Urinary tract infections (UTI) are one of the most common infections in solid organ transplant (SOT) recipients. A systematic review was performed to assess the management of UTI in SOT recipients.
Recommendations are provided on the management of asymptomatic bacteriuria, and prophylaxis and treatment of UTI in SOT recipients. The diagnostic–therapeutic management of recurrent UTI and the role of infection in kidney graft rejection or dysfunction are reviewed. Finally, recommendations on antimicrobials and immunosuppressant interactions are also included.
Las infecciones del tracto urinario (ITU) son muy frecuentes en los receptores de un trasplante de órgano sólido (TOS). Hemos realizado una revisión sistemática para determinar el abordaje de la ITU en receptores de TOS.
Se realizan recomendaciones sobre el abordaje de la bacteriuria asintomática y sobre la profilaxis y tratamiento de las ITU en receptores de TOS. Se han revisado el abordaje diagnóstico-terapéutico de las ITU recurrentes y el papel de la ITU en el rechazo o disfunción del injerto renal. Finalmente, se incluyen recomendaciones sobre las interacciones entre antimicrobianos e inmunosupresores.
The use of solid organ transplantation (SOT) has been established as accepted therapy for end-stage disease of the kidneys, liver, heart, and lungs for nearly 30 years. Intestinal and pancreas transplantation are also generally available but are provided on a more limited basis.
Infections remain a major cause of morbidity and mortality in transplant recipients. Urinary tract infections (UTI) are one of the most common infections in SOT, with a high prevalence, reaching 75% in some series involving kidney recipients. Experienced SOT researchers and clinicians have developed and implemented this consensus document in support of the optimal management of these patients.
The target population of this document are adults receiving SOT. The intended guideline audience is physicians involved in the care of SOT recipients (including primary care physicians). Here we report a consensus with the objective of assessing the overall available evidence and to propose recommendations on the following key issues:
- 1.
Definitions.
- 2.
Epidemiology and risk factors for UTI in SOT recipients.
- 3.
Should SOT recipients receive primary prophylaxis for UTI?
- 4.
What should be the management of asymptomatic bacteriuria in SOT recipients?
- 5.
What is the best empirical treatment of UTI in SOT recipients?
- 6.
What is the best definitive treatment of UTI in SOT recipients?
- 7.
How long should SOT recipients receive antibiotics for a UTI?
- 8.
What should be the management of UTI caused by Candida spp. in SOT recipients?
- 9.
What should be the diagnostic–therapeutic management of recurrent UTI in SOT recipients?
- 10.
What role does UTI play in kidney graft rejection or dysfunction?
- 11.
Antimicrobial and immunosuppressant interactions.
We conducted a systematic review to assess the management of UTI in SOT recipients. Data for this document were identified through a search of PubMed and references from relevant articles using the search terms “transplant” and “urinary tract infection”. The search criteria included articles in English that involved human participants. We selected and revised a total of 3043 articles from 1968 to June 2014.
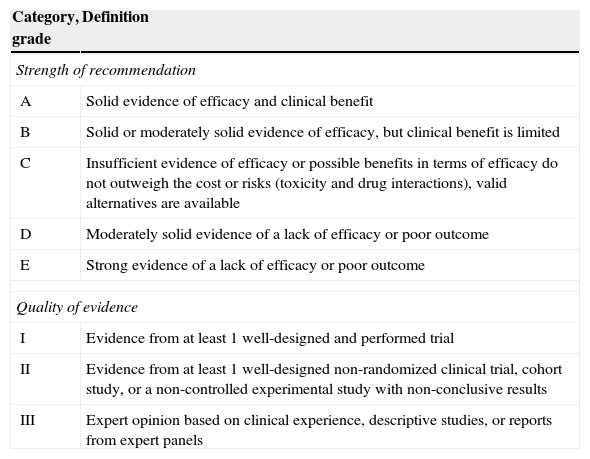
The evidence level based on the available literature is given for each recommendation to assess the strength of the evidence for risk and benefits of the procedure. This article was written in accordance with international recommendations on consensus statements (Table 1) and the recommendations of the Appraisal of Guidelines for Research and Evaluation II (AGREE II). The authors met twice to discuss the consensus and establish formal recommendations. The coordinators and authors agree on the content and conclusions. The consensus statement was sent to the 96 members of GESITRA for external revision of the manuscript. The board of directors of GESITRA will designate the coordinators to update the statements within 5 years. The full version of the consensus document of this executive summary is available at Ref. 1.
Classification of the recommendations of this consensus document based on the strength and quality of the evidence analyzed.
| Category, grade | Definition |
|---|---|
| Strength of recommendation | |
| A | Solid evidence of efficacy and clinical benefit |
| B | Solid or moderately solid evidence of efficacy, but clinical benefit is limited |
| C | Insufficient evidence of efficacy or possible benefits in terms of efficacy do not outweigh the cost or risks (toxicity and drug interactions), valid alternatives are available |
| D | Moderately solid evidence of a lack of efficacy or poor outcome |
| E | Strong evidence of a lack of efficacy or poor outcome |
| Quality of evidence | |
| I | Evidence from at least 1 well-designed and performed trial |
| II | Evidence from at least 1 well-designed non-randomized clinical trial, cohort study, or a non-controlled experimental study with non-conclusive results |
| III | Expert opinion based on clinical experience, descriptive studies, or reports from expert panels |
Bacteriuria is defined according to the criteria proposed by the Infectious Diseases Society of America guidelines. For asymptomatic women, bacteriuria is defined as 2 consecutive voided urine specimens with isolation of the same bacterial strain in quantitative counts ≥105 colony-forming units (cfu)/ml. A single, clean-catch voided urine specimen with 1 bacterial species isolated in a quantitative count ≥105cfu/ml identifies bacteriuria in men. A single catheterized urine specimen with 1 bacterial species isolated in a quantitative count ≥102cfu/ml identifies bacteriuria in women or men. Asymptomatic bacteriuria (AB) is defined by the presence of bacteriuria in the absence of any symptoms of lower or upper UTI.
CystitisCystitis is defined by the presence of bacteriuria and clinical manifestations such as dysuria, frequency, or urinary urgency in the absence of pyelonephritis criteria.
PyelonephritisPyelonephritis is defined by the simultaneous presence of a urine bacteria count ≥105cfu/ml and/or bacteremia and fever with one or more of the following four categories: costovertebral angle pain (if native kidney involved), renal allograft tenderness (if transplanted kidney involved), chills, criteria for cystitis (bacteriuria and clinical manifestations such as dysuria, frequency, or urinary urgency).
ReinfectionReinfection is defined by a new episode of infection with the isolation of bacterium other than the one that caused the previous infection or the same bacteria with a different antibiotic sensitivity pattern.
RelapseRelapse is defined as the isolation of the same microorganism that caused the preceding infection, with the same antibiotic sensitivity pattern, in a urine culture obtained ≥2 weeks after finishing the previous treatment.
Recurrent infectionRecurrent infection is commonly defined as three or more episodes of symptomatic UTIs over a 12-month period or two episodes in the previous six months.
Complicated urinary tract infectionA complicated UTI is defined as an infection that is associated with structural or functional abnormalities of the genitourinary tract, or the presence of an underlying disease that increases the risk of acquiring an infection or of failing therapy.
ProstatitisProstatitis is characterized by discomfort referred to the lower urogenital and perineal and/or ejaculatory discomfort or sexual dysfunction. Acute bacterial prostatitis is presented as fever and chills accompanied by urinary symptoms such as dysuria, frequency, and perineal pain. Chronic bacterial prostatitis has a more prolonged course, usually of at least 3 months. This is usually related to or the result of recurrent urinary infection, or may be a complication of acute prostatitis that is not properly cured, urethritis, or epididymitis. The disease can occur continuously or episodically. The symptoms are milder than in acute prostatitis and sometimes imperceptible. The most common symptoms are perineal or pelvic pain, low back pain, testicular pain, and discomfort when urinating or ejaculating.
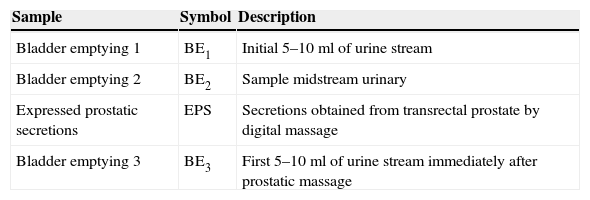
The classification of patients with prostatitis depends on the bacteriological study of lower urinary tract considering sequential urine cultures (Table 2).
Sequential urine cultures for anatomical location within the lower urinary tract.a
| Sample | Symbol | Description |
|---|---|---|
| Bladder emptying 1 | BE1 | Initial 5–10ml of urine stream |
| Bladder emptying 2 | BE2 | Sample midstream urinary |
| Expressed prostatic secretions | EPS | Secretions obtained from transrectal prostate by digital massage |
| Bladder emptying 3 | BE3 | First 5–10ml of urine stream immediately after prostatic massage |
Stamey T. Pathogenesis and Treatment of Urinary Tract Infections. Baltimore: Williams & Wilkins; 1980.
Definitive diagnosis of bacterial prostatitis requires that the number of colonies in the BE3 sample exceeds those in the BE1 sample, preferably by more than 10 times. However, the prostate of many patients with chronic prostatitis contains only small amounts of bacteria. In these patients, a prostatic secretions culture is particularly useful. Microscopic examination of the EPS is useful to identify leukocytes and “oval fat bodies” – large lipid-laden macrophages characteristic of prostatic inflammatory response.
Some risk factors have been described for the development of UTI in SOT recipients (Table 3).
Risk factors for urinary tract infections in solid organ transplantation.
| Female gender |
| Age |
| Mycophenolate mofetil |
| Antithymocyte globulin |
| Need for immediate post-transplant dialysis |
| Ureteral stent placement >30 days |
| Diabetes mellitus |
| Deceased-donor kidneys |
| Number of episodes of acute rejection |
| Reflux kidney disease prior to transplantation |
| Length of hospitalization |
| Length of urinary catheterization |
| Number of episodes of acute rejection |
| Post-transplant urinary obstructions |
| Increase in immunosuppression |
Should SOT recipients receive primary prophylaxis for UTI?
- 1.
Trimethoprim/sulfamethoxazole (TMP/SMX, cotrimoxazole 160–800mg) antibiotic prophylaxis is recommended during the first 3–6 months post-transplant because it significantly decreases AB and symptomatic UTI, and bacteremia in renal transplant recipients (A-I).
- 2.
Antibiotic prophylaxis is not specifically recommended for UTI in non-kidney SOT recipients.
What should be the management of asymptomatic bacteriuria in SOT recipients?
- 3.
Screening for and treatment of AB in kidney transplant recipients (KTR) is recommended in the early postoperative period and up to one month after transplantation (B-III).
- 4.
There is not enough evidence to recommend continued screening for and treatment of AB in a clinically stable KTR beyond one month after transplantation (C-III). However, there is no consensus on whether AB by multidrug resistant (MDR) bacteria, mainly Gram-negative bacilli, should be treated.
- 5.
Screening for and treatment of AB is not currently recommended for other SOT recipients (D-III). In these cases, guidelines for the general population should be applied.
- 6.
Treatment of asymptomatic candiduria is not currently recommended for SOT recipients. Among patients with a urinary catheter, removal of the catheter may be sufficient to eliminate candiduria without specific antifungal therapy (D-III).
- 7.
Urine culture screening of patients awaiting transplantation is not routinely recommended (D-III).
- 8.
Live donors should be screened and treated for bacteriuria before the organ is harvested (A-III).
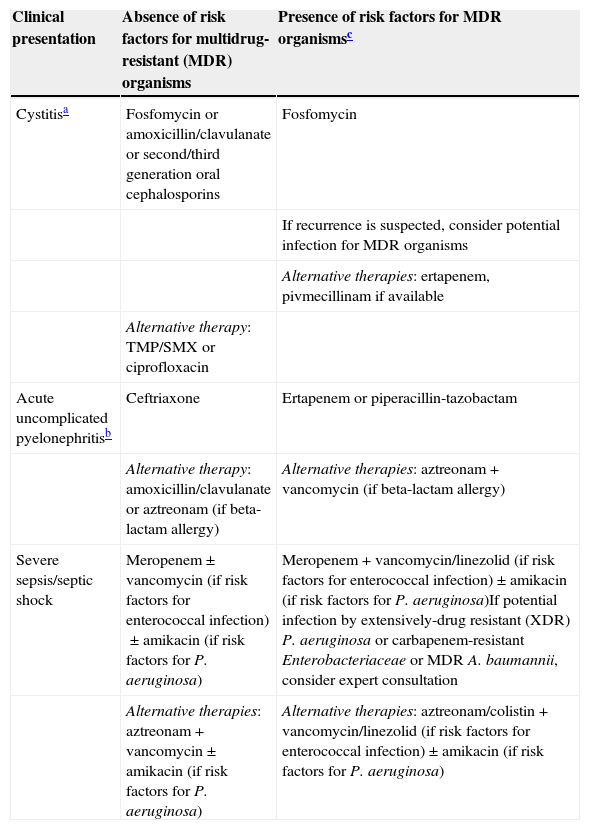
What is the best empirical treatment of UTI in SOT recipients? (Table 4)
Table 4.Empirical treatment of urinary tract infections in solid organ transplant recipients.
Clinical presentation Absence of risk factors for multidrug-resistant (MDR) organisms Presence of risk factors for MDR organismsc Cystitisa Fosfomycin or amoxicillin/clavulanate or second/third generation oral cephalosporins Fosfomycin If recurrence is suspected, consider potential infection for MDR organisms Alternative therapies: ertapenem, pivmecillinam if available Alternative therapy: TMP/SMX or ciprofloxacin Acute uncomplicated pyelonephritisb Ceftriaxone Ertapenem or piperacillin-tazobactam Alternative therapy: amoxicillin/clavulanate or aztreonam (if beta-lactam allergy) Alternative therapies: aztreonam+vancomycin (if beta-lactam allergy) Severe sepsis/septic shock Meropenem±vancomycin (if risk factors for enterococcal infection)±amikacin (if risk factors for P. aeruginosa) Meropenem+vancomycin/linezolid (if risk factors for enterococcal infection)±amikacin (if risk factors for P. aeruginosa)If potential infection by extensively-drug resistant (XDR) P. aeruginosa or carbapenem-resistant Enterobacteriaceae or MDR A. baumannii, consider expert consultation Alternative therapies: aztreonam+vancomycin±amikacin (if risk factors for P. aeruginosa) Alternative therapies: aztreonam/colistin+vancomycin/linezolid (if risk factors for enterococcal infection)±amikacin (if risk factors for P. aeruginosa) In all cases, once culture susceptibility results are available, complete therapy with the most narrow-spectrum antibiotic available.
- 9.
The treatment strategy depends on the time elapsed since transplantation and the severity of the illness (B-III).
- 10.
The choice of empirical antimicrobial agents should be based on local epidemiological data and the patient's history of previous resistant organisms (A-II).
- 11.
Antibiotic therapies prescribed in the previous months should be taken into account (B-III).
- 12.
Review if the patient has recurrent episodes of UTI. The incidence of resistant organisms can rise progressively with the number of episodes (C-III).
- 13.
Especially if resistant organisms are found, expanded antimicrobial testing should be requested from the microbiology lab to identify treatment options for completion of therapy (B-III).
- 14.
Consider removal or replacement of urinary tract instruments such as urethral catheters or urologic stents (B-III).
- 15.
Progression of upper urinary tract disease to a renal or perinephric abscess or emphysematous pyelonephritis usually requires a multidisciplinary approach to treatment, including urologist and/or interventional radiology consultation for percutaneous or surgical drainage of abscesses (A-I).
- 16.
Once culture susceptibility results are available, switch to the narrowest spectrum antibiotic available to complete course of therapy (B-III).
- 17.
Adjust the antibiotic dosage according to the patient's renal function (A-I).
- 18.
In the event of severe infection with sepsis, consider the option of reducing/discontinuing the immunosuppression therapy (B-III).
What is the best definitive treatment of UTI in SOT recipients?
- 19.
To choose an appropriate antibiotic for the treatment of cystitis caused by Enterobacteriaceae, the recommendations for the general population are adapted to organ transplant patients. For hospitalized patients, we recommend using cotrimoxazole or second- or third-generation oral cephalosporin or amoxicillin–clavulanate or fosfomycin trometamol for susceptible strains (B-I). For outpatients, we recommend ciprofloxacin or fosfomycin trometamol (B-I). For the treatment of cystitis caused by extended-spectrum beta-lactamase (ESBL)-producing E. coli, we recommend fosfomycin trometamol (B-I). For cystitis caused by carbapenem-resistant Enterobacteriaceae, we recommend using either fosfomycin trometamol or aminoglycosides (gentamicin or amikacin) (B-II). Contrary to most recommendations for the general population, nitrofurantoin is not recommended as a first-line treatment of cystitis due to the potential occurrence of adverse effects in patients with SOT (D-III).
- 20.
For the treatment of hospitalized patients with acute pyelonephritis caused by Enterobacteriaceae, we recommend the use of a beta-lactam, either third-generation cephalosporins or amoxicillin–clavulanate (B-I). After discharge or in outpatients, we recommend the use of fluoroquinolones (B-I). For pyelonephritis caused by ESBL-producing Enterobacteriaceae, we recommend ertapenem (B-I). Monotherapy with a carbapenem is not recommended for patients with invasive infections caused by carbapenemase-producing Enterobacteriaceae but may be considered in cases of mild invasive infections if adequate source control is readily achieved and the isolate is susceptible (C-III). For patients in which combination therapy is indicated, a regimen with a carbapenem plus one or two fully active drugs (including colistin, an aminoglycoside, or fosfomycin) is recommended if the carbapenem minimum inhibitory concentration (MIC) is ≤8mg/L; this applies mainly to patients with severe infections caused by KPC-producing Klebsiella pneumoniae (B-II). There are not enough data to recommend including a carbapenem in combination regimens if MIC is >8mg/L. If this is the case, carbapenems are probably useless. Particularly if MIC is >16mg/L, we recommend including at least two fully active drugs in the combination regimen according to susceptibility testing results (drugs to be considered: colistin, aminoglycosides, and fosfomycin) (C-III).
- 21.
For the treatment of cystitis caused by Pseudomonas aeruginosa, we recommend ciprofloxacin for susceptible strains (B-III). For pyelonephritis by P. aeruginosa we recommend the use, when possible, of beta-lactams active against P. aeruginosa in hospitalized patients and quinolones in outpatients (B-III). For the treatment of pyelonephritis by multidrug-resistant P. aeruginosa, we recommend colistin or amikacin with monitoring of renal function when no other options are available (C-III).
- 22.
For ampicillin-susceptible enterococci strains, we recommend oral amoxicillin for the treatment of cystitis (B-III) and intravenous ampicillin for the treatment of pyelonephritis (C-III). For ampicillin-resistant Enterococcus faecium, we recommend glycopeptides (C-III). For vancomycin-resistant Enterococcus strains, the treatment should be guided by antibiogram and we recommend the use of quinolones, cotrimoxazole, fosfomycin, nitrofurantoin, and linezolid in order of preference (B-III).
- 23.
For the treatment of infected cysts in patients with renal polycystic disease, we recommend the use of fluoroquinolones or TMP/SMX when possible and percutaneous drainage if necessary (B-III).
- 24.
For the treatment of acute prostatitis we recommend intravenous beta-lactams until apyrexia and consolidation treatment with fluoroquinolones or TMP/SMX when possible (B-I).
How long should SOT recipients receive antibiotics for a UTI?
- 25.
Kidney recipients presenting AB within the first month of transplantation should receive an oral antibiotic selected according to the susceptibility of the isolated microorganism for a period of 5–7 days (BIII). In other SOT recipients, guidelines for the general population should be applied (AII).
- 26.
Cystitis in SOT recipients should be treated for 5–7 days with an oral antibiotic. Early post-transplant cystitis in renal transplant recipients may require longer treatment, especially if a ureteral stent is present (BIII). Short courses of therapy (single dose or three days) have not been studied in SOT recipients (CIII).
- 27.
KTR with allograft pyelonephritis should undergo a 14-day course of antibiotics. However, patients with allograft pyelonephritis in the early post-transplant period presenting with sepsis should be treated for at least 14–21 days (BIII). Late uncomplicated allograft pyelonephritis occurring more than six months after kidney transplantation may be treated with antibiotic therapy for 10–14 days (BIII). At least initially, intravenous antibiotic therapy is recommended in kidney recipients with allograft pyelonephritis (AIII).
- 28.
In non-kidney SOT recipients with uncomplicated pyelonephritis, a 10- to 14-day course of antibiotics is recommended (BIII). At least initially, these patients should be treated with intravenous antibiotics (AIII).
- 29.
No data are available on short courses (7 days) of antibiotic therapy for pyelonephritis in SOT recipients. Therefore, short-term treatment is not recommended in SOT (CIII).
- 30.
For SOT recipients with complicated pyelonephritis, an antibiotic course of at least two weeks is recommended and should be extended until abscesses are adequately drained and patient improvement has been achieved (BIII).
- 31.
For SOT recipients with acute bacterial prostatitis, a 2- to 4-week course of antibiotics is recommended. However, antibiotic therapy can be continued for up to four weeks in patients with severe illness, concomitant bacteremia, and undrained abscesses (BIII).
- 32.
In SOT recipients with polycystic kidney disease and infected cysts, treatment of not less than 14 days is recommended and may be extended depending on patient evolution, cyst diameter, and possibility of drainage (BIII).
What should be the management of UTI caused by Candida spp. in SOT recipients?
What should be the initial diagnostic approach to a SOT recipient with candiduria?
- 33.
SOT recipients with candiduria should be classified according to the presence of risk factors for disseminated candidiasis, indications for obtaining a urine culture (surveillance or infection suspicion), and according to their clinical situation (asymptomatic, with urinary tract symptoms or with general manifestations of sepsis) (A-III).
- 34.
Predisposing risk factors should be eliminated or controlled (antibiotic use, malnutrition, hyperglycemia) and urinary catheters should be removed or at least changed if possible. The presence of candiduria should be verified with a second, clean-voided urine culture (A-II).
- 35.
Disseminated candidiasis should be considered in all hospitalized SOT with candiduria. If clinical manifestations are compatible, blood cultures, a second urine culture after removal or replacement of the urinary catheter, fundoscopy, cultures from any other significant site (vascular accesses, peritoneal fluid, etc.), and a kidney imaging study should be obtained (AII).
- 36.
Patients with persistent candiduria and no indwelling bladder catheter should undergo imaging of the kidneys and collecting system to exclude renal abscess, fungus balls, or other urologic abnormalities (A-II).
- 37.
SOT recipients in whom Candida contamination of the preservation fluid is demonstrated or suspected (donors with ruptured abdominal viscus at the time of multiorgan recovery) should undergo urgent diagnostic evaluation including Doppler ultrasound, blood and urine cultures, and cultures from any other significant site (B-III).
Which patients should receive antifungal drugs?
- 38.
Asymptomatic candiduria in SOT patients that are not neutropenic or undergoing a urologic procedure should not be treated with antifungal therapy (D-II).
- 39.
Candiduria in an unstable SOT should be initially considered as a potential marker of disseminated candidiasis. Prompt effective antifungal therapy has to be provided until an alternative diagnosis is obtained (A-III).
- 40.
Candida cystitis or pyelonephritis should be treated with systemic antifungals for 2–4 weeks (B-III).
- 41.
Fungus balls or casts in the pelvis or urinary bladder need surgery and systemic and/or local antifungal therapy (A-III).
- 42.
KTR with contamination of the preservation fluid or with a donor with digestive tract rupture should receive early effective antifungal therapy (B-II).
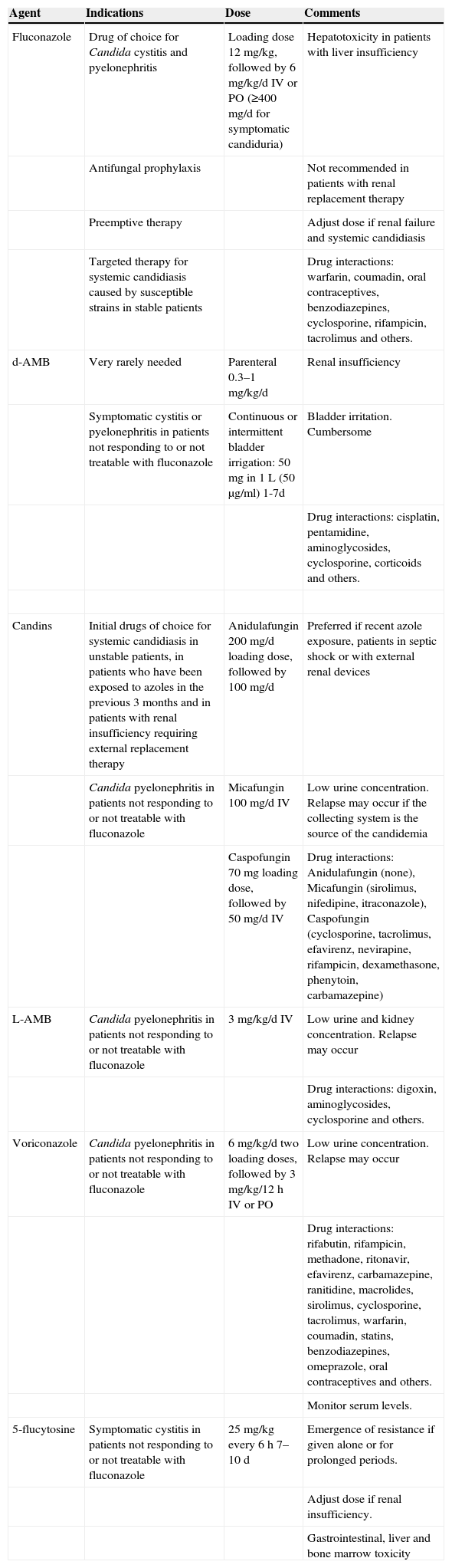
Which drug should be prescribed and for how long? (Table 5)
Table 5.Antifungal drugs.
Agent Indications Dose Comments Fluconazole Drug of choice for Candida cystitis and pyelonephritis Loading dose 12mg/kg, followed by 6mg/kg/d IV or PO (≥400mg/d for symptomatic candiduria) Hepatotoxicity in patients with liver insufficiency Antifungal prophylaxis Not recommended in patients with renal replacement therapy Preemptive therapy Adjust dose if renal failure and systemic candidiasis Targeted therapy for systemic candidiasis caused by susceptible strains in stable patients Drug interactions: warfarin, coumadin, oral contraceptives, benzodiazepines, cyclosporine, rifampicin, tacrolimus and others. d-AMB Very rarely needed Parenteral 0.3–1mg/kg/d Renal insufficiency Symptomatic cystitis or pyelonephritis in patients not responding to or not treatable with fluconazole Continuous or intermittent bladder irrigation: 50mg in 1L (50μg/ml) 1-7d Bladder irritation. Cumbersome Drug interactions: cisplatin, pentamidine, aminoglycosides, cyclosporine, corticoids and others. Candins Initial drugs of choice for systemic candidiasis in unstable patients, in patients who have been exposed to azoles in the previous 3 months and in patients with renal insufficiency requiring external replacement therapy Anidulafungin 200mg/d loading dose, followed by 100mg/d Preferred if recent azole exposure, patients in septic shock or with external renal devices Candida pyelonephritis in patients not responding to or not treatable with fluconazole Micafungin 100mg/d IV Low urine concentration. Relapse may occur if the collecting system is the source of the candidemia Caspofungin 70mg loading dose, followed by 50mg/d IV Drug interactions: Anidulafungin (none), Micafungin (sirolimus, nifedipine, itraconazole), Caspofungin (cyclosporine, tacrolimus, efavirenz, nevirapine, rifampicin, dexamethasone, phenytoin, carbamazepine) L-AMB Candida pyelonephritis in patients not responding to or not treatable with fluconazole 3mg/kg/d IV Low urine and kidney concentration. Relapse may occur Drug interactions: digoxin, aminoglycosides, cyclosporine and others. Voriconazole Candida pyelonephritis in patients not responding to or not treatable with fluconazole 6mg/kg/d two loading doses, followed by 3mg/kg/12h IV or PO Low urine concentration. Relapse may occur Drug interactions: rifabutin, rifampicin, methadone, ritonavir, efavirenz, carbamazepine, ranitidine, macrolides, sirolimus, cyclosporine, tacrolimus, warfarin, coumadin, statins, benzodiazepines, omeprazole, oral contraceptives and others. Monitor serum levels. 5-flucytosine Symptomatic cystitis in patients not responding to or not treatable with fluconazole 25mg/kg every 6h 7–10 d Emergence of resistance if given alone or for prolonged periods. Adjust dose if renal insufficiency. Gastrointestinal, liver and bone marrow toxicity - 43.
Fluconazole is the agent of choice for most patients with Candida UTI due to the high concentration achieved in urine (>100μg/ml, which is 10-fold the simultaneous plasma level) (A-II).
- 44.
Other antifungal agents should only be considered for patients in unstable clinical condition, allergic to fluconazole, or in whom therapy has clearly failed despite maximum fluconazole doses and optimal management of urologic abnormalities or other predisposing conditions (B-III).
- 45.
A single dose of parenteral amphotericin B (AMB) deoxycholate, with or without oral 5-flucytosine, reach high concentrations in urine, and may be used to treat Candida cystitis in patients not responding to or not treatable with fluconazole. Candida pyelonephritis can also be treated with AMB. However, potential kidney toxicity limits its use in the transplant population (B-I).
- 46.
Liposomal AMB, with or without 5-flucytosine, may be used to treat Candida pyelonephritis in patients not responding to or not treatable with fluconazole. However, due to the low concentration reached in urine, a relapse may occur if the collecting system is infected (C-III).
- 47.
AMB deoxycholate bladder irrigation may be used in patients with symptomatic cystitis that cannot be treated with other drugs (C-II).
- 48.
Echinocandins are the preferred initial agents for systemic candidiasis in unstable patients, in patients who have been exposed to azoles in the previous 3 months, and in patients with renal insufficiency requiring external replacement therapy (A-I).
- 49.
Echinocandins achieve low concentrations in the urinary tract but may be used in patients not responding to or not treatable with fluconazole. If the collecting system is infected, relapse may occur (C-III).
- 50.
All symptomatic UTIs due to Candida species in KTR should be considered complicated and treated for at least 14 days (B-II).
What should be the diagnostic–therapeutic management of recurrent UTI in SOT recipients?
- 51.
The diagnostic approach in transplant patients with recurrent UTI must be meticulous in order to rule out the existence of anatomical or functional changes (A-III).
- 52.
If possible, treatment aimed at the sensitivity of the isolated microorganisms must be used in patients with recurrent UTI. TMP/SMX is a good option (B-III). Quinolones must be avoided as empirical therapy (D-II).
- 53.
Duration of antibiotic treatment for recurrent UTIs in transplant patients is not well-defined. At least a 6-week treatment period may be recommendable (B-III), although other authors suggest prolonging treatment more than three months. Indefinite treatment may be evaluated in diabetic patients, patients with a history of UTIs before or soon after transplantation and those receiving high-dose immunosuppressive treatment (equivalent to secondary prophylaxis) (B-II).
- 54.
Anatomical changes related with recurrent UTI must be corrected if possible (A-II).
- 55.
The use of non-antibiotic therapies, such as cranberry extract, l-methionine, topical estrogens, or topical application of Lactobacillus, could be provided to transplant patients with recurrent UTI (C-II).
What role does UTI play in kidney graft rejection or dysfunction?
- 56.
Kidney transplant patients are particularly vulnerable to infections, and this is one of the reasons for which primary prophylaxis has been established (A-I) and early aggressive treatment of symptomatic UTI is recommended (A-II).
- 57.
Although UTI has been associated with induction of acute rejection in kidney transplant patients (A-II), there is controversy about the final impact on the graft in terms of chronic rejection or dysfunction (B-II).
- 58.
Late-onset UTIs, which were traditionally associated with a good prognosis, have also been recently related with a risk of rejection or dysfunction of the kidney graft (B-II).
- 59.
The association between AB and graft loss is unclear.
Antimicrobial and immunosuppressant interactions (Tables 6–11)
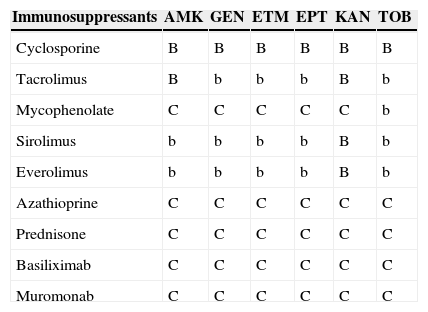
Table 6.Interactions between aminoglycosides and immunosuppressants.a
Immunosuppressants AMK GEN ETM EPT KAN TOB Cyclosporine B B B B B B Tacrolimus B b b b B b Mycophenolate C C C C C b Sirolimus b b b b B b Everolimus b b b b B b Azathioprine C C C C C C Prednisone C C C C C C Basiliximab C C C C C C Muromonab C C C C C C AMK: amikacin; GEN: gentamicin; ETM: streptomycin; EPT: spectinomycin; KAN: kanamycin; TOB: tobramycin.
aA/a: these drugs should not be co-administered; B/b: potential interaction – may require monitoring of plasma levels and graft function and/or change in dose; C/c: no clinically relevant interactions; A, B, C: indicate that interaction has been described; a, b, c: indicate that interaction is based on a prediction guided by the pharmacokinetic characteristics of the product.
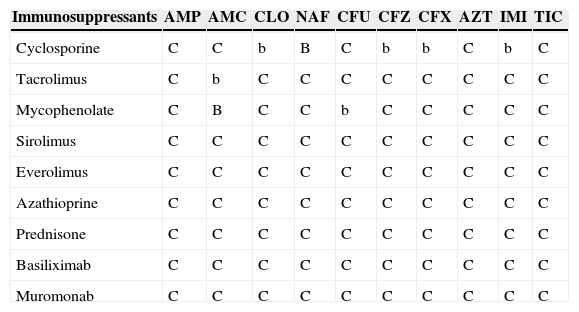
Table 7.Interactions between beta-lactams and immunosuppressants.a
Immunosuppressants AMP AMC CLO NAF CFU CFZ CFX AZT IMI TIC Cyclosporine C C b B C b b C b C Tacrolimus C b C C C C C C C C Mycophenolate C B C C b C C C C C Sirolimus C C C C C C C C C C Everolimus C C C C C C C C C C Azathioprine C C C C C C C C C C Prednisone C C C C C C C C C C Basiliximab C C C C C C C C C C Muromonab C C C C C C C C C C AMP: ampicillin; AMC: amoxicillin–clavulanate; CLO: cloxacillin; NAF: nafcillin; CFU: cefuroxime; CFZ: ceftazidime; CFX: ceftriaxone; AZT: aztreonam; IMI: imipenem; TIC: ticarcillin.
aA/a: these drugs should not be co-administered; B/b: potential interaction – may require monitoring of plasma levels and graft function and/or change in dose; C/c: no clinically relevant interactions; A, B, C: indicate that interaction has been described; a, b, c: indicate that interaction is based on a prediction guided by the pharmacokinetic characteristics of the product.
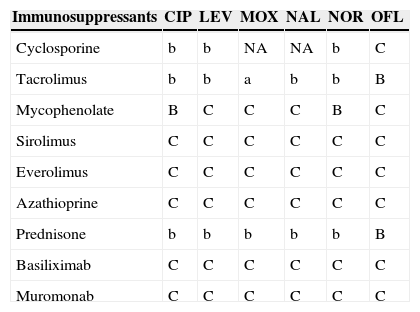
Table 8.Interactions between quinolones and immunosuppressants.a
Immunosuppressants CIP LEV MOX NAL NOR OFL Cyclosporine b b NA NA b C Tacrolimus b b a b b B Mycophenolate B C C C B C Sirolimus C C C C C C Everolimus C C C C C C Azathioprine C C C C C C Prednisone b b b b b B Basiliximab C C C C C C Muromonab C C C C C C CIP: ciprofloxacin; LEV: levofloxacin; MOX: moxifloxacin; NAL: nalidixic acid; NOR: norfloxacin; OFL: ofloxacin.
aA/a: these drugs should not be co-administered; B/b: potential interaction – may require monitoring of plasma levels and graft function and/or change in dose; C/c: no clinically relevant interactions; NA: data not available; A, B, C: indicate that interaction has been described; a, b, c: indicate that interaction is based on a prediction guided by the pharmacokinetic characteristics of the product.
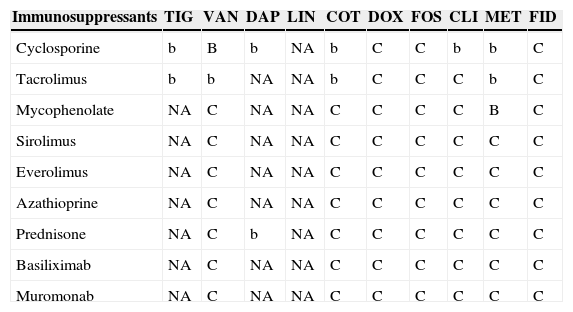
Table 9.Interactions between other antibiotics and immunosuppressants.a
Immunosuppressants TIG VAN DAP LIN COT DOX FOS CLI MET FID Cyclosporine b B b NA b C C b b C Tacrolimus b b NA NA b C C C b C Mycophenolate NA C NA NA C C C C B C Sirolimus NA C NA NA C C C C C C Everolimus NA C NA NA C C C C C C Azathioprine NA C NA NA C C C C C C Prednisone NA C b NA C C C C C C Basiliximab NA C NA NA C C C C C C Muromonab NA C NA NA C C C C C C TIG: tigecycline; VAN: vancomycin; DAP: daptomycin; LIN: linezolid; COT: cotrimoxazole; DOX: doxycycline; FOS: fosfomycin; CLI: clindamycin; MET: metronidazole; FID: fidaxomicin.
NA: Not analyzed.
aA/a: these drugs should not be co-administered; B/b: potential interaction – may require monitoring of plasma levels and graft function and/or change in dose; C/c: no clinically relevant interactions; NA: data not available; A, B, C: indicate that interaction has been described; a, b, c: indicate that interaction is based on a prediction guided by the pharmacokinetic characteristics of the product.
Table 10.Interactions between azoles, echinocandins and immunosuppressants.a
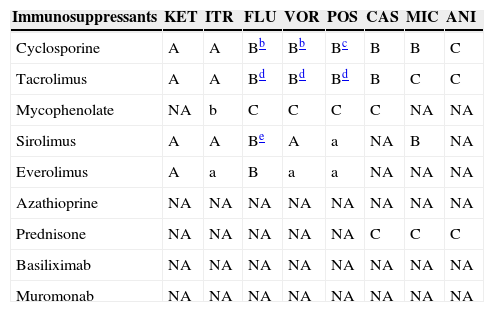
Immunosuppressants KET ITR FLU VOR POS CAS MIC ANI Cyclosporine A A Bb Bb Bc B B C Tacrolimus A A Bd Bd Bd B C C Mycophenolate NA b C C C C NA NA Sirolimus A A Be A a NA B NA Everolimus A a B a a NA NA NA Azathioprine NA NA NA NA NA NA NA NA Prednisone NA NA NA NA NA C C C Basiliximab NA NA NA NA NA NA NA NA Muromonab NA NA NA NA NA NA NA NA KET: ketoconazole; ITR: itraconazole; FLU: fluconazole; VOR: voriconazole; POS: posaconazole; CAS: caspofungin; MIC: micafungin; ANI: anidulafungin.
aA/a: these drugs should not be co-administered; B/b: potential interaction – may require monitoring of plasma levels and graft function and/or change in dose; C/c: no clinically relevant interactions; NA: data not available; A, B, C: indicate that interaction has been described; a, b, c: indicate that interaction is based on a prediction guided by the pharmacokinetic characteristics of the product.
Table 11.Interactions between flucytosine, polyenes and immunosuppressants.a
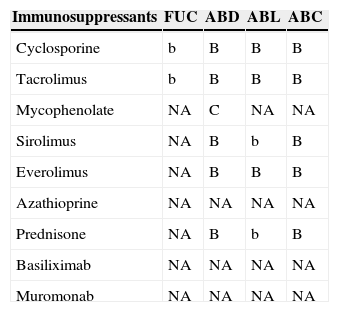
Immunosuppressants FUC ABD ABL ABC Cyclosporine b B B B Tacrolimus b B B B Mycophenolate NA C NA NA Sirolimus NA B b B Everolimus NA B B B Azathioprine NA NA NA NA Prednisone NA B b B Basiliximab NA NA NA NA Muromonab NA NA NA NA FUC: flucytosine; ABD: amphotericin B deoxycholate; ABL: liposomal amphotericin B; ABC: amphotericin lipid complex.
aA/a: these drugs should not be co-administered; B/b: potential interaction – may require monitoring of plasma levels and graft function and/or change in dose; C/c: no clinically relevant interactions; NA: data not available; A, B, C: indicate that interaction has been described; a, b, c: indicate that interaction is based on a prediction guided by the pharmacokinetic characteristics of the product.
- 60.
The treatment of UTIs in SOT recipients is more complex due to interactions between antimicrobials and immunosuppressants.
- 61.
The interactions may jeopardize the transplanted organ and also increase the specific adverse effects of each drug.
- 62.
Key measures to avoid the consequences of these interactions are to know and to prevent them by monitoring the plasma levels of these drugs, monitoring graft function and characteristic adverse effects, and avoiding contraindicated combinations (AII).
JMC has received a conference grant from Astellas, Astra-Zeneca, MSD, Novartis, and Pfizer. All other authors have no conflict of interest to declare.
We thank Jesús Rodríguez-Baño MD, PhD and member of SEIMC for his comments on the manuscript.