To describe the, incidence, the changes in the etiology and the prognosis of lower respiratory tract infection (LRTI) in HIV infected patients, presenting by the first time to the Emergency Department (ED), during years 2000-2010.
Study designProspective collection of data.
MetbodsData were collected on the first visit of HIV-infected patients at our ED due to a LRTI, (defined according to the criteria of the European Respiratory Society), between 1/1/2000 and 31/12/2010. A series of epidemiological and laboratory variables as well as the need for admission to the intensive care unit (ICU). LRTI etiology were also collected. The influence ofthe mentioned variables on 30-day mortality were analyzed.
ResultsOne hundred thirty one patients were included. LRTI represented 27% of visits to the ED by HIV-infected patients. Mean age was 39±9 years. 72% of patients were males. 18% required admission to the ICU. The most frequent LRTI was pneumonia by P. jiroveci in 35 cases, bacterial penumonia in 27 and pulmonary tuberculosis in 20. LRTI incidence gradually reduced significantly over time from 6.13 × 1000 patients/year in year 2000 to 0.23 × 1000 patients/year in 2010 (p<0.05). Overall mortality was 14%. Logistic regression analysis showed that admission to ICU (p<0.004) and viral load (p<0.029) were independent variables predicting mortality.
ConclusionLRTI is a pathology with a decreasing incidence, probably related to the widespread utilization increased of HAART regimens. lts etiology has also been changing, but with a non negligible mortality, mostly when ICU admission was required.
Describir la incidencia, la etiología y el pronóstico de la infección de las vías respiratorias bajas (IVRB) en los pacientes VIH, que acudieron a un Servicio de Urgencias (SU), durante el período del 2000–2010.
Diseño del estudioEstudio prospectivo de 10 años de evolución.
MétodosSe recogió únicamente el primer episodio del paciente que acude al SU por IRVB (definida según la European Respiratory Society). Se analizaron una serie de variables epidemiológicas y de laboratorio, así como la necesidad de ingreso en una unidad de cuidados intensivos (UCI). Se estudió la etiología de la IRVB y la incidencia. Finalmente se analizaron la influencia de las variables con la mortalidad a 30 días.
ResultadosSe incluyeron un total de 131 pacientes. La edad media fue de 39±9 años. El 72% de los pacientes eran varones y el 18% de los pacientes requirieron ingreso en la UCI. La IRVB más frecuente fue la neumonía por P. jirovecci, seguida de la neumonía bacteriana en 27 y la tuberculosis pulmonar en 20. La incidencia de IRVB se ha ido reduciendo gradualmente de forma significativa, 6,13×1.000 pacientes/año en 2000 a 0,23×1.000 pacientes/año en 2010 (p<0,05). El análisis de regresión logística mostró que la única variable que predijo mortalidad fue el ingreso en UCI (p<0,05; OR: 73,01).
ConclusiónLa IRVB es una enfermedad cuya incidencia y etiología han ido disminuyendo y cambiando respectivamente, probablemente en relación con la utilización generalizada del TAR. Sin embargo, todavía presenta una mortalidad nada despreciable, que es mayor cuando el paciente requiere ingreso en la UCI.
The morbidity and mortality associated to infection by the human immunodeficiency virus (HIV)1 has dramatically decreased since highly active antiretroviral therapy (HAART) was first introduced in the mid-1990s. Respiratory pathology and, specifically, acute lower respiratory tract infections (LRTI), have been one of the most frequent problems in HIV-infected patients with a life-threatening prognosis in a large number of cases.2
In the pre-HAART era, the most common cause of community-acquired pneumonia (CAP) was Pneumocystis jiroveci (PCP) occurring mainly in patients with <200CD4/mm3 lymphocytes.3 After the introduction of HAART and subsequent immune recovery, the incidence of PCP has gone down but bacterial infections like Streptococcus pneumoniae have emerged as the first cause of CAP.4 Conversely, a reduction in the incidence of S. peumoniae invasive disease (bacteremia) has been observed.5 Pulmonary tuberculosis (TBC) has always been a worldwide health problem with a great impact in the pre-HAART era, in HIV/AIDS population6–9 even in the developed world. Nowadays, pulmonary tuberculosis develops in HIV patients with relatively good immunological control and its incidence has become close to that of the immunocompetent population of the same geographical area.
Emergency departments (ED) used to be the health resource that HIV patients utilize to receive acute medical care.10 In the setting of antiretroviral therapy, most of ED consultations in HIV-infected patients are due to reasons, not directly related with HIV infection. The changing situation of HIV patients in the HAART era has led to changes in the reasons to seek emergency care along the years.11 However, these changes may not be well understood. We have been prospectively collecting data on ED admissions at Hospital Clinic, a major hospital located in the center of Barcelona delivering emergency care for a wide urban area.
We herewith describe the evolution of the incidence, etiology and prognosis of LRTI in HIV patients presenting for the first time to an ED, in the HAART era.
Patients and methodsProspective study (2000–2010), collecting data using a predefined form of the first consultation to lower ED of patients previously diagnosed with HIV or newly diagnosed patients during the patient's admission to the ED that presented, with a LRTI. Patients diagnosed with LRTI derived from other hospitals were excluded. Patients diagnosed with LRTI transferred from other hospitals were excluded. LRTI was defined according to the criteria of the European Respiratory Society.12–14 AIDS criteria, including pneumonia by PCP and TB, were determined based on the 1993 CDC15 recommendations. HAART was defined as a combination antiretroviral therapy including at least three drugs. CAP was defined according to IDSA criteria.16,17
Mortality was considered related with the episode of LRTI if it occurred within 30 days.
Our hospital is both a referral center and also a primary care center, for an area of 500,000 inhabitants, 125,000 emergency visits per year of, which 42,00018 in the area of medicine. Data collection was carried out by reviewing the emergency department medical records, which were subsequently compared with the database of HIV patients followed up in our hospital on an outpatient basis (at present about 5000 patients). The study was approved by the Ethics Committee of our hospital and all patients participating in the study signed an informed consent during their admission at the ED. To calculate the incidence, HIV patients not controlled in our hospital were excluded.
Blood cultures were performed using BACTEC 9240, Becton Dickinson, NJ, EE.UU; good quality sputum samples, according to Murray's criteria,19 were gram stained and cultured. Urine antigen for S. pneumoniae (Binax NOW S pneumoniae Urinary Antigen Test) and L. Pneumophila (Binax NOW Legionella Urinary Antigen Test) were also determined. When the patient presented with radiological infiltrate and clinical symptoms compatible with TB, Ziehl–Neelsen staining of the sputum, stools and gastric juice were performed. Bronchoalveolar lavage (BAL) was performed in patients with respiratory insufficiency and radiological features of suspected infection by PCP or Cytomegalovirus. If the patient required mechanical ventilation a bronchoaspiration procedure was performed. A nasopharyngeal smear from patients was performed to study respiratory viruses. It was processed for antigen detection by indirect immunofluorescence (IFI) (Chemicon internacional Temula, California, EE.UU.), and for the detection of nucleic acids was performed by means of two multiple and independent reverse transcription nested polymerase chain reactions (RT-PCR). When a fungal infection was suspected, a fresh exam of the sputum samples was performed using Sabouraud's agar culture or Giemsa staining. The detection of AGA (anti transglutaminase antibodies) in BAS or in BAL was determined by the sandwich-type immunoenzymatic assay in microplaques (“Platelia Aspergillus EIA” technique).
A series of epidemiological and laboratory variables such as age, sex, HAART, cD4 lymphocyte count and viral load (VL) were assessed prior to admission. In addition, meeting AIDS criteria, the patient's need for admission to the intensive care unit (ICU) and LRTI etiology were also assessed. Lastly, the influence of these variables on 30-day mortality in our hospital was analyzed. Patients requiring admission to the ICU are those that meet at least one of the following two criteria: vasoactive support or need for non-invasive or invasive mechanical ventilation. The days of hospital stay were collected but not finally included in the data analysis, in order to reduce bias, since many patients had social pathology and did not fit the medical discharge with the actual discharge. Categorical variables were expressed as frequencies and percentages. All continuous variables were expressed as mean±standard deviation unless otherwise specified. Differences between groups were evaluated using parametric tests if the variables were normally distributed and the non-parametric tests if the variables were not normally distributed. Proportions were compared with the Chi-square test and the Fisher's exact test when necessary. Stepwise logistic regression analysis was performed to model the relationship between mortality and each of the variables, including sex and age as potential confounders. A bilateral value of p<0.05 was considered significant. All statistical calculations were performed using the SPSS version 18.0 (Chicago, IL, USA).
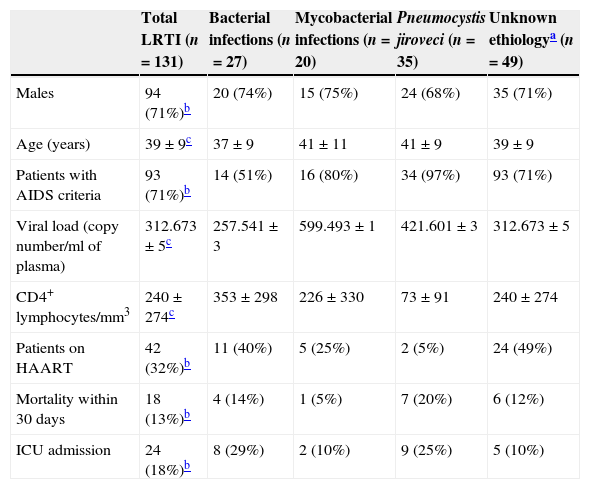
ResultsOne hundred and thirty-one (27%) patients had a LRTI diagnosed among the 445 HIV infected patients with a first visit to ED during the 10 years study period. Mean age of these 131 patients was 39±9 years. 71% of the patients were males, 33% were receiving HAART, 71% met AIDS criteria and 24 (18%) had to be admitted to the ICU, including 10 with mechanical invasive and 4 with noninvasive ventilation (NIV). All patients admitted to the ICU, pointed vasoactive drugs. Six patients required NIV but most were managed in the ED to the unavailability of ICU beds. The diagnosis of HIV infection was done during the ED admission in 25 (19%) of these 131 patients. The rest of epidemiological and laboratory variables are shown in Table 1.
Characteristics of the 131 study patients stratified by the etiology of the LRTI.
| Total LRTI (n=131) | Bacterial infections (n=27) | Mycobacterial infections (n=20) | Pneumocystis jiroveci (n=35) | Unknown ethiologya (n=49) | |
|---|---|---|---|---|---|
| Males | 94 (71%)b | 20 (74%) | 15 (75%) | 24 (68%) | 35 (71%) |
| Age (years) | 39±9c | 37±9 | 41±11 | 41±9 | 39±9 |
| Patients with AIDS criteria | 93 (71%)b | 14 (51%) | 16 (80%) | 34 (97%) | 93 (71%) |
| Viral load (copy number/ml of plasma) | 312.673±5c | 257.541±3 | 599.493±1 | 421.601±3 | 312.673±5 |
| CD4+ lymphocytes/mm3 | 240±274c | 353±298 | 226±330 | 73±91 | 240±274 |
| Patients on HAART | 42 (32%)b | 11 (40%) | 5 (25%) | 2 (5%) | 24 (49%) |
| Mortality within 30 days | 18 (13%)b | 4 (14%) | 1 (5%) | 7 (20%) | 6 (12%) |
| ICU admission | 24 (18%)b | 8 (29%) | 2 (10%) | 9 (25%) | 5 (10%) |
LTRI, lower respiratory tract infections; HAART, highly active antiretroviral therapy; ICU, intensive care unit.
The most frequent LRTI was pneumonia by PCP in 35 cases (27%), followed by bacterial pneumonia in 27 cases (21%) and TB in 20 cases (15%). Five patients having being previously diagnosed with chronic obstructive pulmonary disease were diagnosed with CAP. Bacterial pneumonia was suspected in 66 cases but only microbiological isolation was obtained in 27. The most frequently identified microorganism was PCP in 35 cases, which was diagnosed by BAL in 33 (94%) patients, followed by induced sputum in 2 (6%) patients. In one patient with high suspicion of PCP pneumonia no microorganism could be identified. The most frequently diagnosed bacterium was S. pneumoniae in 21 patients, 9 (43%) of which developed bacteriemia. Other bacteria identified were Sapht aureus, H influenzae and Streptococcus viridans in 3, 2 and 1 patient, respectively. In 10 (50%) patients with TBC, bacilloscopy was negative and the diagnosis was established by pathological examination of the pleural biopsy, the growth of mycobacterium in stool, urine or gastric juice. In the remaining patients without microbiological diagnosis, 49 cases (37%), attending the clinical and radiological suspicion, 39 cases were treated as bacterial infection, 1 as PCP and 9 as TB. No fungal or viral isolation was obtained in any patient.
The clinical course of the different LRTI pathologies is shown in Table 2.
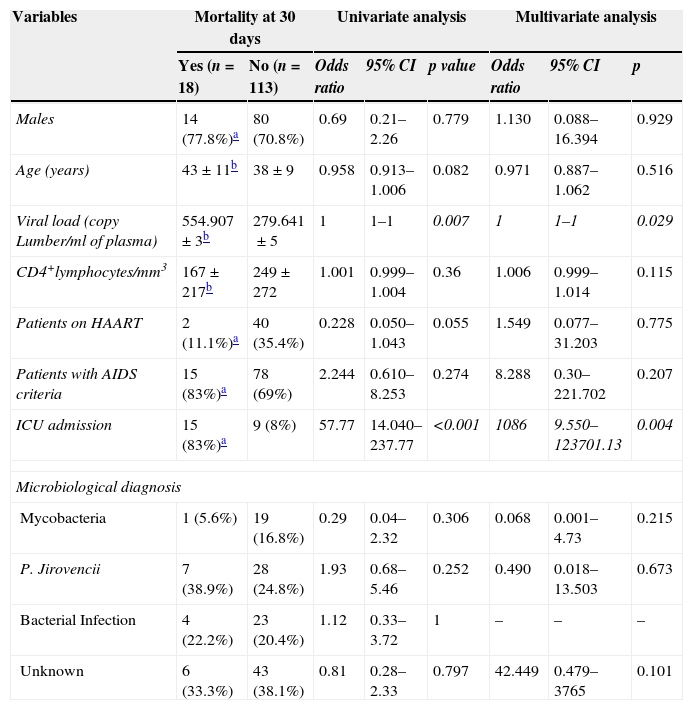
Predictors of mortality within 30 days.
| Variables | Mortality at 30 days | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Yes (n=18) | No (n=113) | Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p | |
| Males | 14 (77.8%)a | 80 (70.8%) | 0.69 | 0.21–2.26 | 0.779 | 1.130 | 0.088–16.394 | 0.929 |
| Age (years) | 43±11b | 38±9 | 0.958 | 0.913–1.006 | 0.082 | 0.971 | 0.887–1.062 | 0.516 |
| Viral load (copy Lumber/ml of plasma) | 554.907±3b | 279.641±5 | 1 | 1–1 | 0.007 | 1 | 1–1 | 0.029 |
| CD4+lymphocytes/mm3 | 167±217b | 249±272 | 1.001 | 0.999–1.004 | 0.36 | 1.006 | 0.999–1.014 | 0.115 |
| Patients on HAART | 2 (11.1%)a | 40 (35.4%) | 0.228 | 0.050–1.043 | 0.055 | 1.549 | 0.077–31.203 | 0.775 |
| Patients with AIDS criteria | 15 (83%)a | 78 (69%) | 2.244 | 0.610–8.253 | 0.274 | 8.288 | 0.30–221.702 | 0.207 |
| ICU admission | 15 (83%)a | 9 (8%) | 57.77 | 14.040–237.77 | <0.001 | 1086 | 9.550–123701.13 | 0.004 |
| Microbiological diagnosis | ||||||||
| Mycobacteria | 1 (5.6%) | 19 (16.8%) | 0.29 | 0.04–2.32 | 0.306 | 0.068 | 0.001–4.73 | 0.215 |
| P. Jirovencii | 7 (38.9%) | 28 (24.8%) | 1.93 | 0.68–5.46 | 0.252 | 0.490 | 0.018–13.503 | 0.673 |
| Bacterial Infection | 4 (22.2%) | 23 (20.4%) | 1.12 | 0.33–3.72 | 1 | – | – | – |
| Unknown | 6 (33.3%) | 43 (38.1%) | 0.81 | 0.28–2.33 | 0.797 | 42.449 | 0.479–3765 | 0.101 |
Italics values denote statistical significance.
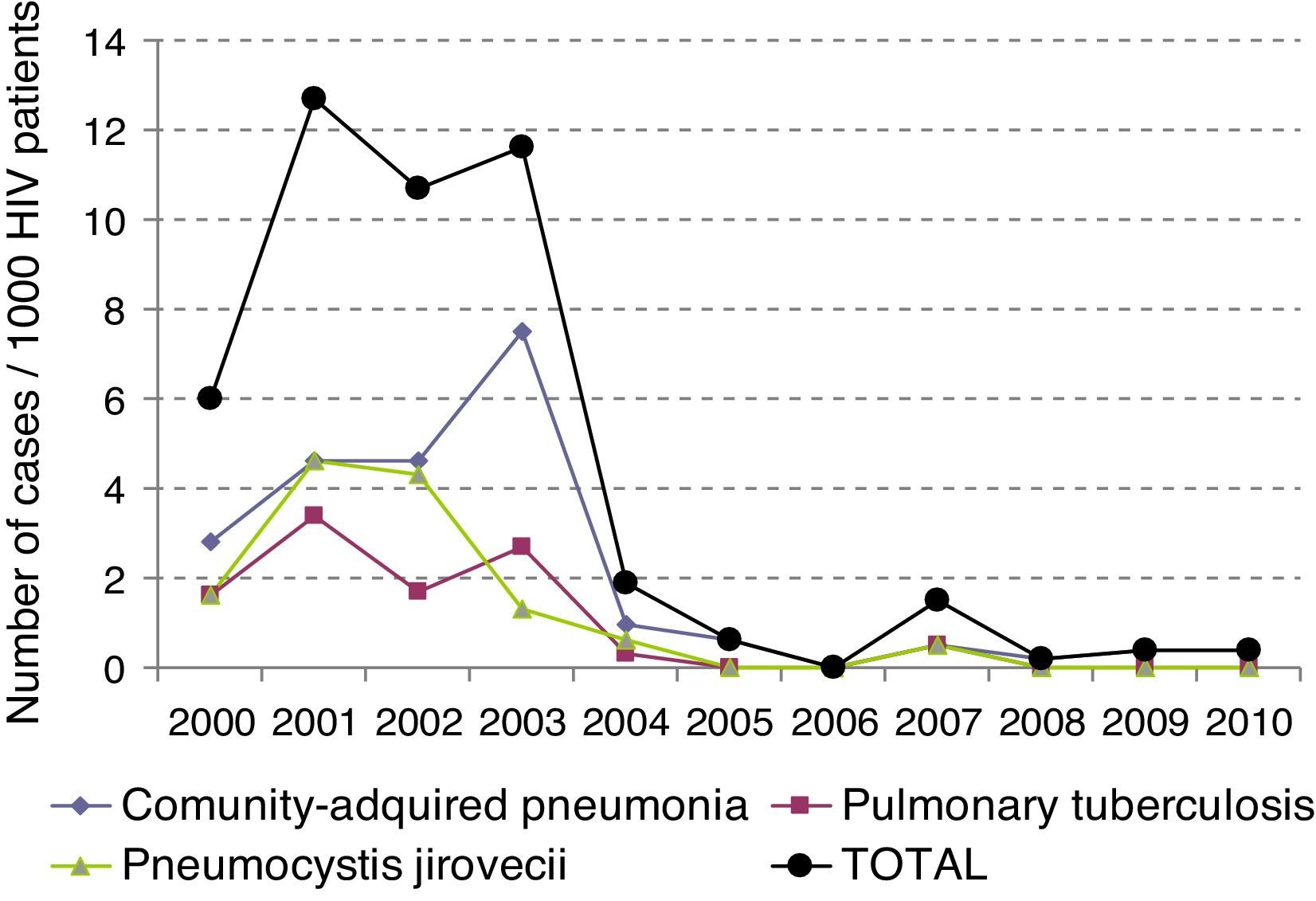
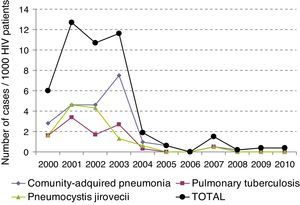
The incidence of LRTI has decreased over time (p<0.05), and has dropped from initially being 6.13×1000patients/year to being 0.23×1000patients/year in 2010 (Fig. 1).
The total mortality of the series was 14%, mainly due to PCP in 50% of the cases. The variables that predicted a bad prognosis were the viral load (p<0.007), absence of treatment with HAART (p<0.040), and admission to the ICU (p<0.001). Interestingly though, after logistic regression analysis, the variables that showed to be a predictor of death were admission to the ICU (p<0.004) and the viral load (p<0.029). Mortality from LRTI has been decreasing during the last decade, from a 14.2% in the period 2000–2004 to a 10% in the period 2005–2010 (data not shown). For all patients who died in our study, death took place at ICU.
DiscussionIn view of our results, LRTI accounts for 27% of the visits to the ED in the HIV population and is one of the highest motives for ED frequentation in the HIV population.20 These patients have a non-negligible mortality that reaches 14%. Attending the global cases, CAP continues to be nowadays the most frequent LRTI in the HAART, with S. pneumoniae being the most common bacterial etiology.21 Infections classically known as atypical, such as PCP infection, that appear in patients that have a poor immunological control (CD4<200cells/mcL)3 are rapidly falling in HIV patients receiving HAART, even though in our study, atypical infections continue to be the leading cause of mortality from LRTI. It is not always possible to determine the immunological status of the patient at the ED, and even more so, if the patient is not followed up in the same hospital, which can give rise to diagnostic doubts on whether the etiology of pneumonia may or may not be caused by PCP. Because of this, Napoli et al.22 found that a total lymphocyte value of <1700cells/mm is a predisposing factor, but not the only one to suffer an infection by PCP. Nowadays, LRTI by PCP is frequently seen in non-HIV immunodepressed patients, such as those with a transplanted solid organ, oncological patients and patients that suffer from connective tissue diseases. This makes it necessary to give this pathology more consideration and improve diagnostic testing in order to start treatment against PCP in this type of patients as early as possible.23,24 In our study we have seen that the CAP and PCP had similar mortality knowing that they exhibit different immune status, probably this result reinforces the study of Bordon et al.25 which asserts that neither the levels of CD4 or viral load is a factor of poor prognosis in HIV patient CAP hospitalized. The management of CAP in patients with HIV infection should not be based on CD4+ cell counts or HIV-RNA levels of the HIV infection.
TB was the third LRTI pathology accounting for 22% of the visits, being more common in the HIV/AIDS population and in continents such as Africa where it continues to have a high mortality.9 After decades of decline, TB continues to be a worldwide health problem in immunocompromised HIV patients. Microbiological diagnosis is not always easy. Not even when the most modern techniques are applied (such as the determination of the lipoarabinomannan enzyme by the ELISA assay in induced sputum),26 it is always possible to isolate mycobacterium. The standard tests to diagnose mycobacterium tuberculosis are less sensitive when the patient has low CD4 levels,27 which may, perhaps, explain the lack of microbiological diagnosis of some patients in our series. Early diagnosis of TB, as well as the use of HAART, improves prognosis, as its late onset in patients with a previously confirmed diagnosis of TB increases mortality; which highlights the importance of good immunological control in HIV patients.6,8 TB in Spain may be more common in immigrants. In our study there were 2 patients from Pakistan and one from Eastern Europe, although the only patient that died was native. During the last 30 years pulmonary involvement has played a major role in the history of HIV infection, being associated with greater impairment in self-reported physical function in these patients.28,29 With the introduction of HAART and subsequent immunological recovery of patients, pathology as cronical obstructive pulmonary diseases (COPD), a widely spread disease in the general population, caused most significantly by smoking,30 will increase and will probably become a new and significant comorbidity for HIV patients, especially where there is airway colonization by PCP.31 Hence, it would be advisable to conduct early diagnosis screening of COPD in HIV patients with predisposing risk factors,32 aimed at taking preventive measures. In a future health-care providers should be aware of the increased likelihood of airway obstruction among HIV patients.
Nowadays, as discussed above, HIV patients come to the ED more often due to pathologies that are not directly associated with HIV. This will continue to be the trend in patients with good immunological control, which, in turn, will entail longer hospital stays and higher utilization of health resources,10,11,33 with the aim of reducing their morbimortality and related healthcare costs.34 Finally, our study shows a decrease in the incidence of LRTI and associated mortality, even though it has not remained significant (14%) in the last ten years. These improvements are owed probably to HAART and to a wider range of antibiotics that were not available before. Our study has a series of limitations as it was conducted in a single hospital, although our hospital is one of the most important healthcare centers in Spain, not only in terms of number of HIV patients in follow-up but also in terms of emergency visits volume. Despite this limitation, the sample is large enough to draw some valid conclusions. The study of respiratory viruses was not carried out in its entirety. This is because the techniques that were available in our hospital until the mid-2000s, such as virus cultures and indirect immunofluorescence, provided low sensitivity in contrast with currently available molecular biology techniques.35. Consequently, viral pathology would have been underdiagnosed in the referred period of study. The availability of a specific daycare center in our hospital has made it possible to treat some uncomplicated LRTI without to referral to the ED. In addition, patients referred from other hospitals were excluded. Therefore, the incidence rates reported may be likely underestimating the real ones.
In summary our study, shows that LRTI is a condition in HIV-infected patients seeking acute care showing a decreasing incidence, a changing etiology to bacterial infections, and a trend to decreasing mortality (not significantly).
Elena Dopino (edopino@hotmail.com), Editing and Translation in Science and Medicine.