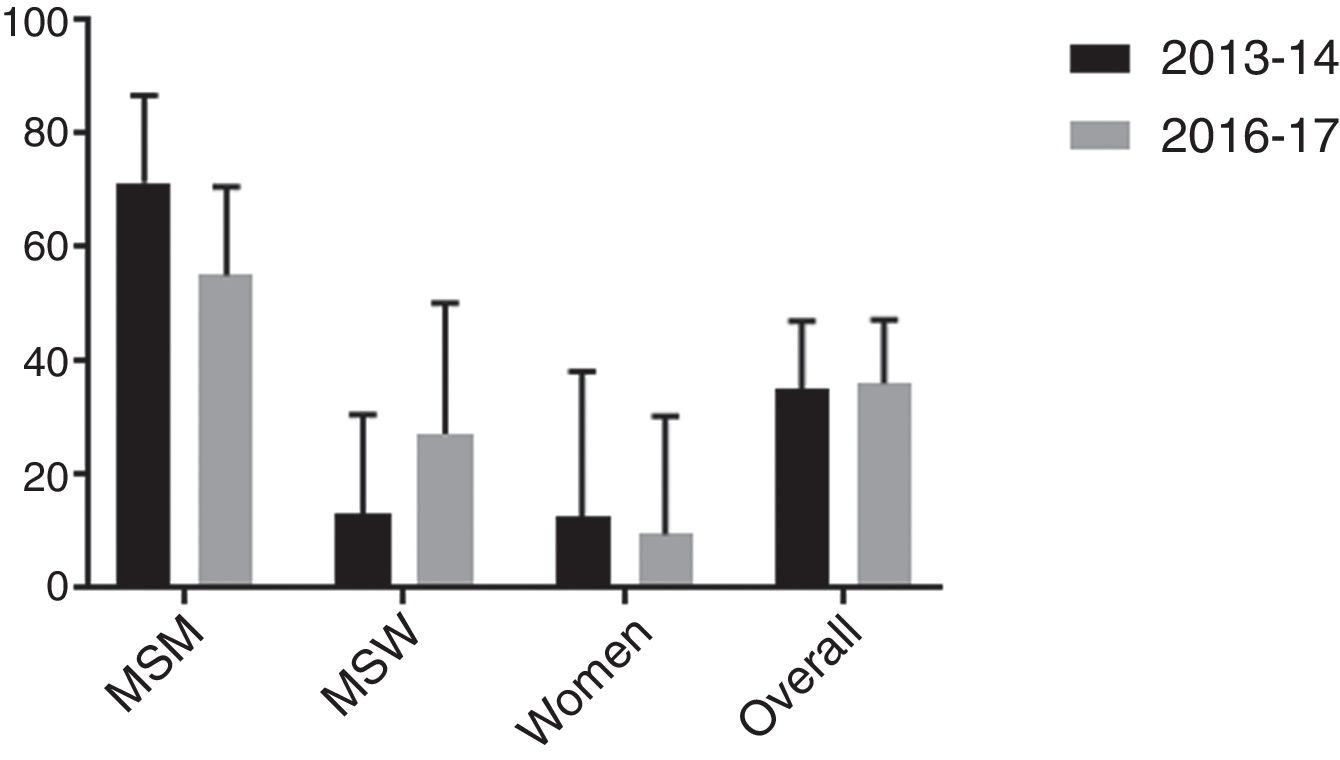
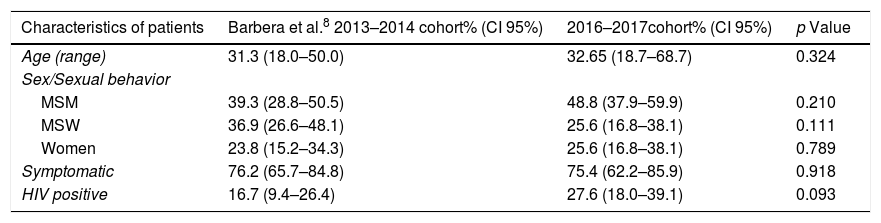
Firstly, similarity between both populations was evaluated in terms of age, sex/sexual behavior, presence/absence of symptoms and proportion of HIV positive patients. As shown in Table 4, no significant differences were found between cohorts although proportion of HIV positive patients was slightly higher in the 2016–2017 study. Secondly, macrolide resistance rates of both cohorts were calculated overall and stratified by sex/sexual behavior (Fig. 1). There were no significant differences between the 2013 and 2014 population cohort and the current one, neither from an overall approach (p=0.895) nor between MSM (p=0.170), MSW (p=0.208) or women (p=0.773).
Comparison between the characteristics of patients from the 2013 to 2014 cohort (Barberá et al.8) and the 2016–2017 cohort.
| Characteristics of patients | Barbera et al.8 2013–2014 cohort% (CI 95%) | 2016–2017cohort% (CI 95%) | p Value |
|---|---|---|---|
| Age (range) | 31.3 (18.0–50.0) | 32.65 (18.7–68.7) | 0.324 |
| Sex/Sexual behavior | |||
| MSM | 39.3 (28.8–50.5) | 48.8 (37.9–59.9) | 0.210 |
| MSW | 36.9 (26.6–48.1) | 25.6 (16.8–38.1) | 0.111 |
| Women | 23.8 (15.2–34.3) | 25.6 (16.8–38.1) | 0.789 |
| Symptomatic | 76.2 (65.7–84.8) | 75.4 (62.2–85.9) | 0.918 |
| HIV positive | 16.7 (9.4–26.4) | 27.6 (18.0–39.1) | 0.093 |
MSM indicates men who have sex with men; MSW, men who have sex with women; HIV, human immunodeficiency virus; CI, confidence intervals.
M. genitalium was first isolated in 1981 from men with urethritis,29 however the first PCRs for detection of M. genitalium were not developed until the early 1990s.30 Thenceforth, the role of M. genitalium in urethritis and many other anogenital disorders has been established. In addition, the antibiotic resistance profile of M. genitalium has been rigorously studied, especially after the appearance of the first AZM treatment failures in M. genitalium associated with SNPs in domain V of the 23S rRNA gene.6,11 Despite these efforts, M. genitalium remains still underdiagnosed in some areas due to the lack of easy access to diagnostic tools. Additionally, the alarming capability of this pathogen to develop antimicrobial resistance has complicated the current clinical management of the infection.31
This research provides further evidence regarding macrolide resistance in Spain, where epidemiological data remains limited.8,23,27,28 In the current study, the rate of macrolide resistance in M. genitalium was 36% overall. However, macrolide resistance was significantly more frequent among MSM (55%) compared to MSW or women (27% and 10%, respectively), p<0.001; findings previously reported.8 Additionally, results from this 2016 to 2017 cohort were compared with a previous report published by Barberá et al., 8 where macrolide resistance in M. genitalium was estimated in similar settings in Barcelona during 2013–2014 with comparable methodology. Inferences from this analysis indicate that macrolide resistance did not significantly increase during this period; neither from an overall approach nor within specific subpopulations (MSM, MSW and women).
Emerging macrolide resistance in M. genitalium has been widely reported in the last decade, approaching or even exceeding 50% in many countries.8–10,13–18 Nevertheless, most of these cross-sectional studies focus on very specific populations at risk for STIs. So, there is limited longitudinal data regarding the overall evolution of macrolide resistance among general population in the medium term.17 Our research suggests that macrolide resistance may be stabilizing around 35%, at least in our local epidemiology. Several factors could be influencing this situation. Firstly, there is increasing concern about M. genitalium and antibiotic resistance worldwide since first treatment failures appeared a decade ago.6,11 Consequently, medical societies are fully aware of this issue leading to a significant improvement on the diagnosis and management of M. genitalium infections in many settings.5,25 Secondly, since May 2016, as a response to the increasing macrolide resistance in M. genitalium, European guidelines changed to recommend DOX as the first line treatment for non-filiated NGU as the initial empirical approach.24 Thus, this statement also recommends the posterior investigation for further infectious aetiologies (including M. genitalium and macrolide resistance, if available) in order to refine the empirical therapy. Indeed, this treatment change may have contributed to control the spread of macrolide resistance in M. genitalium. In fact; Jensen, in a recent comment in Lancet Infect Dis,31 highlighted the case of Sweden where DOX has been and remains the only recommended antimicrobial treatment for C. trachomatis and where the prevalence of macrolide resistance in M. genitalium is among the lowest in western Europe, at less than 20%.15 Finally, macrolide resistance in our settings may have reached the plateau leading its stabilization around 35%.
Some limitations to our report must be addressed. First, we should remark that many of the patients included, especially MSM, attended the STI Unit Vall d’Hebron-Drassanes. This clinic is particularly specialized in high risk populations where we could expect higher proportions of macrolide resistance. So, our study may slightly overestimate the real prevalence of macrolide resistance in M. genitalium among general population in our region. Second, due to the retrospective design of the research, many registrations (mainly regarding treatment outcomes) are missing and limit the power of the results. Finally, it should be noted that M. genitalium was not unlimitedly tested but only on certain clinical presentations as mentioned in methodology.
No selection of resistance mutations was reported among the WT cases treated with AZM although TOC was only performed in 5/15 cases (33%). Additionally, no treatment failures were detected using MXF. Regarding symptoms, there were no cases of proctitis. Nevertheless, it is noteworthy the high proportion of disrupted vaginal biome in women with M. genitalium infections in our cohort (92%).
In conclusion, this study provides further data regarding macrolide resistance in M. genitalium in Spain. Additionally, a comparison between the current data and previous investigations indicates that macrolide resistance in M. genitalium did not significantly increase, at least between 2013 and 2017 in our study area, Barcelona. This evidence contrasts with the increasing emergence of antimicrobial resistance in M. genitalium worldwide and could provide a second opportunity for the adequate utilization of AZM against M. genitalium based on novel approaches such as sequential-resistance-guided and/or dual therapy.26,32 Nevertheless, AZM resistance continues to be a major concern in M. genitalium since therapeutic alternatives remain scarce. A strict local surveillance of M. genitalium and its antibiotic resistance is evidently required to facilitate the optimization of antibiotic administration for NGU and M. genitalium infections, and reduce the selection and transmission of resistance. Indeed, the implementation of combined diagnostic-resistance tests for M. genitalium plays a key role in this purpose.
FundingNone.
Conflicts of interestSpeeDx Pty Ltd. supplied all the reagents for molecular testing of M. genitalium. CFN is a researcher who has received partial salary support from SpeeDx Pty Ltd. MFH and ME have participated in symposiums organized by SpeeDx Pty Ltd.
We thank the laboratory team of the STI Unit at the Microbiology Department of the Vall d’Hebron University Hospital. We also thank Dr. Andrés Antón. Finally, we would like to thank SpeeDx Pty Ltd for their support although they had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.