Nasal swab culture is used to identify Staphylococcus aureus colonization, as this is a major risk factor for surgical site infection (SSI) in patients who are going to undergo major heart surgery (MHS). We determined nasal carriage of S. aureus in patients undergoing MHS by comparing the yield of a conventional culture with that of a rapid molecular test (Xpert® SA Nasal Complete, Cepheid).
MethodsFrom July 2015 to April 2017, all patients who were to undergo MHS were invited to participate in the study. We obtained two nasal cultures from each patient just before entering the operating room, independently of a previous test for the determination of nasal colonization by this microorganism performed before surgery. One swab was used for conventional culture in the microbiology laboratory, and the other was used for the rapid molecular test. We defined nasal colonization as the presence of a positive culture for S. aureus using either of the two techniques. All patients were followed up until hospital discharge or death.
ResultsOverall, 57 out of 200 patients (28.5%) were colonized by S. aureus at the time of surgery. Thirty-three patients had both conventional culture- and PCR-positive results. Twenty-four patients had a negative culture and a positive PCR test. Only twenty-one percent (12/57) of colonized patients had undergone an attempt to decolonise before the surgical intervention.
ConclusionA significant proportion of patients undergoing MHS are colonized by S. aureus in the nostrils on entering the operating room. New strategies to prevent SSI by this microorganism are needed. Rapid molecular tests immediately before MHS, followed by immediate decolonisation, must be evaluated.
Trial Registration Clinical Trials.gov NCT02640001.
Los cultivos nasales se usan para identificar colonización por Staphylococcus aureus, ya que la colonización es un factor de riesgo para la infección de la herida quirúrgica en pacientes que van a ser sometidos a cirugía cardiaca mayor (CCM). En este trabajo, identificamos portadores de S. aureus en el momento quirúrgico en pacientes que van a ser sometidos a CCM, comparando el resultado del cultivo convencional con un test molecular rápido (Xpert® SA Nasal Complete, Cepheid).
MétodosDesde julio del 2015 hasta abril del 2017, a todos los pacientes que iban a ser intervenidos con CCM se les invitó a participar en el estudio. Se obtuvieron 2 cultivos nasales de cada paciente, justo antes de entrar en el quirófano, independientemente de si había un test previo de colonización nasal realizada. Una torunda fue usada en el laboratorio de microbiología para cultivo convencional y la otra para el test molecular rápido. Se definió colonización nasal como la positividad para S.aureus por cualquiera de las 2 técnicas. Todos los pacientes fueron seguidos hasta el alta hospitalaria o éxitus.
ResultadosUn total de 57 de 200 pacientes (28,5%), estaban colonizados por S. aureus en el momento de la cirugía. En total, 33 pacientes tuvieron ambas muestras positivas (convencional y PCR); 24 pacientes tuvieron cultivo negativo y PCR positiva. Solo el 21% (12/57) de los pacientes colonizados habían tenido un intento de descolonización antes de la cirugía.
ConclusiónUn porcentaje alto de pacientes están colonizados por S. aureus en el momento de ser sometidos a CCM. Son necesarias nuevas estrategias para prevenir la infección de la herida quirúrgica por este microorganismo. Un test molecular rápido inmediatamente antes de la CCM y descolonización posterior inmediata debe ser evaluado.
Trial Registration Clinical Trials.gov NCT02640001.
Nasal carriage of Staphylococcus aureus (whether methicillin-resistant [MRSA] or methicillin-sensitive [MSSA]) before major heart surgery (MHS) is a risk factor for postoperative infection.1–4 Carriers are usually identified by nasal cultures, but results from the microbiology laboratory are available only 24 to 72h after sampling.5
Nasal decolonization of S. aureus carriers is standard of care in many MHS units and consists of nasal mupirocin ointments for five days before surgery.6,7 However, carriage is not always evaluated, and patients can become recolonized after a former decolonization, performed too early. Also, cultures obtained after admission are not usually reported shortly enough to be clinically useful. Finally, given that some patients undergo emergency surgery, it is impossible to determine the real situation of nasal carriage of S. aureus at the time of MHS. Therefore, more rapid and readily available procedures performed immediately before surgery are necessary.
We determined nasal carriage of S. aureus in patients entering the operating room for MHS by comparing the yield of conventional culture with that of a rapid molecular tests in order to assess the real frequency of nasal carriage of S. aureus during surgery.
Material and methodsHospital setting and patientsOur institution is a general referral hospital with 1,550 beds and approximately 50,000 admissions/year. The Department of Heart Surgery performs around 500 procedures per year. Consecutive patients admitted to undergo MHS during the study period (July 2015 to April 2016) were enrolled in the study if they consented to participate.
Prospective quasi-experimental study. All data of patients undergoing MHS were prospectively collected in an anonymous database.
Laboratory procedureSample processing by the microbiology laboratoryOne nasal swab was taken for culture in the microbiology laboratory, and a second for the Xpert® SA Nasal Complete assay. The nasal swab was plated on a mannitol-salt agar plate and a chromogenic medium for the isolation of MRSA (chromID™ MRSA, bioMérieux, Craponne, France), and processed for a semiquantitative count. Plates were incubated for 48h at room temperature.
Samples for PCR were processed according to the manufacturer's instructions, as detailed in previous publications.8,9 For samples testing positive, the amplification threshold cycle for positivity (Ct) was registered.
All patients received daily bathing with clorhexidine-impregnated wipes (Clinell) during ICU stay and oral hygiene care with clorhexidine 0.12%.
Patients colonized by S. aureus received three-daily intranasal mupirocin for 5 days.
The study end-points were as follows:
- -
Primary: frequency of nasal colonization by S. aureus as determined by comparing isolation and PCR diagnostic methods.
- -
Secondary: Rate of colonization by S. aureus (MRSA and MSSA), at the time of surgery.
- -
Comparison of the Ct of the PCR assy and the semiquantitative culture results.
Nasal colonization by S. aureus: a positive PCR result, or a positive culture result with S. aureus in nasal secretions, or both.
Patients were followed up until hospital discharge or death.
Statistical analysisValues are expressed as the mean (standard deviation, SD) or median (interquartile range, IQR) for continuous variables, and as percentages, with a 95% confidence interval (95% CI), when applicable, for categorical variables. Categorical variables were evaluated using the chi-square test or a 2-tailed Fisher exact test. Statistical significance was set at p<0.05 (2-tailed).
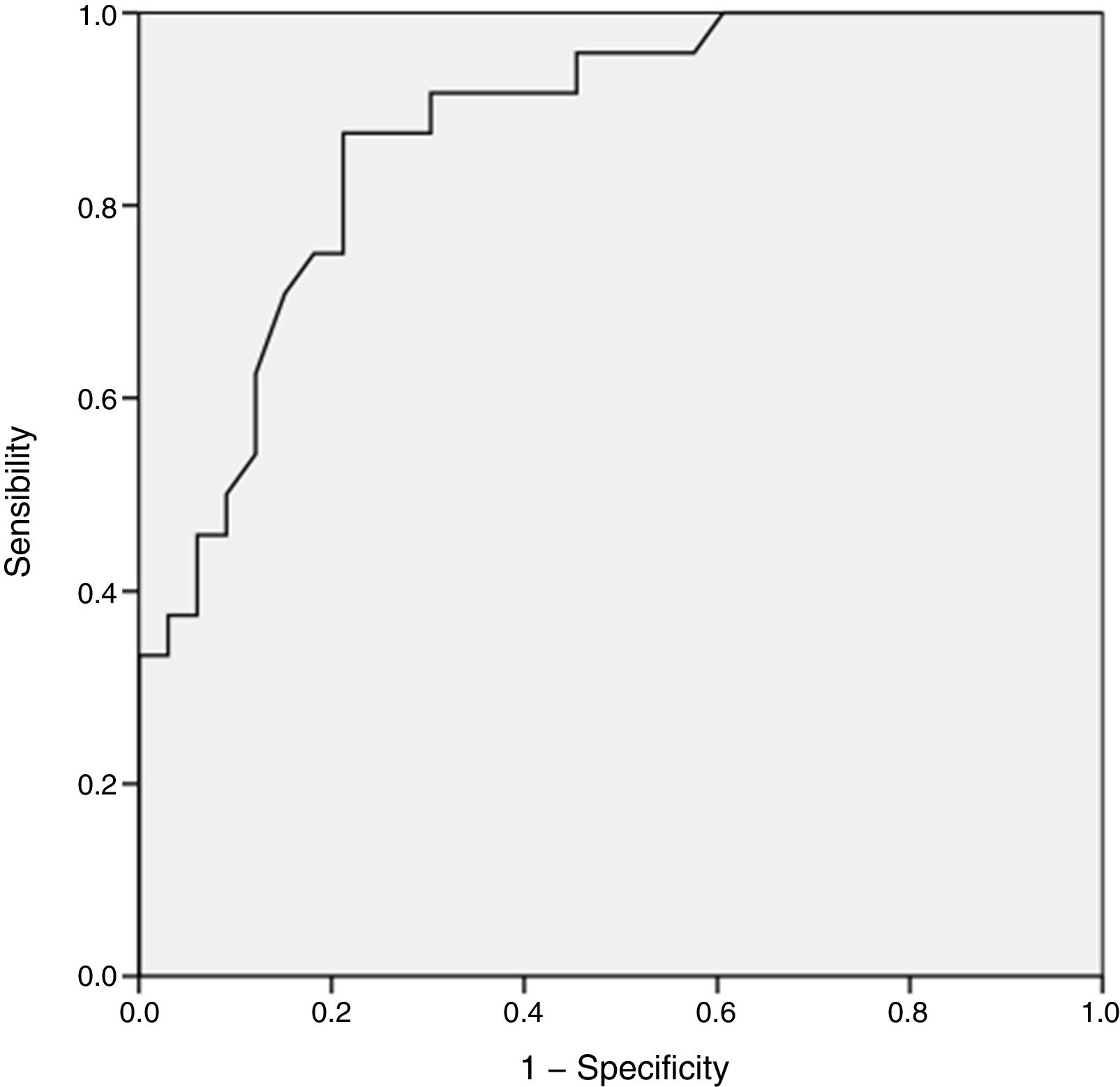
To determine the prediction of positive S. aureus cultures by the number of the Ct, we constructed a ROC curve to determine the optimal cut-off point.
For an area under the ROC curve of 80%, the sample size that gives a precision of 15% with a 95% confidence interval, is 190 patients.
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp, Armonk, New York, USA).
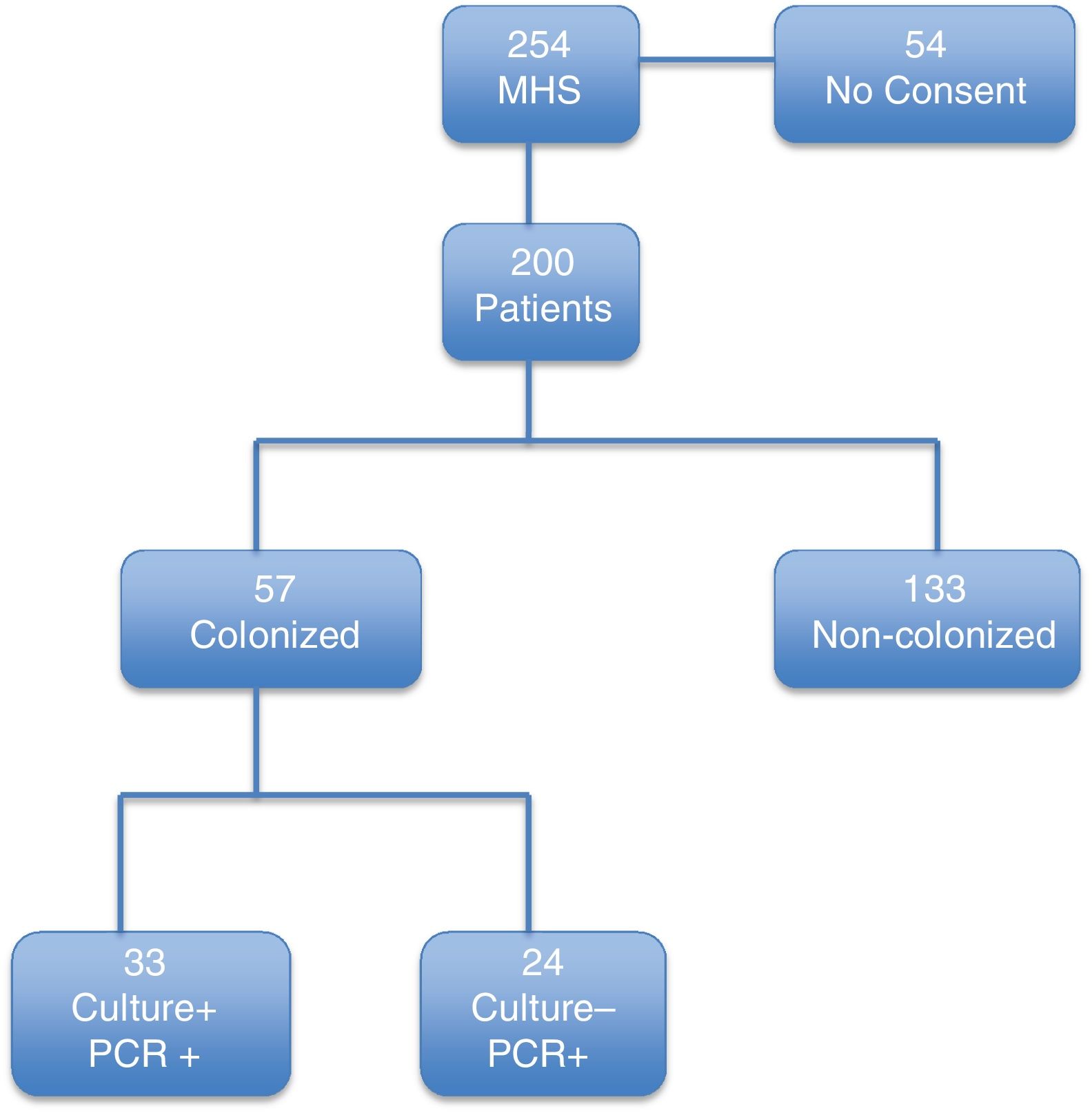
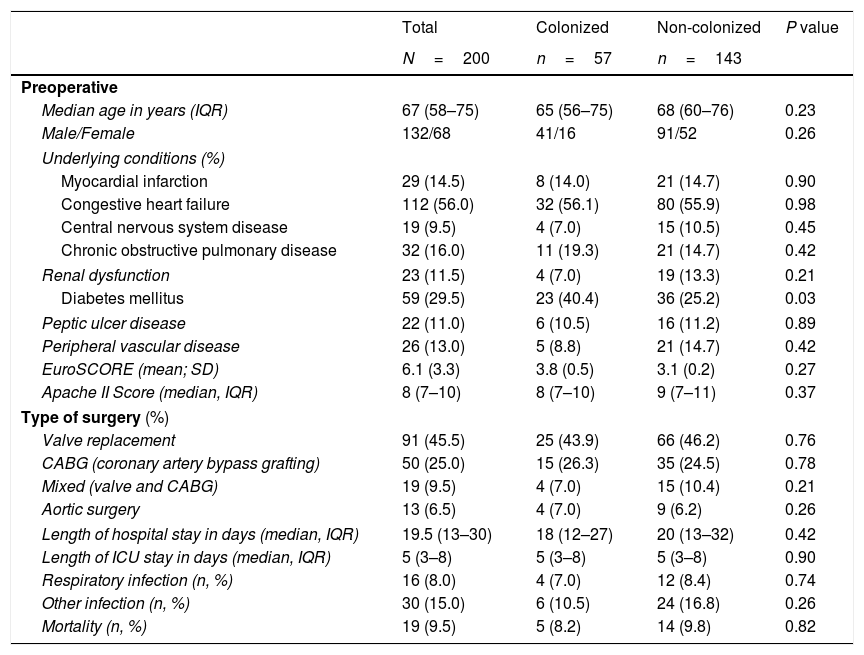
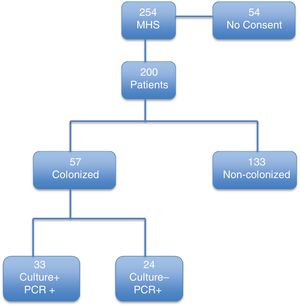
ResultsDuring the study period, 254 patients underwent MHS. Of these, 200 patients fulfilled the inclusion criteria (Fig. 1). The median (IQR) age was 67 (58–75) years. The main underlying conditions were congestive heart failure, diabetes mellitus, chronic obstructive pulmonary disease, and ischemic heart disease. The most frequent surgical procedure was valve replacement (Table 1). The mean (SD) EuroSCORE and median (IQR) of APACHE II score at inclusion were, respectively, 6.1 (3.3) and 8.0 (7–10) (Table 1).
Baseline characteristics and surgical variables of patients.
| Total | Colonized | Non-colonized | P value | |
|---|---|---|---|---|
| N=200 | n=57 | n=143 | ||
| Preoperative | ||||
| Median age in years (IQR) | 67 (58–75) | 65 (56–75) | 68 (60–76) | 0.23 |
| Male/Female | 132/68 | 41/16 | 91/52 | 0.26 |
| Underlying conditions (%) | ||||
| Myocardial infarction | 29 (14.5) | 8 (14.0) | 21 (14.7) | 0.90 |
| Congestive heart failure | 112 (56.0) | 32 (56.1) | 80 (55.9) | 0.98 |
| Central nervous system disease | 19 (9.5) | 4 (7.0) | 15 (10.5) | 0.45 |
| Chronic obstructive pulmonary disease | 32 (16.0) | 11 (19.3) | 21 (14.7) | 0.42 |
| Renal dysfunction | 23 (11.5) | 4 (7.0) | 19 (13.3) | 0.21 |
| Diabetes mellitus | 59 (29.5) | 23 (40.4) | 36 (25.2) | 0.03 |
| Peptic ulcer disease | 22 (11.0) | 6 (10.5) | 16 (11.2) | 0.89 |
| Peripheral vascular disease | 26 (13.0) | 5 (8.8) | 21 (14.7) | 0.42 |
| EuroSCORE (mean; SD) | 6.1 (3.3) | 3.8 (0.5) | 3.1 (0.2) | 0.27 |
| Apache II Score (median, IQR) | 8 (7–10) | 8 (7–10) | 9 (7–11) | 0.37 |
| Type of surgery (%) | ||||
| Valve replacement | 91 (45.5) | 25 (43.9) | 66 (46.2) | 0.76 |
| CABG (coronary artery bypass grafting) | 50 (25.0) | 15 (26.3) | 35 (24.5) | 0.78 |
| Mixed (valve and CABG) | 19 (9.5) | 4 (7.0) | 15 (10.4) | 0.21 |
| Aortic surgery | 13 (6.5) | 4 (7.0) | 9 (6.2) | 0.26 |
| Length of hospital stay in days (median, IQR) | 19.5 (13–30) | 18 (12–27) | 20 (13–32) | 0.42 |
| Length of ICU stay in days (median, IQR) | 5 (3–8) | 5 (3–8) | 5 (3–8) | 0.90 |
| Respiratory infection (n, %) | 16 (8.0) | 4 (7.0) | 12 (8.4) | 0.74 |
| Other infection (n, %) | 30 (15.0) | 6 (10.5) | 24 (16.8) | 0.26 |
| Mortality (n, %) | 19 (9.5) | 5 (8.2) | 14 (9.8) | 0.82 |
According to our definition (PCR- or culture-positive), 57 of 200 patients (28.5%) were colonized at the time of surgery. Of these, 24 cases were positive only by the molecular test and had a negative culture result. The remaining 33 patients had both culture- and PCR-positive results.
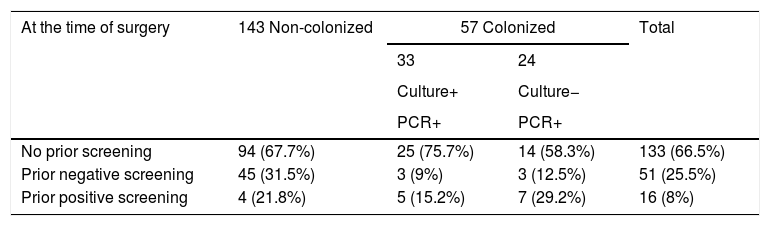
Patients included in the study.
| At the time of surgery | 143 Non-colonized | 57 Colonized | Total | |
|---|---|---|---|---|
| 33 | 24 | |||
| Culture+ | Culture− | |||
| PCR+ | PCR+ | |||
| No prior screening | 94 (67.7%) | 25 (75.7%) | 14 (58.3%) | 133 (66.5%) |
| Prior negative screening | 45 (31.5%) | 3 (9%) | 3 (12.5%) | 51 (25.5%) |
| Prior positive screening | 4 (21.8%) | 5 (15.2%) | 7 (29.2%) | 16 (8%) |
Column percentages are given.
Of the 57 patients colonized with S. aureus, 5 were MRSA (8.8%).
Patients with concordant resultsThe 33 patients with positive S. aureus cultures at the time of surgery were distributed as follows: 25 had not been previously screened (4 because of emergency surgery), 3 had had a pre-admission nasal test that was negative a median of 13 days before surgery (range 6.0–35.09) and, consequently, were not offered decolonization. The remaining 5, had previously been decolonized a median of 11 days before surgery (range 5.0–26.5).
Patients with discordant resultsA total of 24 patients were PCR-positive and culture-negative. Of these, 7 had previously been culture-positive and were decolonized (a median of 9 days before surgery range 8.0–22.0), 14 had never been screened before surgery (3 had undergone emergency surgery), and 3 had had a negative result in a nasal screening at a median of 13 days before surgery (range 11.0–32.0).
Non-colonized patientsThe situation of the 143 non-colonized patients was as follows: 45 had previously had a negative nasal culture result, 4 were positive, and 94 had never been screened (20 because of emergency surgery).
Semiquantitative cultures and PCR amplification cyclesPatients with positive cultures had PCR Ct ranging from 19.9 to 32.7 (median 26.10 (23.5–28.3)), while patients with negative cultures had Ct ranging from 25.7 to 35.0 (median 30.9 (29.2–33.5); p<0.001).
Analysis of the semiquantitative cultures in the 33 patients with both positive PCR and culture results revealed a range of 103 to 105 colony-forming units (CFUs) per plate.
We also recorded a correlation between Ct for positivity of cultures and CFUs (Spearman rho, 0.60; p<0.001).
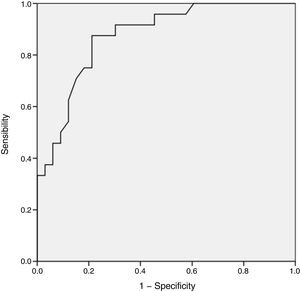
The ROC curve, which had a cut-off Ct of 32 for a positive result (area under the curve, 0.87; 95% CI, 0.78–0.96) showed a sensitivity of 99.7% and specificity of 64.0% for predicting positive cultures (Fig. 2).
Outcome of casesAll patients with either a positive nasal PCR result or a positive nasal culture result were decolonized with nasal mupirocin for 5 days on leaving the operating room.
There were three post-operative infections caused by S. aureus. In one of the three infections, the results of screening were negative before, during, and after surgery, and the patient was never decolonized. The remaining two patients were PCR-positive and culture-negative at the time of surgery and were decolonized.
Two patients had post-surgical deep wound infection (mediastinitis) and the third developed ventilator-associated pneumonia. Two of the 3 infections occurred in previously decolonized patients and occurred on days +50 and +20 after surgery.
None of the S. aureus isolated from nasal samples of our patients had high level resistance to mupirocin. High-level resistant to mupirocin in our institution keeps under 2%.
DiscussionOur study shows that, despite the existence of a program for S. aureus nasal screening and decolonization in patients undergoing MHS at our center, a high proportion of patients are colonized on entering the operating room.
Between 20% and 30% of the population carry S. aureus in the upper airway10,11 and decolonization programs have been associated with a reduction in the incidence of postoperative staphylococcal infections in patients undergoing clean surgery.6,12–15
Decolonization is performed at different times before surgery, yet we were not able to find data showing how frequently decolonization fails owing to circumstances such as recolonization after an original negative test result or after decolonization. Furthermore, patients who are undergoing imminent MHS are not able to benefit from decolonization if they have to wait for the results of a nasal culture.
The process of decolonization often depends on the degree of adherence by physicians, patients or relatives.16,17
The decolonization process should last for a minimum of five days and start before surgery. However, reported data suggest that shorter interventions or interventions started immediately after surgery may be worthwhile.12,18 We decolonized all patients based on a positive result in either culture or PCR and had only three staphylococcal severe infections in the 200 study patients, 1 of whom had never been colonized in the nares or carried S. aureus in the airway.
A PCR rapid screening method allows for the identification of colonized patients immediately before surgery and to start then the decolonization process. We found that the PCR amplification cycle can reasonably anticipate the presence of a positive nasal culture, as already found by other groups.19,20
The results of culture-based and nucleic acid amplification tests (NAATs) are sometimes discordant, and the interpretation of a PCR-positive, culture-negative result is challenging. Some explanations of “false” positive results may include the presence of non-viable organisms, as NAATs do not confirm the presence of live microorganisms. Also, low bacterial densities in the nostrils can produce a negative culture and a positive PCR, or vice versa, because this will cause both methods to provide sporadic positive or negative results under the parameters described by the statistical phenomenon known as the Poisson effect. Besides, patients with a positive PCR and a negative nasal culture can be colonized elsewhere in the body.21
Conventional, culture-based techniques, take 24–72h to provide a significant result, and NAATs provide a result in less than 3h. PCR-based techniques are also considered to be more sensitive than culture, although the frequency of discordant results (PCR-positive and culture-negative) is estimated at between 2.4% and 13.7%, as in our study.22,23 We were not able to find information on proper interpretation of a discordant test result in patients who had recently undergone decolonization.
One of the limitations of this study is that our results cannot be extrapolated to areas other than MHS. In addition, this study has been carried out in a single center and may not reflect the situation of other units where decontamination is done systematically.
A high proportion of patients undergoing MHS are colonized with S. aureus when they enter the operating room despite programs to address this problem and previous decolonization attempts. The value of a rapid PCR-based assessment of nasal colonization immediately after admission to hospital, even in the immediate preoperative period, followed by decolonization after surgery, should be evaluated to assess the clinical impact of this “delayed” complementary approach.
ConclusionA significant proportion of patients undergoing major heart surgery are colonized by S. aureus in the nares on entering the operating room. New strategies to prevent surgical site infection by this microorganism, including molecular tests immediately before surgery followed by decolonization, must be evaluated.
FundingSupported in part by CIBER de Enfermedades Respiratorias-CIBERES (CB06/06/0058), Madrid, Spain, the Rafael del Pino Foundation, and by grants from the Fondo de Investigacion Sanitaria of the Instituto de Salud Carlos III (FIS PI10/02869), which was partially financed by the European Regional Development Fund (FEDER) “A way of making Europe”. Cepheid® (Sunnyvale, US) supplied the Xpert® SA Nasal Complete cartridges.
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors’ contributionsEB and MJPG participated in the conception and design of the study, carried out the analysis, interpreted the data, and drafted the manuscript. AB, JH, JMB and PM participated in the conception and design of the study, performed the statistical analysis, and participated in the drafting of the manuscript. TV and VDE collected the samples and data and participated in drafting the manuscript. All of the authors read and approved the final version of the manuscript.
Conflict of interestsThe authors declare no conflict of interest.
We thank Thomas O’Boyle for his help in the preparation of the manuscript.