Acute cholangitis is one of the most frequent complications in patients carrying biliary stents. The aim of our study is to analyze the demographic and clinical characteristics, as well as the microbiological profile and evolution of patients with acute bacteremic cholangitis, comparing them based upon they were or not biliary stent carriers.
MethodsWe performed a retrospective analysis of all consecutive patients over 18 years-old with a stent placement in our center between 2008 and 2017 were included. We compared them with our prospective cohort of patients with a diagnosis of acute bacteremic cholangitis. Primary outcome was 30-day mortality. Secondary outcome was clinical cure at day 7, 14-day mortality and 90-day recurrence.
ResultsTwo hundred and seventy-three patients were analyzed, including 156 in the stent-related (SR) and 117 in the stent not-related (SNR) group, respectively. Stent-related colangitis patients were younger, with more comorbidities and with a greater severity of infection. Escherichia coli and Klebsiella pneumonia were the most frequent isolation. Enterococcus spp. was the third most frequent isolation in SR group but were uncommon in SNR patients; where E. coli was the most prevalent microorganism. Septic shock (HR 3.44, 95% [CI 1.18–8.77]), inadequate empirical treatment (HR 2.65, 95% CI [1.38–.7.98]) and advanced neoplasia (HR 2.41, 95% CI [1.55–6.44]) were independent 30-day mortality risk factors. The 90-day recurrence rate significantly higher in those patients with stent-related cholangitis (29% vs. 13%, p=0.016) and stent replacement was associated with lower recurrence rate (HR 0.38, 95% CI [0.11–0.77]).
ConclusionsClinical and microbiological profile, as well as outcome of patients with SR and SNR cholangitis were different. In SR group, recurrence rate was high and stent replacement was associated with a lower risk.
La colangitis aguda es una de las complicaciones más frecuentes en los pacientes portadores de stents biliares. El objetivo de nuestro estudio es analizar las características demográficas y clínicas, así como el perfil microbiológico y la evolución de los pacientes con colangitis bacteriana aguda, comparándolos en función de si eran o no portadores de stents biliares.
MétodosSe realizó un análisis retrospectivo de todos los pacientes consecutivos mayores de 18 años con un stent colocado en nuestro centro entre 2008 y 2017. Los comparamos con nuestra cohorte prospectiva de pacientes con diagnóstico de colangitis bacteriana aguda. El criterio principal de valoración fue la mortalidad a los 30 días. Los criterios secundarios de valoración fueron la curación clínica el día 7, la mortalidad a los 14 días y la recidiva a los 90 días.
ResultadosSe analizaron 273 pacientes, incluyendo 156 en el grupo relacionado con el stent (RS) y 117 en el grupo no relacionado con el stent (NRS). Los pacientes con colangitis RS eran más jóvenes, con más enfermedades concomitantes y con una mayor intensidad de la infección. Las cepas aisladas más frecuentes fueron Escherichia coli y Klebsiellapneumoniae. Enterococcus spp. fue la tercera cepa aislada más frecuente en el grupo RS, pero no fue frecuente en los pacientes NRS, en los que E. coli fue el microorganismo más prevalente. El choque septicémico (HR: 3,44; IC del 95%: 1,18-8,77), el tratamiento empírico inadecuado (HR: 2,65; IC del 95%: 1,38-7,98) y la neoplasia avanzada (HR: 2,41; IC del 95%: 1,55-6,44) fueron factores de riesgo de mortalidad a los 30 días independientes. La tasa de recidiva a los 90 días fue significativamente más elevada en aquellos pacientes con colangitis RS (29 frente al 13%; p=0,016) y el reemplazo del stent se asoció a una menor tasa de recidiva (HR: 0,38; IC del 95%: 0,11-0,77).
ConclusionesEl perfil clínico y microbiológico, así como el resultado de los pacientes con colangitis RS y NRS, fue diferente. En el grupo RS la tasa de recidiva fue alta y la sustitución del stent se asoció con un menor riesgo.
Biliary stents are commonly used as a palliative treatment of obstructive jaundice in patients with unresectable biliary or pancreatic neoplasias. It has also been indicated in patients with non-malignant obstruction of the bile duct or as a previous step to surgery in patients with resectable neoplasias.1 There are different types of biliary stents (plastic vs. metallic, covered vs. uncovered), with different safety profile and risk of complications. Over time and inevitably, these stents are colonized by microorganisms (fungi and bacteria) that promote the occlusion of the device and increase the risk of cholestasis and acute cholangitis.2,3
Acute bacteremic cholangitis continues to be a frequent cause of morbidity and mortality in these patients. Elderly patients with solid organ tumors are particularly susceptible,4,5 with a crude mortality rate of around 11–26%.6,7 Although some authors have pointed out a greater frequency of colonization of biliary stents by Enterococcus sp. and yeast,8,9 there is very little information regarding the etiology, management, as well as, prognosis of patients with acute cholangitis related to the presence of a biliary stent.10 In the largest published series,11 a multicenter retrospective study conducted in Japan and Taiwan included more than 6,400 patients with acute cholangitis included, only 16% were carriers of a biliary stent and comparative analysis with non-carriers was not performed.
The aims of our study were to analyze the demographic and clinical characteristics, as well as the microbiological profile and evolution of patients with acute bacteremic cholangitis, comparing patients based upon they were or not biliary stent carriers.
MethodsAll consecutive patients over 18 years-old with a stent placement in our center between January 1st 2008 and December 31st 2017 were included. Data were collected retrospectively by reviewing medical electronic records. Only patients with acute bacteremic cholangitis (those who met diagnostic criteria of Tokyo Guidelines 201812) were analyzed. Moreover, we also included all patients with a diagnosis of acute bacteremic cholangitis prospectively collected between October 1st 2015 and October 31st 2017. Only the first episode of cholangitis of each patient was included in the analysis.
Patients who did not receive antibiotic treatment and/or those who died in the first 24h since blood cultures extraction and/or with limitation of therapeutic efforts were excluded. Besides, patients with a definitive diagnosis of acute cholecystitis (based on Tokyo Guidelines 2018 criteria13) but had missing data in the retrospective cohort were also excluded. The items collected were demographic data including age, sex, Charlson Comorbidity Index, clinical symptoms, etiologies of acute cholangitis, healthcare-associated factors, therapy characteristics (appropriate of empiric antimicrobial and duration of therapy) and outcome (such as 30-day all-cause mortality and 90-day recurrence rate) and management factors (need to perform a source control procedure as stent replacement and/or withdrawal). Primary outcome was 30-day mortality defined as all-cause mortality within 30 days after index blood cultures. Secondary outcome was clinical cure at day 7, 14-day mortality and 90-day recurrence (within 90 days after index blood cultures).
SettingUniversity Hospital Complex of Vigo is a 1300-bed tertiary care hospital which attends a potential population of 600,000 inhabitants; located in the North West of Spain.
DefinitionsThe Charlson comorbidities index was determined at admission.14 Patients were classified as having sepsis or septic shock as described elsewhere in Sepsis-3 consensus.15 Pitt bacteremia score was calculated at the moment of blood cultures extraction.16
Appropriate empiric therapy was considered when at least one in vitro active drug was administered within 24h after drawing blood cultures and before susceptibility report was available. Duration of antimicrobial treatment was chosen by the responsible physician of the patient; considering as adequate at least 7 days of therapy. Definitive antibiotic therapy was defined as the antibiotic treatment administered after the susceptibility results were known. Nosocomial acquisition was considered if symptoms of infection began after 48h of hospital admission. Otherwise, community acquired infection was considered.
Clinical cure was defined as resolution of signs and symptoms of infection within 7 days of treatment initiation. Relapse was defined as acute cholangitis after resolution of symptoms and completion of antibiotic treatment for the initial episode.
Microbiological methodsIsolations in blood cultures were processed in the Laboratory of Microbiology of the University Hospital Complex of Vigo according to the current standardized procedures. The antibiotic susceptibility was determined by the automatic method VITEK2 (BioMérieux, France) which includes combinations of cephalosporins with clavulanic acid for the detection of extended-spectrum β-lactamases. For confirmation, a phenotypic method with double Etest strip and/or modified double disc was used. The detection of carbapenemases was carried out following the EUCAST protocol: meropenem disc diameter<25mm or minimum inhibitory concentration>0.12mg/L in all Enterobacteriaceae. Confirmation was carried out with chromID medium CARBA SMART (BioMérieux, rancid) and the type of carbapenemase by PCR (Cepheid Xpert Carba-R.) There was no change in the method used for the identification of microorganisms or analysis of antibiotic susceptibility during the study period.
Statistical analysisThe statistical package SPSS v24.0 (SPSS, Chicago, IL) was used to analyze the data. Continuous variables were compared using t-Student test or U Mann–Whitney test and were described as mean±standard deviation (SD) or as median and interquartile range (IQR) according to whether the distribution of the variables was normal or non-normal. Chi-square test (χ2) and Fisher's Exact Test were used to compare categorical variables. A multivariate analysis using a Cox regression model was carried out to identify factors independently associated with outcome. All variables with a p-value<0.20 in the univariate analysis were included in the multivariate analysis. Variables with a two-sided p-value<0.05 were considered statistically significant.
EthicsThe study was approved by the Local Ethics Committee (2018/378) which waived the need to obtain written informed consent. STROBE recommendations were followed.
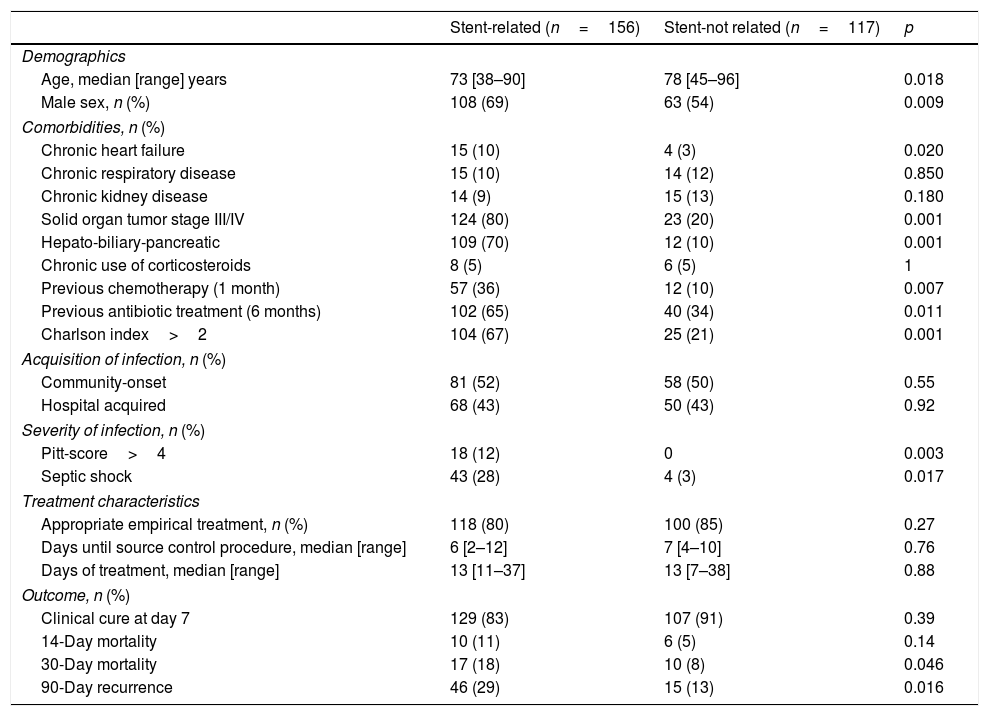
ResultsDuring the study period 273 patients met eligibility criteria (8 patients were excluding due to missing data), including 156 in the stent-related group (SR) and 117 in the stent not-related (SNR) group. The main demographic, clinical, treatment and outcome characteristics of both group of patients are shown in Table 1. Stent-related acute cholangitis affected more commonly to male and younger patients, with more comorbidities and with a greater severity of infection. The type of stent used was plastic in 83 patients (53%) and metallic in 73 (47%). Prophylaxis with ciprofloxacin (400mg intravenously) was started in all patients before stent placement and replacement.
Demographic and clinical characteristics of patients according to biliary stent carrier status.
| Stent-related (n=156) | Stent-not related (n=117) | p | |
|---|---|---|---|
| Demographics | |||
| Age, median [range] years | 73 [38–90] | 78 [45–96] | 0.018 |
| Male sex, n (%) | 108 (69) | 63 (54) | 0.009 |
| Comorbidities, n (%) | |||
| Chronic heart failure | 15 (10) | 4 (3) | 0.020 |
| Chronic respiratory disease | 15 (10) | 14 (12) | 0.850 |
| Chronic kidney disease | 14 (9) | 15 (13) | 0.180 |
| Solid organ tumor stage III/IV | 124 (80) | 23 (20) | 0.001 |
| Hepato-biliary-pancreatic | 109 (70) | 12 (10) | 0.001 |
| Chronic use of corticosteroids | 8 (5) | 6 (5) | 1 |
| Previous chemotherapy (1 month) | 57 (36) | 12 (10) | 0.007 |
| Previous antibiotic treatment (6 months) | 102 (65) | 40 (34) | 0.011 |
| Charlson index>2 | 104 (67) | 25 (21) | 0.001 |
| Acquisition of infection, n (%) | |||
| Community-onset | 81 (52) | 58 (50) | 0.55 |
| Hospital acquired | 68 (43) | 50 (43) | 0.92 |
| Severity of infection, n (%) | |||
| Pitt-score>4 | 18 (12) | 0 | 0.003 |
| Septic shock | 43 (28) | 4 (3) | 0.017 |
| Treatment characteristics | |||
| Appropriate empirical treatment, n (%) | 118 (80) | 100 (85) | 0.27 |
| Days until source control procedure, median [range] | 6 [2–12] | 7 [4–10] | 0.76 |
| Days of treatment, median [range] | 13 [11–37] | 13 [7–38] | 0.88 |
| Outcome, n (%) | |||
| Clinical cure at day 7 | 129 (83) | 107 (91) | 0.39 |
| 14-Day mortality | 10 (11) | 6 (5) | 0.14 |
| 30-Day mortality | 17 (18) | 10 (8) | 0.046 |
| 90-Day recurrence | 46 (29) | 15 (13) | 0.016 |
The most frequent empirical therapy was a combination of β-lactam plus β-lactamase inhibitor in both groups (72 patients; 62% in SR and 69 patients; 58% in SNR), followed by a carbapenem in SR group (35 patients; 22%) and third-generation cephalosporin in SNR group (27 patients; 23%). A fluoroquinolone was the initial treatment in 11 (7%) and 8 (7%) patients in SR and SNR groups, respectively.
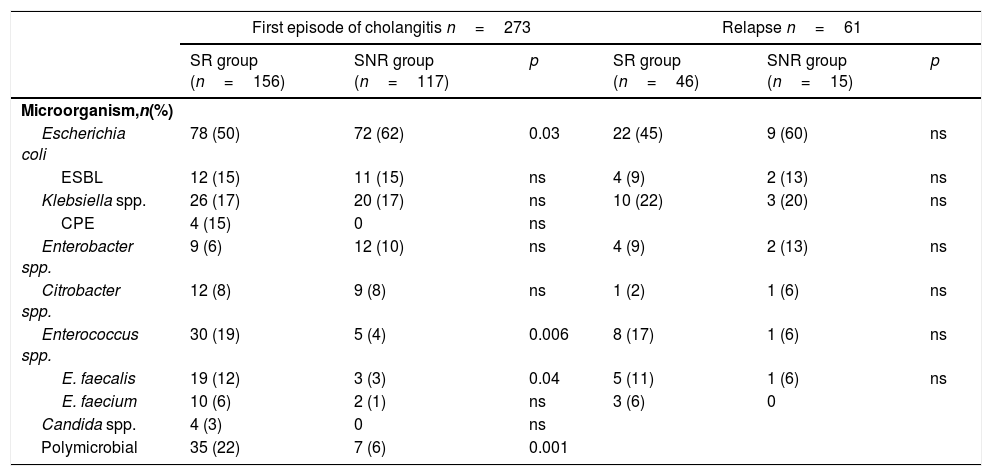
Microorganisms isolated in both group of patients are shown in Table 2. Other isolates with low frequency were Pseudomonas aeruginosa (3 patients in SR and 2 patients in SNR group), Streptococcus sp. (5 patients, only in SR group), Serratia marcescens (2 patients in SR group) and Bacteroides stercolaris (2 patients in SNR group).
Microbiological data of both groups of patients comparing first episode of cholangitis and relapses.
| First episode of cholangitis n=273 | Relapse n=61 | |||||
|---|---|---|---|---|---|---|
| SR group (n=156) | SNR group (n=117) | p | SR group (n=46) | SNR group (n=15) | p | |
| Microorganism,n(%) | ||||||
| Escherichia coli | 78 (50) | 72 (62) | 0.03 | 22 (45) | 9 (60) | ns |
| ESBL | 12 (15) | 11 (15) | ns | 4 (9) | 2 (13) | ns |
| Klebsiella spp. | 26 (17) | 20 (17) | ns | 10 (22) | 3 (20) | ns |
| CPE | 4 (15) | 0 | ns | |||
| Enterobacter spp. | 9 (6) | 12 (10) | ns | 4 (9) | 2 (13) | ns |
| Citrobacter spp. | 12 (8) | 9 (8) | ns | 1 (2) | 1 (6) | ns |
| Enterococcus spp. | 30 (19) | 5 (4) | 0.006 | 8 (17) | 1 (6) | ns |
| E. faecalis | 19 (12) | 3 (3) | 0.04 | 5 (11) | 1 (6) | ns |
| E. faecium | 10 (6) | 2 (1) | ns | 3 (6) | 0 | |
| Candida spp. | 4 (3) | 0 | ns | |||
| Polymicrobial | 35 (22) | 7 (6) | 0.001 | |||
Abbreviations: SR: stent-related; SNR: stent not-related; ESBL: extended-spectrum beta-lactamase; CPE: carbapenemase-producing Enterobacteriaceae; ns: not statistically significant.
Regarding therapeutic management, a source control procedure was performed in 95 patients in SR group (61%). Among these patients stent obstruction was documented in 58 patients (61%) and migration in 13 (14%). Biliary stent replacement was performed in the first 7 days after diagnosis of bloodstream infection in 73 patients (47%) and biliary drainage surgery was performed in 22 (14%). The rest of patients were managed conservatively according to its treating physician (due to poorer short-term outcome and/or technical difficulties in replacing the stent). We did not observe differences in treatment duration depending on wether or not a stent replacement was performed (12 days vs 13 days; p=0.66). In the SNR group, endoscopic retrograde cholangiopancreatography (ERCP) was performed for biliary drainage/stent placement in 88 patients (75%) with biliary obstruction documented in 45 patients (51%). The median time from the extraction of blood cultures to the performance of ERCP was 7 days [range 5–12]. The most frequent etiology of cholangitis in the SNR group was bile duct stones in 82 patients (70%), followed by the presence of a tumor in 19 patients (16%) and no definitive diagnosis in the rest.
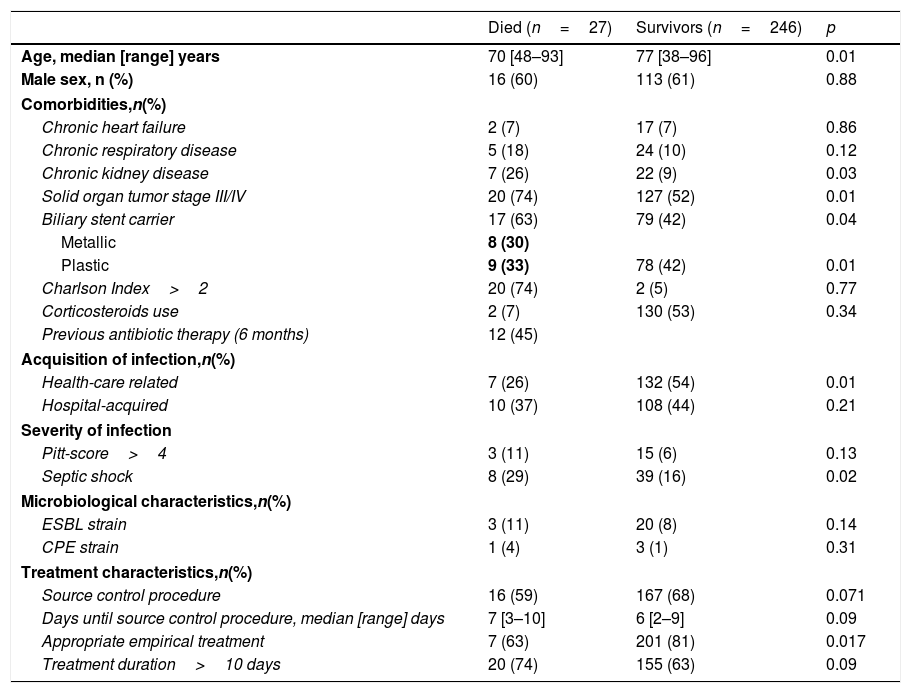
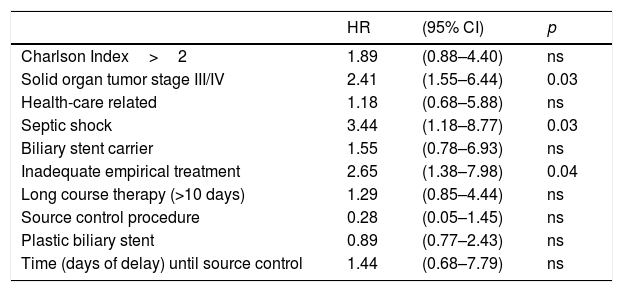
Considering to the primary and secondary outcomes we did not observed differences between the two groups in terms of 7-day clinical cure but a higher 30-day mortality and 90-day recurrence were seen in SR group (Table 1). Table 3 shows the main characteristics of patients with and without 30-day mortality. Risk factors associated with 30-day mortality were shown in Table 4. Presence of septic shock at diagnosis (HR 3.44, 95% [CI 1.18–8.77]; p=0.03) and advanced solid organ tumor (HR 2.41, 95% CI [1.55–6.44]; p=0.03) were independent 30-day mortality risk factors. An inadequate empirical treatment was associated with higher 30-day mortality (HR 2.65, 95% CI [1.38–.7.98]; p=0.04).
Comparison of patients with and without 30-day mortality.
| Died (n=27) | Survivors (n=246) | p | |
|---|---|---|---|
| Age, median [range] years | 70 [48–93] | 77 [38–96] | 0.01 |
| Male sex, n (%) | 16 (60) | 113 (61) | 0.88 |
| Comorbidities,n(%) | |||
| Chronic heart failure | 2 (7) | 17 (7) | 0.86 |
| Chronic respiratory disease | 5 (18) | 24 (10) | 0.12 |
| Chronic kidney disease | 7 (26) | 22 (9) | 0.03 |
| Solid organ tumor stage III/IV | 20 (74) | 127 (52) | 0.01 |
| Biliary stent carrier | 17 (63) | 79 (42) | 0.04 |
| Metallic | 8 (30) | ||
| Plastic | 9 (33) | 78 (42) | 0.01 |
| Charlson Index>2 | 20 (74) | 2 (5) | 0.77 |
| Corticosteroids use | 2 (7) | 130 (53) | 0.34 |
| Previous antibiotic therapy (6 months) | 12 (45) | ||
| Acquisition of infection,n(%) | |||
| Health-care related | 7 (26) | 132 (54) | 0.01 |
| Hospital-acquired | 10 (37) | 108 (44) | 0.21 |
| Severity of infection | |||
| Pitt-score>4 | 3 (11) | 15 (6) | 0.13 |
| Septic shock | 8 (29) | 39 (16) | 0.02 |
| Microbiological characteristics,n(%) | |||
| ESBL strain | 3 (11) | 20 (8) | 0.14 |
| CPE strain | 1 (4) | 3 (1) | 0.31 |
| Treatment characteristics,n(%) | |||
| Source control procedure | 16 (59) | 167 (68) | 0.071 |
| Days until source control procedure, median [range] days | 7 [3–10] | 6 [2–9] | 0.09 |
| Appropriate empirical treatment | 7 (63) | 201 (81) | 0.017 |
| Treatment duration>10 days | 20 (74) | 155 (63) | 0.09 |
Abbreviations: ESBL: extended-spectrum beta-lactamase; CPE: carbapenemase-producing Enterobacteriaceae.
30-Day mortality risk-factors in multivariate analysis.
| HR | (95% CI) | p | |
|---|---|---|---|
| Charlson Index>2 | 1.89 | (0.88–4.40) | ns |
| Solid organ tumor stage III/IV | 2.41 | (1.55–6.44) | 0.03 |
| Health-care related | 1.18 | (0.68–5.88) | ns |
| Septic shock | 3.44 | (1.18–8.77) | 0.03 |
| Biliary stent carrier | 1.55 | (0.78–6.93) | ns |
| Inadequate empirical treatment | 2.65 | (1.38–7.98) | 0.04 |
| Long course therapy (>10 days) | 1.29 | (0.85–4.44) | ns |
| Source control procedure | 0.28 | (0.05–1.45) | ns |
| Plastic biliary stent | 0.89 | (0.77–2.43) | ns |
| Time (days of delay) until source control | 1.44 | (0.68–7.79) | ns |
Abbreviations: ns: not statistically significant; HR: hazard ratio.
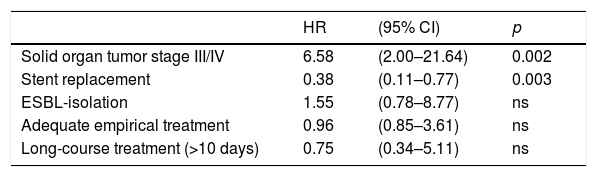
Relapse within 90 days after index blood cultures occurred in 61 patients (46 in SR and 15 in SNR group). The microorganisms isolated during recurrences were similar to those isolated in the cases that only had one episode (Table 2). Multivariate analysis of associated risk factors is shown in Table 5. Patients with advanced solid organ tumor had a higher risk of relapsed (HR 6.58, 95% CI [2.00–21.64]; p=0.002). Stent replacement was associated with a lower risk of recurrence (HR 0.38, 95% CI [0.11–0.77]; p=0.003). No differences were observed in terms of mortality and or recurrence regarding the type of stent placed (plastic vs metallic).
90-Day recurrence risk-factors in multivariate analysis.
| HR | (95% CI) | p | |
|---|---|---|---|
| Solid organ tumor stage III/IV | 6.58 | (2.00–21.64) | 0.002 |
| Stent replacement | 0.38 | (0.11–0.77) | 0.003 |
| ESBL-isolation | 1.55 | (0.78–8.77) | ns |
| Adequate empirical treatment | 0.96 | (0.85–3.61) | ns |
| Long-course treatment (>10 days) | 0.75 | (0.34–5.11) | ns |
Abbreviations: ns: not statistically significant; HR: hazard ratio; ESBL: extended spectrum beta-lactamase-producing Enterobateriaceae.
In this study, we have performed a comparative analysis of a cohort of patients with and without biliary stent with acute bacteremic cholangitis.
It was observed that the group of patients with biliary stent were younger, but with more comorbidities, a higher prevalence of advanced solid organ tumors and with a greater severity of infection. In previous studies10,11 comparing the etiology of both groups of patients (SR vs. SNR groups), differences in demographic, clinical characteristics, as well as management and prognosis, were not analyzed.
In the work of Royo-Cebrecos et al.,7 where a cohort of patients with solid organ tumors and acute bacteremic cholangitis was analyzed, the independent factors associated with 30-day all cause mortality rate were the concomitant treatment with corticosteroids and the complications related to the neoplasia.
In our series, the 30-day mortality risk factors (which was globally 10%) were the presence of septic shock and advanced neoplasia at the time of diagnosis, as well as, an inadequate empirical treatment. It is important to highlight the fact that an inadequate empirical treatment is associated with a worse prognosis, even though classically the biliary focus has been associated with a lower risk of mortality.17 In general terms, the rate of adequate empirical treatment in our series was high (80–85%) and there were no differences in the presence or absence of a biliary stent.
We have not demonstrated an association between the presence of a biliary stent and increased mortality. We also did not show reduction in mortality in those patients who underwent procedures of source control. This may be in part due to that in our center ERCP is only performed twice a week (with the exception of patients with septic shock) and this delay can reduce the beneficial impact of the technique in the prognosis of patients.
An interesting aspect to note is the duration of antibiotic treatment. In our study the median duration was 13 days, in both groups. In two retrospective studies,18,19 it was observed that a short antibiotic treatment (even a 3 day course in the first of them18) was as effective as a longer treatment, especially in those cases in which adequate biliary drainage was achieved, in most cases less than 24hours. In our study, the main limitation to achieve a shorter antibiotic duration was the delay until the performance of biliary drainage procedures (ERCP in most cases), that was 7 days [range 5–12].
The main etiology of biliary tract infections are Enterobacteriaceae (Escherichia coli and Klebsiella pneumoniae), being Enterococcus spp. much less frequent (rates between 7.6–10.5% according to the series published in patients with bacteremia7,11). In our cohort of patients with biliary stent Enterococcus spp. was the main etiology in 19% of patients, being the third most frequent isolation. This finding had already been described in other studies. Weber et al.10 in 2013 published that Enterococcus sp. was the main etiology of 447 episodes of cholangitis (with liquid biliary cultures and/or blood cultures) and this rate was even higher in those patients with biliary stent. In addition, in a study on microbiology of liquid biliary cultures in patients with cholangitis with and without a biliary stent,20Enterococcus sp. was the second most frequent etiology (31%). This fact can be explained by the presence of virulence and adhesion factors and by the synergistic effect that it has together with gramnegative bacilli in the formation of biofilm in biliary stents.21,22 Even though in the work of Lubbert et al. an increase rate of colonization of biliary stents due to Candida spp. was also pointed out, in the majority of published works it is not isolated in blood cultures.7,10,11,20 In our series, only 4 episodes of candidemia were identified, all of them in the SR group. The rate of P. aeruginosa isolates was less than 2%, which differs from the previously published reports where it was isolated up to 7% of the cases associated with biliary stent.10 In addition, as in previous studies,7 polymicrobial infections were significantly more frequent in the SR group.
According to our results and after analyzing the microbiological profile of our isolates, a modification of our local prophylaxis guidelines has been made, replacing ciprofloxacin by the association of a beta-lactam with a beta-lactamase inhibitor.
The 90-day recurrence rate was 22% in the global cohort, and significantly higher in those patients with stent-related cholangitis (29% vs. 13%, p=0.016). The associated factor was the presence of neoplasia. Moreover, stent replacement was associated with a significant reduction in the risk of recurrence, a finding not described in previous studies. In the study by Royo-Cebrecos et al 7they analyzed the microbiological isolates also in the recurrences of cholangitis and they observed a higher frequency of multidrug resistant microorganisms, mainly Enterococcus faecium and extended-spectrum beta-lactamases producing Enterobacteriaceae, than in patients with only one episode of cholangitis. In our work, information has been collected up to 90 days from index blood cultures and we did not find an increase of multidrug resistant microorganisms.
Although our study has some strengths, such as analyzing patients with biliary stent for a long period of time (2008–2017). Also, we included only patients with positive blood cultures, which facilitates the comparison between both cohorts. However, it has several limitations. On the one hand, it is a single-center study and SR group patients are analyzed in a retrospective manner and for a larger period of time compared to SNR group (with also different number of patients), so the biases of this type of study could not be avoided. In addition, we were unable to analyze the impact of the delay in carrying out source control procedures (ERCP) on the outcome, since we did not have data in the SR group.
In summary, we have observed that episodes of acute bacteremic cholangitis in patients with biliary stent present a different etiology than non-carriers, with a higher incidence of enterococcal infection. Also, these patients used to show a greater severity of infection and have more comorbidities, frequently with advanced solid tumors. Factors associated with a worse prognosis were the presence of septic shock, having an advanced neoplasia at diagnosis and an inadequate empirical antibiotic treatment. The recurrence rate of the infection was relatively high and stent replacement was associated with a lower risk.
Authors contributionsAS, AAH, MTPR conceived and designed the study. AS, AAH, OL, AO, MS performed the scientific literature search, collected data, interpreted data. AS, AAH and MTPR wrote the report. All the authors approved the final version of the manuscript. As corresponding author AS had full access to all the data in the study and takes responsibility for the decision to submit for publication.
FundingNo external funding was received for the submitted work.
Conflicts of interestAll the authors have completed the ICMJE form for Disclosure of Potential Conflicts of Interest and declare that (1) AS reports personal fees from Pfizer, Angelini and Merck, outside the submitted work. MTPR reports personal fees from Pfizer and Merck, Astellas and Angelini outside the submitted work. The others authors have nothing to disclose; (2) None of the authors have had relationships in the previous two years with companies that might have an interest in the submitted work; (3) None of the authors’ spouses, partners, or children have any financial relationship that may be relevant to the submitted work; and (4) None of the authors have any non-financial interests that may be relevant to the submitted work.
Partial data of the submitted work was presented at 29th European Congress on Clinical Microbiology and Infectious Diseases (ECCMID 2019) with abstract numbers 1580 and 1585.