To study antibiotic susceptibility in bacterial keratitis (BK), its profile over 10 years and its influence on ophthalmological practice.
MethodsRetrospective review of BK with positive corneal scraping over a 10-year period. Risk factors for keratitis, visual acuity (VA), empirical topical treatment, corneal infection characteristics and outcomes were analyzed for BK due to Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, Pseudomonas aeruginosa and Propionibacterium acnes.
Results389 positive corneal scrapings were collected. All Gram-positive bacteria were susceptible to vancomycin. P. aeruginosa demonstrated >90% sensitivity to the most-commonly-used topical antibiotics. Susceptibility to methicillin was 90.2% for S. aureus and 66.3% for S. epidermidis. The results of 215 patients were available. 1.9% required enucleation and 2.8% required surgical treatments. Final VA improved after treatment in keratitis due to S. aureus (p=0.026) and S. epidermidis (p=0.005). There was a correlation between S. aureus resistance to methicillin (p=0.002) and levofloxacin (p=0.043) and enucleation (20% and 10%, respectively) compared with a 0% rate of enucleation in S. aureus-susceptible keratitis.
ConclusionsBK due to S. pneumoniae is very aggressive irrespective of antibiotic sensitivity. S. aureus was frequently isolated in patients with systemic diseases. It causes severe keratitis and remains moderately resistant to methicillin and levofloxacin. For this reason, keeping vancomycin in empirical regimens is believed to be necessary.
Estudiar la susceptibilidad antibiótica en queratitis bacteriana (QB), el perfil temporal a lo largo de 10 años y su influencia en la clínica ocular.
MétodosRevisión retrospectiva durante un periodo de 10 años de QB con raspado corneal positivo. Se analizaron los factores de riesgo de queratitis, la agudeza visual (AV), el tratamiento empírico tópico, las características de la infección corneal y el resultado clínico para QB por Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, Pseudomonas aeruginosa y Propionibacterium acnes.
ResultadosSe recogieron 389 raspados corneales positivos. Todas las bacterias grampositivas fueron susceptibles a la vancomicina. P. aeruginosa presentó sensibilidad mayor del 90% a los antibióticos tópicos más comúnmente utilizados. La susceptibilidad a la meticilina fue del 90,2% para S. aureus y del 66,3% para S. epidermidis. Los resultados clínicos estaban disponibles para 215 pacientes. El 1,9% requirieron enucleación y el 2,8% tratamientos quirúrgicos. La AV final mejoró después del tratamiento en queratitis por S. aureus (p=0,026) y por S. epidermidis (p=0,005). Hubo correlación entre la resistencia de S. aureus a la meticilina (p=0,002) y levofloxacino (p=0,043) y enucleación (20 y 10%, respectivamente) en comparación con una tasa de enucleación del 0% en S. aureus susceptible.
ConclusionesLas QB por S. pneumoniae son muy agresivas independientemente de la sensibilidad antibiótica. S. aureus se aisló con frecuencia en pacientes con enfermedades sistémicas, causa queratitis severa y permanece moderadamente resistente a la meticilina y a levofloxacino; debido a esto, consideramos necesario mantener la vancomicina en la pauta empírica.
Bacterial infectious keratitis (BK) is a common reason of consulting in ophthalmology and is associated with high morbidity. All cases of moderate to severe keratitis require a detailed laboratory work-up, which ensures that if there is partial or no response to initial therapy, the antimicrobial treatment can be modified based on the results of culture and susceptibility tests. Generally, broad-spectrum antibiotics are used as empiric first-line treatment for presumed BK after obtaining appropriate corneal scrapes. The drugs chosen as initial therapy are either commercially available quinolone or a combination of fortified antibiotics, topical solutions prepared from parenteral antibiotics by reconstituting them with sterile injection water1 or Balanced Salt Solution (BSS)2 with one agent largely directed against Gram-positive and the other against Gram-negative organisms.
The maintenance of the effectiveness of the empiric therapy requires a low resistance rate of the bacteria that can cause keratitis. Longitudinal epidemiologic studies provide clinicians with vital information on the changing microbiological pattern of keratitis in their specific area concerning causative organisms and their antibiotic sensitivities,3–5 which is critical for choosing the most suitable empirical regimen. These descriptive studies are gaining importance because monotherapy quinolone is currently being proposed as the main empirical option for bacterial keratitis although caution seems advisable when using monotherapy for any serious bacterial corneal ulcer.6
The main purpose of this study is to know the causative bacteria of corneal infection in our area, and their susceptibility profile. Secondary purpose are to analyze the temporal profile of the antibiotic susceptibility to the most relevant topical antibacterial agents, to describe the clinical presentation of the most frequent bacterial keratitis groups and to study if antibiotic resistance has any association with the clinical outcome. Significant trends in these factors over the 10 years of the study were sought to help to select the most convenient empiric initial regimen for BK in our area.
MethodsA retrospective audit was performed of the isolate records of different episodes of patients with symptoms and biomicroscopic signs of BK who had a positive corneal scrape from January 2006 to December 2015 at a tertiary Hospital in Madrid, Spain. All the corneal smears and cultures were typically indicated in our hospital in cases of corneal infiltrates with at least one of the following criteria: dense infiltrate, epithelial ulcer of central location, association with anterior chamber cells 1+ (10 cells or greater in a 1-mm beam), absence or partial response to broad spectrum antibiotic therapy and or any infiltrate with clinical features suggestive of fungal, amoebic or mycobacterial keratitis. As well, samples were obtained usually in case of infiltrates or epithelial ulcers in relation with contact lens (CL) users.
Specimens were collected in the cornea unit, emergency room or surgery room by corneal scraping procedure and direct inoculation onto the appropriate culture media by the 24h on call microbiologist at any day or night time. Gram stains were immediately performed on smears, whereas blood, chocolate blood agar plate with hemin and vitamin K1 and Sabouraud agar plates were inoculated. Incubation time for plates varied between 7 and 10 days from blood and chocolate agar plates, and up to 4 weeks for Sabouraud agar plates. Blood and chocolate agar plates were incubated in carbon dioxide environments at 35°C, whereas two Sabouraud agar plates were incubated in oxygen environments at 25°C and 35°C. A second blood agar plate with hemin and vitamin K1 was incubated in an anaerobic environment at 35°C for 14 days.
To exclude accidental contaminants, the minimum criterion for a positive culture was the growth of at least 3 colonies on one solid medium with similar morphology to the Gram stain, if this was positive. Cultures that isolated multiple organisms were analyzed separately. Antibiotic resistance was determined by broth microdilution to determinate the Minimum Inhibitory Concentrations. The antibiotics tested for the isolated microorganisms are described in Table 1. The interpretations for sensitive, intermediate and resistant were in accordance with EUCAST 4.02014 standards.7
Antibiotics tested for isolated microorganisms.
| Isolated bacteria | Isolated bacteria | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abiotrophia defectiva | P | CTX | MER | VAN | ERI | CLI | LEV | RI | |||||||||||||||||||
| Acinetobacter baumannii | CF | CTX | IMI | MER | DOR | COL | G | TO | AK | TIG | CIP | LEV | T/S | NIT | |||||||||||||
| Alcaligenes faecalis | TIC | A/S | P/T | CF | CTZ | CFP | C/C | AZT | IMI | MER | COL | G | TO | AK | NET | MIN | CIP | LEV | TS | FOS | |||||||
| Arthrobacter aurescens | OX | A/C | CX | VAN | G | TO | AK | ERI | CLI | TE | CIP | LEV | T/S | FOS | |||||||||||||
| Corynebacterium macginleyi | P | AM | CFX | CTX | CFP | MER | VAN | TEI | ERI | CLI | TE | CIP | T/S | RI | LEV | ||||||||||||
| Corynebacterium propinquum | P | CTX | CFP | S | VAN | ERI | CLI | TE | CIP | T/S | RI | ||||||||||||||||
| Corynebacterium pseudodiphthericum | P | AM | A/C | CFX | CTX | CFI | CFP | MER | VAN | TEI | ERI | CL | CLI | TE | CIP | LEV | T/S | C | RI | ||||||||
| Corynebacterium spp. | P | CXT | CFP | MER | VAN | ERI | CLI | TE | CIP | T/S | RI | ||||||||||||||||
| Corynebacterium tuberculostearicum | P | MER | VAN | ERI | CLI | CIP | T/S | RI | |||||||||||||||||||
| Eikenella corrodens | BLA | AMX | |||||||||||||||||||||||||
| Enterobacter cloacae | TIG | AM | A/C | P/T | CF | CFZ | CFX | CX | CTX | CTZ | CFP | C/C | C/C | AZT | ETP | IMI | COL | G | TO | AK | AL | CIP | T/S | NIT | |||
| Enterococcus faecalis | P | AM | OX | CX | A/C | VAN | TEI | DAP | G | TO | AK | 100 | G5 | ERI | CLI | Q/D | LNZ | MIN | TE | CIP | LEV | T/S | FOS | NIT | RI | ||
| Escherichia coli | AMX | A/C | P/T | CF | CFX | CX | CTX | CTZ | CFP | C/C | C/C | AZT | ETP | IMI | MER | COL | G | TO | AK | MIN | NAL | CIP | T/S | FOS | NIT | ||
| Granulicatella adiacens | P | CTX | MER | VAN | ERI | CLI | LEV | RI | |||||||||||||||||||
| Haemophilus influenzae | BLA | P | AM | AMX | A/C | CFX | CTX | CFI | CFP | MER | VAN | TEI | ERI | CL | CLI | TE | CIP | LEV | T/S | C | RI | ||||||
| Lactobacillus gasseri | TIC | A/S | P/T | CF | CTZ | CFP | AZT | IMI | MER | COL | G | TO | AK | NET | MIN | NAL | CIP | LEV | T/S | FOS | NIT | ||||||
| Moraxella lacunata | BLA | P | AM | AMX | A/C | CFX | CTX | CFI | CFP | MER | VAN | TEI | ERI | CL | CLI | LEV | TE | CIP | T/S | C | RI | ||||||
| Paenibacillus spp. | P | AM | OX | A/C | VAN | G | 100 | G5 | ERI | CLI | LEV | TS | FOS | ||||||||||||||
| Propionibacterium acnes | P | AMX | PIP | A/C | P/T | CX | IMI | CLI | C | MTR | |||||||||||||||||
| Proteus mirabilis | TIG | AM | A/C | P/T | CF | CFZ | CFX | CX | CTX | CTZ | CFP | C/C | C/C | AZT | ETP | IMI | COL | G | TO | AK | NAL | CIP | T/S | NIT | |||
| Pseudomonas aeruginosas | TIC | A/S | P/T | CF | CTZ | CFP | AZT | IMI | MER | COL | G | TO | AK | NET | MIN | NAL | CIP | LEV | T/S | FOS | NIT | ||||||
| Serratia marcescens | TIG | AM | A/C | P/T | CF | CFZ | CFX | CX | CTX | CTZ | CFP | AZT | ETP | IMI | COL | G | TO | AK | NAL | CIP | T/S | NIT | |||||
| Staphilococcus hominis | P | AM | OX | A/C | VAN | TEI | DAP | G | TO | AK | 100 | G5 | ERI | CLI | Q/D | LNZ | AFU | MUP | MIN | LEV | T/S | FOS | NIT | RI | |||
| Staphylococcus aureus | P | AM | OX | A/C | CX | VAN | TEI | DAP | G | TO | AK | 100 | G5 | ERI | CLI | Q/D | LNZ | AFU | MUP | MIN | TE | CIP | LEV | T/S | FOS | NIT | RI |
| Staphylococcus capitis | P | AM | OX | A/C | CX | VAN | TEI | DAP | G | TO | AK | ERI | CLI | LNZ | AFU | MUP | TE | CIP | LEV | T/S | FOS | NIT | RI | ||||
| Staphylococcus cohnii | P | OX | A/C | CX | VAN | TEI | DAP | G | TO | AK | ERI | CLI | LNZ | AFU | MUP | TE | CIP | LEV | T/S | FOS | NIT | RI | |||||
| Staphylococcus epidermidis | P | AM | OX | A/C | VAN | TEI | DAP | G | TO | AK | 100 | G5 | ERI | CLI | Q/D | LNZ | AFU | MUP | MIN | LEV | T/S | FOS | NIT | RI | |||
| Staphylococcus haemolyticus | P | AM | OX | A/C | VAN | TEI | DAP | G | TO | AK | 100 | G5 | ERI | CLI | Q/D | LNZ | AFU | MUP | TE | CIP | MIN | LEV | T/S | FOS | NIT | RI | |
| Staphylococcus intermedius | P | AM | A/C | CFX | CTX | CFI | CFP | MER | VAN | TEI | ERI | CL | CLI | TE | CIP | LEV | T/S | C | RI | ||||||||
| Staphylococcus warneri | P | AM | OX | A/C | VAN | TEI | DAP | G | TO | AK | 100 | G5 | ERI | CLI | Q/D | LNZ | AFU | MUP | MIN | LEV | T/S | FOS | NIT | RI | |||
| Stenotrophomonas maltophila | AMX | A/C | P/T | CF | CFX | CX | CTX | CTZ | CFP | C/C | C/C | AZT | ETP | IMI | MER | COL | G | TO | AK | MIN | CIP | T/S | FOS | ||||
| Streptococco gordonii | P | AM | AC | CFX | CTX | CFR | CFP | MER | VAN | ERI | CL | CLI | T/S | ||||||||||||||
| Streptococcus agalactiae | P | AM | A/C | CFX | CTX | CFI | CFP | MER | VAN | TEI | ERI | CL | CLI | TE | LEV | T/S | C | RI | |||||||||
| Streptococcus anginosus | TIC | A/S | P/T | CF | CTZ | CFP | AZT | IMI | MER | COL | G | TO | AK | NET | MIN | NAL | CIP | LEV | T/S | FOS | NIT | ||||||
| Streptococcus constellatus | P | AM | A/C | CFX | CTX | CFI | CFP | MER | VAN | TEI | ERI | CL | CLI | TE | CIP | LEV | T/S | C | RI | ||||||||
| Streptococcus mitis | P | AM | A/C | CFX | CTX | CFI | CFP | MER | VAN | TEI | ERI | CL | CLI | TE | CIP | LEV | T/S | C | RI | ||||||||
| Streptococcus oralis | P | AM | A/C | CFX | CTX | CFI | CFP | MER | VAN | TEI | ERI | CL | CLI | TE | CIP | LEV | T/S | C | RI | ||||||||
| Streptococcus pneumoniae | P | AM | A/C | CFX | CTX | CFP | MER | VAN | TEI | ERI | CL | CLI | TE | CIP | LEV | T/S | C | RI | |||||||||
| Streptococcus pyogenes | P | AM | A/C | CFX | CTX | CFI | CFP | MER | VAN | TEI | ERI | CL | CLI | TE | CIP | LEV | T/S | C | RI | ||||||||
| Streptococcus salivarius | P | AM | A/C | CFX | CTX | CFI | CFP | MER | VAN | TEI | ERI | CL | CLI | TE | CIP | LEV | T/S | C | RI | ||||||||
| Streptococcus sanguinis | P | AM | A/C | CFX | CTX | CFI | CFP | MER | VAN | TEI | ERI | CL | CLI | TE | CIP | LEV | T/S | C | RI | ||||||||
| Streptococcus viridans | P | AM | A/C | CFX | CTX | CFI | CFP | MER | VAN | TEI | ERI | CL | CLI | TE | CPI | LEV | T/S | C | RI | ||||||||
Risk factors for keratitis, initial and final visual acuity (VA) (final VA was the VA on the last visit before medical discharge for the episode), topical empirical treatment, characteristics of the corneal infection and clinical outcome were analyzed from patients with a positive corneal scrape for Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, Pseudomonas aeruginosa and Propionibacterium acnes keratitis. Influence of antibiotic susceptibility to clinical outcome (enucleation, corneal surgery and number of days of antibiotic treatment) was analyzed.
The statistical analysis was performed using SPSS 11.5.8 Causative bacteria of keratitis and their susceptibility profile were described with their relative frequency and counts. Lineal by lineal association test was used to analyze the temporal profile of the antibiotic susceptibility to the most relevant topical antibacterial agents between 3 periods, over the 10 years of the study. For the description of the keratitis episodes clinical data, quantitative variables were described with their mean and the standard deviation (SD) and the days of treatment variable with median and interquartile range. The qualitative variables were described with their relative frequency and counts. Finally, to study if antibiotic resistance has any effect in the clinical outcome, a comparative statistical analysis has been done: the Fisher Test was used in 2×2 cross tables, in any other case Chi-square was considered. For quantitative variable (days of treatment) Mann–Whitney test was used. To compare the two related samples of visual acuity (VA), the Wilcoxon Test was used.
ResultsIsolates from patients with bacterial keratitisOf a total of 389 positive bacterial corneal scrapings, Gram-positive bacteria (n=305, 78.4%) were the most common group of organisms; predominantly Gram-positive cocci of the genera Staphylococcus and Streptococcus. Gram-negative bacteria grew in 85 cultures (21.9%), being Pseudomonas aeruginosa (n=38; 9.7%) the most common isolate. The Gram-positive bacilli were the second group in frequency, being P. acnes (n=32, 8.2%) and Corynebacterium macginleyi (n=27, 6.9%) the most common isolates (Table 2).
Isolated bacteria in corneal scrapps in patients with bacterial keratitis from January 2006 to December 2015. Each isolated bacterium is counted separately and the total number of isolates is indicated. The associations are described at the end of the table.
| Isolated bacteria | N° (%) |
|---|---|
| Gram-positive cocci | 225 |
| Staphylococci (173) | |
| Staphylococcus epidermidis | 104 (27.01) |
| Staphylococcus aureus | 51 (13.24) |
| Staphylococcus capitis | 6 (1.55) |
| Staphylococcus warneri | 4 (1.03) |
| Staphylococcus haemolyticus | 4 (1.03) |
| Staphylococcus intermedius | 2 (0.51) |
| Staphylococcus cohnii | 1 (0.25) |
| Staphilococcus hominis | 1 (0.25) |
| Streptococci (45) | |
| Streptococcus pneumoniae | 22 (5.71) |
| Streptococcus sanguinis | 6 (1.55) |
| Enterococcus faecalis | 6 (1.55) |
| Streptococcus oralis | 6 (1.55) |
| Streptococcus mitis | 2 (0.51) |
| Streptococcus salivarius | 1 (0.25) |
| Streptococcus constellatus | 1 (0.25) |
| Streptococcus anginosus | 1 (0.25) |
| Streptococcus agalactiae | 1 (0.25) |
| Streptococcus viridans | 1 (0.25) |
| Streptococcus pyogenes | 1 (0.25) |
| Streptococcus gordonii | 1 (0.25) |
| Abiotrophia defectiva | 2 (0.51) |
| Granulicatella adiacens | 1 (0.25) |
| Gram-negative cocci | 2 |
| Moraxella catarrhalis | 2 (0.51) |
| Gram-negative cocobacilli | 19 |
| Moraxella lacunata | 9 (2.33) |
| Moraxella osloensis | 2 (0.51) |
| Moraxella nonliquefaciens | 1 (0.25) |
| Haemophilus influenzae | 7 (1.81) |
| Gram-positive bacilli | 78 |
| Propionibacterium acnes | 32 (8.31) |
| Corynebacterium (43) | |
| Corynebacterium macginleyi | 27 (7.01) |
| Corynebacterium spp. | 5 (1.29) |
| Corynebacterium pseudodiphthericum | 4 (1.03) |
| Corynebacterium tuberculostearicum | 3 (0.77) |
| Corynebacterium propinquum | 2 (0.51) |
| Corynebacterium kroppenstedtii | 1 (0.25) |
| Corynebacterium accolens | 1 (0.25) |
| Paenibacillus spp. | 2 (0.51) |
| Lactobacillus gasseri | 1 (0.25) |
| Gram-negative bacilli | 64 |
| Pseudomonas aeruginosas | 38 (9.7) |
| Serratia marcescens | 9 (2.33) |
| Escherichia coli | 4 (1.03) |
| Serratia liquefaciens | 2 (0.51) |
| Proteus mirabilis | 2 (0.51) |
| Enterobacter cloacae | 2 (0.51) |
| Alcaligenes faecalis | 2 (0.51) |
| Moxarella catarrhalis | 1 (0.25) |
| Stenotrophomonas maltophila | 1 (0.25) |
| Eikenella corrodens | 1 (0.25) |
| Citrobacter koseri | 1 (0.25) |
| Acinetobacter baumannii | 1 (0.25) |
| Gram-positive cocobacilli | 1 |
| Arthrobacter aurescens | 1 (0.25) |
| Total isolated bacteria | 389 (100%) |
| Polybacterial infections | |
| Staphylococcus epidermidis+Streptococcus sanguinis | 3 |
| Staphylococcus epidermidis+Streptococcus salivarius | 1 |
| Staphylococcus epidermidis+Stenotrophomonas maltophila | 1 |
| Staphylococcus epidermidis+Serratia marcescens | 1 |
| Staphylococcus epidermidis+Staphylococcus aureus | 1 |
| Staphylococcus epidermidis+Serratia liquefaciens | 1 |
| Staphylococcus epidermidis+Staphylococcus warneri | 1 |
| Staphylococcus epidermidis+Pseudomonas aeruginosas | 2 |
| Staphylococcus epidermidis+Moraxella lacunata+Staphylococcus aureus | 1 |
| Staphylococcus epidermidis+Corynebacterium spp. | 1 |
| Staphylococcus epidermidis+Corynebacterium pseudodiphthericum+Corynebacterium macginleyi | 1 |
| Staphylococcus aureus+Staphylococcus capitis | 1 |
| Staphylococcus aureus+Proteus mirabilis | 1 |
| Staphylococcus aureus+Corynebacterium macginleyi | 1 |
| Pseudomonas aeruginosas+Escherichia coli | 1 |
| Pseudomonas aeruginosas+Staphylococcus warneri | 1 |
| Moxarella catarrhalis+Stenotrophomonas maltophila | 1 |
The most frequently isolated bacteria were selected for the analysis of the results, only studied in S. aureus, S. epidermidis, S. pneumoniae, P. aeruginosa and P. acnes.
In vitro susceptibility of five of the most common bacterial pathogens against relevant antibacterial agents is described in Table 3. All Gram-positive cocci bacteria were susceptible to vancomycin. 51 isolates were S. aureus and 104 were S. epidermidis: 9.8% of the S. aureus were methicillin-resistant (MRSA) and 33.7% were methicillin-resistant S. epidermidis (MRSE). Their resistance rate was close to 20% for levofloxacin. All 22 isolates of S. pneumoniae were sensitive to vancomycin, penicillin, levofloxacin, and amoxicillin. Nearly of the entirety of P. aureuginosa colonies (n=38) were sensitive to the most common antipseudomonal agents. All P. acnes analyzed (n=10) were sensitive to penicillin, amoxicillin-clavulanic acid, piperacillin/tazobactam, imipenem, and clindamycin.
In vitro susceptibility of the four most common bacterial pathogens isolated in corneal scrapps against relevant antibacterial agents from the total of analyzed cases [penicillin (P), oxacillin (OX), ceftazidime (CTZ), cefepime (CFP), imipenem (IMI), meropenem (MER), aztreonam (AZT), colistin (COL), levofloxacin (LEV), ciprofloxacin (CIP), gentamicin (GEN), tobramycin (TO), amikacin (AK), vancomycin (VAN) and rifampycin (RIF)]. Period of study 2005–2015.
| Antibiotics | S. aureus (n=51) | S. epidermidis (n=104) | S. pneumoniae (n=22) | P. aeruginosa (n=38) | P. acnés (n=32) |
|---|---|---|---|---|---|
| P | 21.6% (n=51) | 15.8% (n=101) | 100% (n=22) | 100% (n=26) | |
| OX | 90.2% (n=51) | 66.3% (n=101) | |||
| CTZ | 94.7% (n=38) | ||||
| CFP | 94.7% (n=38) | ||||
| IMI | 94.7% (n=38) | 100% (n=26) | |||
| MER | 100% (n=38) | ||||
| AZT | 97.1% (n=34) | ||||
| COL | 100% (n=35) | ||||
| LEV | 80.4% (n=51) | 84.2% (n=101) | 100% (n=22) | 94.1% (n=34) | |
| CIP | 94.7% (n=38) | ||||
| AK | 100% (n=38) | ||||
| G | 79.0% (n=100) | 92.1% (n=38) | |||
| TO | 67.4% (n=92) | ||||
| LNZ | 98.0% (n=50) | ||||
| RIF | 95.9% (n=49) | 97.8% (n=92) | |||
| VAN | 100% (n=51) | 100% (n=101) | 100% (n=22) |
Time tracking was divided into 3 periods: 2005 to 2009 (58 isolations); 2010 to 2012 (95 isolations) and from 2013 to 2015 (234 isolations) to study the antibiotic susceptibility over time of the bacteria with some resistance. The rate of antibiotic susceptibility P. aeruginosa remains stable through the period of the study. It has not been possible to demonstrate differences because the sample size is small when making etiological groups (Tables 4–5).
Temporal profile of antibiotic susceptibility in S. aureus and S. epidermidis isolated in corneal scrapps through 3 periods: 2005 to 2009; 2010 to 2012 and from 2013 to 2015.
| Antibiotics | 2005–2009 | 2010–2012 | 2013–2015 | p | ||||
|---|---|---|---|---|---|---|---|---|
| R | S | R | S | R | S | |||
| LEV | S. epidermidis | 3 (30%) | 7 (70%) | 6 (20.7%) | 23 (79.3%) | 7 (12.3%) | 50 (87.7%) | 0.3 |
| S. aureus | 3 (30%) | 7 (70%) | 1 (8.3%) | 11 (91.7%) | 6 (20.7%) | 23 (79.3%) | 0.433 | |
| OX | S. epidermidis | 6 (40%) | 9 (60%) | 12 (41.4%) | 17 (58.6%) | 16 (28.1%) | 41 (71.9%) | 0.398 |
| S. aureus | 2 (20%) | 8 (80%) | 1 (8.3%) | 11 (91.7%) | 2 (6.9%) | 27 (93.1%) | 0.477 | |
| ERI | S. epidermidis | 8 (53.3%) | 7 (46.7%) | 14 (48.3%) | 15 (52.7%) | 32 (56.1%) | 25 (43.9%) | 0.787 |
| S. aureus | 1 (10%) | 9 (90%) | 4 (33.3%) | 8 (66.7%) | 8 (28.6%) | 20 (71.4%) | 0.414 | |
| LNZ | S. epidermidis | 0 | 15 (100%) | 0 | 29 (100%) | 1 (1.8%) | 56 (98.2%) | 0.677 |
| S. aureus | 0 | 10 (100%) | 1 (8.3%) | 11 (91.7%) | 0 | 28 (100%) | 0.199 | |
| RIF | S. epidermidis | 0 | 15 (100%) | 2 (6.9%) | 27 (93.1%) | 0 | 48 (100%) | 0.109 |
| S. aureus | 1 (10%) | 9 (90%) | 1 (9.1%) | 10 (90.9%) | 0 | 28 (100%) | 0.248 | |
| MIN | S. epidermidis | 0 | 11 (100%) | 0 | 26 (100%) | |||
| S. aureus | 0 | 2 (100%) | 0 | 9 (100%) | 0 | 9 (100%) | ||
Temporal profile of antibiotic susceptibility in P. aeruginosa through 3 periods: 2005 to 2009; 2010 to 2012 and from 2013 to 2015.
| Antibiotics | 2005–2009 | 2010–2012 | 2013–2015 | p | |||
|---|---|---|---|---|---|---|---|
| R | S | R | S | R | S | ||
| LEV | 0 | 2 (100%) | 0 | 12 (100%) | 2 (10%) | 18 (90%) | 0.475 |
| CTZ | 0 | 6 (100%) | 1 (8.3%) | 11 (91.7%) | 1 (5%) | 19 (95%) | 0.755 |
| CFP | 0 | 6 (100%) | 1 (8.3%) | 11 (91.7%) | 0 | 20 (100%) | 0.329 |
| IMI | 0 | 6 (100%) | 0 | 12 (100%) | 1 (5%) | 19 (95%) | 0.63 |
| MER | 0 | 6 (100%) | 0 | 12 (100%) | 0 | 20 (100%) | |
| AZT | 0 | 2 (100%) | 1 (8.3%) | 11 (91.7%) | 0 | 20 (100%) | 0.389 |
| CIP | 0 | 6 (100%) | 0 | 12 (100%) | 2 (10%) | 18 (90%) | 0.387 |
| AK | 0 | 6 (100%) | 0 | 12 (100%) | 0 | 20 (100%) | |
| G | 1 (8.3%) | 11 (91.7%) | 2 (10%) | 18 (90%) | 0.876 | ||
| COL | 0 | 3 (100%) | 0 | 12 (100%) | 0 | 20 (100%) | |
Clinical outcome data were available for 215 events of S. aureus, S. epidermidis, S. pnemoniae, P. aeruginosa and P. acnes keratitis. The rate of coinfection (isolation of more than one microorganism in the same corneal scrap) was 5.3%, being more frequent in cases of S. epidermidis (22%) and P. acnes (30.2%) keratitis.
Risk factors for keratitisCorneal traumatism or a corneal foreign body were rare factors in our area (2% and 1% respectively). P. aeruginosa was the most prevalent bacteria in contact lens wearers (44.7% in 38 cases of P. aeruginosa keratitis analyzed), however the most isolated bacteria in patients with a therapeutic contact lens were S. pneumoniae (9.1% of 22 cases analyzed) and S. aureus (4% of 50 cases analyzed). S. pneumoniae was the most frequently associated with corneal surgery, mainly in penetrating keratoplasty (3 of 22 cases) and radial keratotomy (2 of 22 cases). The presence of an ocular surface disease (Stevens Johnson syndrome, ocular penfigoid, Sjögren syndrome, bullous keratopathy) was recorded in 22.5% of the Gram-positive cocci but only in 7.9% of the P. aeruginosa. The presence of meibomitis presented in 35% of 20 patients with S. aureus keratitis, in 28.6% of 21 patients with S. epidermidis, and in 15.4% of 26 patients with P. aeruginosa. Palpebral malposition was associated mainly with S. epidermidis (17.4%). The main risk factors in patients with anaerobic BK (P. acnes) in 18 analyzed cases were contact lenses (41%), previous ocular surgery (16%) and herpetic keratitis (14%). Systemic diseases were associated mainly with S. aureus keratitis: 25% diabetes mellitus, 20% rheumatoid arthritis, 25% immunodepression; 15% dialysis and 15% cognitive deficiency in 20 cases analyzed.
Characteristic of the corneal infectionKeratitis caused by coinfection with other microorganism as Herpesvirus, Acanthamoeba spp., mycobacteria or fungus, and S. epidermidis keratits with the simultaneously isolation of other bacteria were exclude for this analysis.
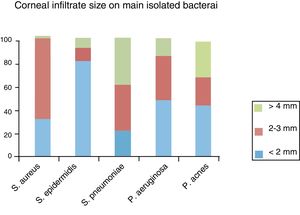
The presence of more than one corneal infiltrate (multiple infiltrates) was present in 4 (22.2%) of P. acnes, 10% of P. aeruginosa and S. epidermidis, and 6% of S. aureus infections. Four (22.2%) P. acnes, two (4%) S. aureus and two (2.3%) S. epidermidis keratitis showed epithelial defect but not stromal infiltrate as initial presentation. The infiltrate position in P. aeruginosa was central or paracentral in 80% of patients, while in Staphylococcus spp. and P. acnes infections; the position most commonly found was peripheral (57 and 56% of cases respectively). S. pneumoniae locates in the center equally as peripheral cornea. The bacteria associated with larger corneal infiltrate (analyzed in 100 patients) were S. pneumoniae and the bacteria associated with smaller infiltrates were S. epidermidis (Fig. 1). S. pneumoniae was also the bacteria most associated with hypopion (37.5%), followed by P. aeruginosa (23.5%), S. aureus (16.7%), P. acnes (11.1%) and S. epidermidis (2.3%).
Corneal infiltrate size on main isolated bacteria: from small infiltrates (<2mm) 24 were caused by S. epidermidis, 9 polimicrobial infection, 6 Pseudomonas, 5 P. acnes, 3 S. aureus and 2 S. pneumoniae. From medium size infiltrates (>2 and < 4mm) 6 were caused by S. aureus, 6 polimicrobial infection, 4 S. pneumoniae, 3 Pseudomonas, 3 S. epidermidis and 3 P. acnes. From infiltrates > 4mm, 4 were caused by S. pneumoniae, 4 by P. acnes, 4 Polimicrobial infections, 2 Pseudomonas and 2 S. epidermidis.
At the time of diagnosis, 25% of S. pneumoniae and 5% of S. aureus keratitis presented as corneal perforation. Stromal thinning was reported in 66.7%, 33.3%, 33%, 19.5% and 13.3% of S. pneumoniae, S. aureus, P. aeruginosa, S. epidermidis, and P. acnes keratitis respectively. S. epidermidis was the only tested bacteria that did not present leucoma in all patients (only 75%).
Antimicrobial regimenMost frequently antibiotics used were the combination of vancomycin and ceftazidime topical eye drops in 54 (25.1%) of cases analyzed. The second more used antimicrobial regimen were quinolone topical eye drops monotherapy in 26 cases (12.1%) (LEV, CIP or MOX) and the combination of tobramycin with a quinolone topical eye drops (LEV, CIP or MOX) in 24 patients (11.2% of cases). A triple empiric therapy (vancomycin plus ceftazidime plus quinolone topical eye drops) was used in 5.3% of the cases only for moderate to big infiltrates. We did not find evidence of an association between the size of the corneal infiltrate and the empiric antibiotic regimen used (Chi2=5.49, p=0.24).
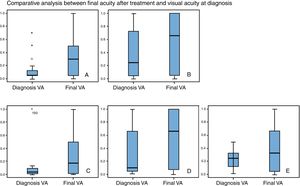
Visual acuityThe worst visual acuity at diagnosis was in patients with S. pneumoniae keratitis. Final visual acuity was significantly higher than visual acuity at diagnosis in patients with S. aureus keratitis (p=0.026) and in patients with S. epidermidis (p=0.005) (“Wilcoxon test”). Changes between initial and final visual acuity were no significative in patients with P. aeruginosa, S. penumoniae or P. acnes (Fig. 2a–e).
A: S. aureus (n=17 cases): The average visual acuity (VA) at diagnosis was 0.16 (DE=0.22) and final VA was 0.36 (DE=0.34). The final VA was significantly higher than VA at diagnosis (Z Wilconxon −2.826, p=005). B: S. epidermidis (n=31 cases): The average VA at diagnosis was 0.42 (DE=0.37) and final VA was 0.59 (DE=0.44). The final VA was significantly higher than VA at diagnosis (Z: Wilconxon −2.826, p=005). C: S. pneumoniae (n=8 cases): The average VA at diagnosis was 0.14 (DE=0.32) and final VA was 0.33 (DE=0.38). The improvement of AV was 1.84 lines (Z Wilconxon −1.841, p=0.066). D: P. aeruginosa (n=12 cases): The average VA at diagnosis was 0.33 (DE=0.35) and final VA was 0.50 (DE=0.41), without significant improvement (1.53 lines) (Z Wilconxon −1.53; p=0.12). E: P. acnes (n=13 cases): The average VA at diagnosis was 0.27 (DE=0.22) and final VA was 0.43 (DE=0.39), without significant improvement (1.53 lines) (Z Wilconxon −1.58; p=0.113).
Clinical response variables were correlated (enucleation, corneal surgery and number of days of antibiotic treatment) with susceptibility to empirical antibiotic used between bacterias with antibiotic resistance; S. aureus and S. epidermidis. This correlation was not analyzed in cases of P. aeruginosa, P. acnes or S. pneumoniae keratitis because they are usually sensitive to commonly antibiotics used.
The antibiotics P, AMP, OXA and LEV were analyzed in 50 cases of S. aureus keratitis and in 37 cases of S. epidermidis keratitis. Oxacillin resistance S. aureus (methicillin-resistant S. aureus, MRSA) was significantly associated with worse prognosis; one (20%) of the 5 MRSA cases suffered enucleation and no case of the 45 MSSA cases analyzed suffered enucleation (p=0.002), and frequency of corneal surgery was also higher in patients with MRSA (one of five patients, 20%) compared to methicillin-sensitive S. aureus (MSSA) (one of 44 patients, 2.2%) (p=0.054). Patients with S. aureus keratitis and resistance to LEV treatment were also associated with greater risk of enucleation: 1 of 10 patients (10%) in MRSA cases and no patient of 40 MSSA analyzed cases (p=0.043).
Corneal surgery frequency was higher in S. epidermidis resistant to rifampicin with 1 of 2 cases (50%) in resistant bacteria compared with 3 of 77 cases (3.9%) in susceptible bacteria (p=0.003). Surgery was more frequent in S. epidermidis resistant to levofloxacin: 2 of 14 (14.3%) in MRSE cases needed corneal surgery compared with 2 of 72 cases (2.8%) in MSSE cases, but without stadistic significance (p=0.061). Patients with this bacteria and LEV resistance were also associated with increased treatment time (50.57 days) compared to 26.43 days of S. epidermidis LEV sensitive treatment (Mann–Whitney U test, Z: −2.56, p=0.009).
DiscussionWe analyzed the BK treated in the cornea unit of a major public hospital in an urban area of Madrid. Because our study has been done retrospectively we cannot affirmed that we performed a corneal scrape of all the suspected infectious keratitis and this could have some influence on our results. Another study limitation could be the impossibility of ensuring a correct therapeutic compliance in all patients. We found a low antibiotic resistance index except in the case of Staphylococcus spp. where we observed the influence of resistance on the clinical prognosis (index of enucleation).
S. epidermidis was the bacteria most commonly isolated in corneal smears, agreeing with the reports from other series.9,10 Although this bacteria has been considered a commensal bacteria, we saw some severe and disabling keratitis; moreover, 33.7% were MRSE and levofloxacin resistant S. epidermidis and those were related to a prolonged antibiotic treatment.
Infectious keratitis caused by MRSA is an increasing problem around the world11,12 and is of great concern because it is related to fluoroquinolones resistance13 and responds poorly to conventional antibiotic treatment.14 Our rate of MRSA is 9.8% and was significantly associated with worse clinical prognosis. The susceptibility rate of MRSA to vancomycin is reportedly still 100%15–19 and thus vancomycin is highly valued for the treatment of MRSA infections. Vancomycin (2μg/mL) had the lowest MIC90 values (μg/mL) for ocular S. aureus and coagulase-negative Staphylococcus isolates recovered from the eye.20 To treat ocular infections, a topical application of vancomycin solution, or as a 1% or 5% ophthalmic ointment, has proven to be useful for the treatment of external ocular MRSA or MRSE infections.21–24 The solution reached high corneal tissue concentrations that significantly exceeded the MIC90 (2–10mg/ml) for most key Gram-positive corneal pathogens.24 We consider topical vancomycin an irreplaceable antibiotic as initial empirical combined therapy of severe BK in patients predisposed to MRS keratitis (previous surgery, previous methicillin-resistant staphylococcus (MRS) ocular infection, immunosuppression or dialysis-patients). Staphylococcus spp. kept a low sensitivity rate to beta-lactams or levofloxacin and we found significant association between the MIC of levofloxacin and methicillin and the prognosis of Staphylococcus spp. BK. Moreover the patients affected by S. aureus keratitis suffered from systemic debilitating conditions, which could compromise the defense host mechanism against infections.
Ulcers from S. pneumoniae occurred in patients wearing therapeutic contact lens and showed the largest abscesses and a high rate of perforation as initial presentation. All the S. pneumoniae isolated showed susceptibility to penicillin, levofloxacin or vancomycin. Regarding fluorquinolones, moxifloxacin25 appears to be more effective against S. pneumoniae. On the other hand, enhanced penetration of levofloxacin into the ocular tissue in addition to the higher concentration in the tear film combined with its lower MICs against streptococci, may allow for higher MIC ratios and a more immediate cure.26 An experimental study evaluating the chemotherapeutic efficacy of topical antibiotic in vivo showed that the combination of gentamicin and vancomycin was most effective against penicillin-resistant pneumococci.27 Due to the lack of penicillin resistance in our hospital we could consider a combination of penicillin and levofloxacin as an appropriate regimen for this aggressive bacteria.
P. aeruginosa was the most frequently isolated Gram-negative bacillus (9.7%). This report shows low levels of resistance of P. aeruginosa over the period 2005 to 2015. In Europe, there may be a rate of resistance of P. aeruginosa to aminoglycosides and ciprofloxacin, which is currently reported at 11%.25P. aeruginosa are often associated with the largest ulcers28 and significantly poorer visual acuity than patients with other bacterial ulcers.29 In our study final visual acuity was 0.5 for P. aeruginosa keratitis, but was lower for S. pneumoniae, and S. aureus. Gram-positive cocci in our series affected patients with other ocular conditions, which could explain this difference. While many options are available for susceptible P. aeruginosa, colistin30 or the synergistic activity between a combination of meropenem/ciprofloxacin are possible treatments for the more resistant strains.31 Our prefer topical final regimen for this aggressive keratitis is the combination of ceftazidime plus ciprofloxacin plus tobramicine or amikacine.
P. acnes, isolated in 8.2%, affected patients with previous surgery, herpetic keratitis or contact lens wearers. This commensal bacteria showed susceptibility to the tested antibiotics and caused peripheral and small infiltrates more frequently, but is also capable to produce largest infiltrates with hypopion and stromal thinning.
Microbial keratitis requires prompt and appropriate management to ensure the best visual outcome for the patient. Culturing allows sensitivity testing to a range of agents so that treatment modifications can be made in an informed manner if the clinical response to initial treatment is inadequate. In our urban community the most common bacteria causing moderate and severe keratitis were Staphylococcus, Streptococcus, and Pseudomonas species. Because of the level of methicillin and levofloxacin resistance found in our study for Staphylococcus spp., we propose to continue the empirical treatment used in our hospital with vancomycin, combined with ceftazidime or fluorquinolones, as an effective regimen for severe central bacterial keratitis when we suspect a MRS. With our standard practice the rate of enucleation or need of surgery remain bellow 5% in bacterial keratitis.
Conflict of interestThe authors declare that they have no conflict of interest.