The use of the matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry has shown to be effective and fast in some clinical specimens for the identification of colonizing microorganisms. The objective of the study was to analyze the validity values for the prediction of colonization and catheter-related bloodstream infection (C-RBSI) of the MALDI-TOF mass spectrometry performed at all intravascular catheters that arrived in the microbiology laboratory.
MethodsCatheter tips (after performing the roll-plate technique) were tested by MALDI-TOF mass spectrometry during a period of 3-months. The gold standard for colonization and C-RBSI were, respectively: the presence of ≥15cfu/plate in the catheter tip culture; and the isolation of the same microorganism(s) in blood cultures as well as in the colonized catheter (during the 7days before or after catheter withdrawal).
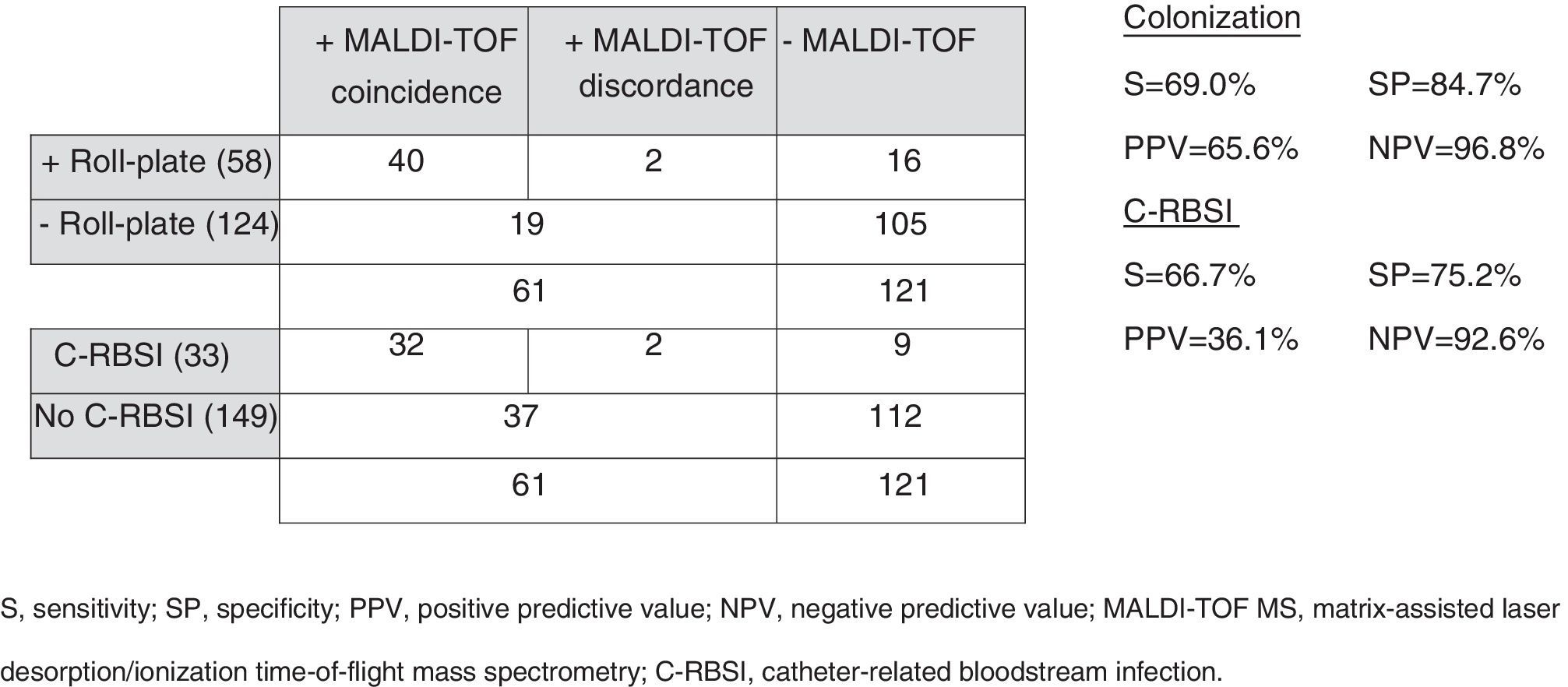
ResultsA total of 182 intravascular catheters were collected. The overall colonization rate detected by roll-plate technique and MAL-TOF mass spectrometry was 31.9% and 32.4%, respectively. Overall, there were 33 (18.1%) episodes of C-RBSI. The validity values of the MALDI-TOF mass spectrometry for the identification of colonization and C-RBSI were, respectively: sensitivity (69.0%/66.7%), specificity (84.7%/75.2%), positive predictive value (65.6%/36.1%), and negative predictive value (86.8%/92.6%). Conclusion MALDI-TOF mass spectrometry could be an alternative diagnostic tool for ruling out C-RBSI. However, despite it showing to be faster than conventional culture, future studies are required in order to improve the pre-analytical process.
La espectrometría de masas MALDI-TOF ha demostrado ser rápida y eficaz en la identificación de microorganismos que colonizan determinadas muestras clínicas. Nuestro objetivo fue analizar los valores de validez de la espectrometría de masas MALDI-TOF para predecir colonización y bacteriemia relacionada con el catéter (BRC) en todos los catéteres que llegaran al laboratorio de Microbiología.
MétodosDurante 3meses, la espectrometría de masas MALDI-TOF se realizó sobre las puntas de catéter recibidas (previo rodamiento para cultivo). Las reglas de oro de colonización y BRC fueron, respectivamente, la presencia de ≥15ufc/placa en el cultivo de la punta de catéter y el aislamiento del (de los) mismo(s) microorganismo(s) tanto en los hemocultivos como en el catéter colonizado (7días antes o después de la retirada del catéter).
ResultadosSe incluyeron un total de 182 catéteres intravasculares. La tasa global de colonización detectada por la técnica del rodamiento y la espectrometría de masas MALDI-TOF fue del 31,9 y del 32,4%, respectivamente. Hubo un total de 33 (18,1%) episodios de BRC. Los valores de validez de la espectrometría de masas MALDI-TOF para predecir colonización y BRC fueron, respectivamente: sensibilidad (69,0/66,7%), especificidad (84,7/75,2%), valor predictivo positivo (65,6/36,1%) y valor predictivo negativo (86,8/92,6%).
ConclusiónLa espectrometría de masas MALDI-TOF puede ser una herramienta de diagnóstico alternativa para descartar BRC. Sin embargo, a pesar de haber demostrado ser más rápida que el cultivo convencional, son necesarios futuros estudios que mejoren el proceso pre-analítico.
In order to confirm a diagnosis of catheter-related bloodstream infection (C-RBSI), it is necessary to isolate the same microorganism from both peripheral blood cultures and the catheter.1 However, routine microbiological analysis of catheter cultures requires at least 48hours before the microorganism is identified, thus leading to a potential delay in antibiotic therapy and adverse outcome. The use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) has proven effective for the rapid identification of isolated microorganisms and microorganisms in clinical samples.2–6
The purpose of our study was to determine validity values for the prediction of colonization and C-RBSI of MALDI-TOF MS performed directly on all intravascular catheters sent to our microbiology laboratory. We also determined whether this approach reduced the time to availability of results compared to conventional culture.
MethodsDuring a 3-month period, our working samples comprised either catheter tips (roll-plate and Gram staining) or sonication fluid from implanted reservoirs (1mL). The sample was introduced into 5mL of brain-heart infusion broth and incubated at 37°C with continuous shaking for 24hours. The tubes were centrifuged at 3,500rpm for 5minutes and the supernatant was discarded. The pellet was incubated with TWEEN-80 0.5% for 10minutes and spotted onto a MALDI-TOF target plate covered with 1μL of formic acid 100% and 1μL of matrix. The gold standards for colonization and C-RBSI were, respectively, ≥15 cfu/plate in the catheter tip culture and isolation of the same microorganism(s) both in blood culture and in the colonized catheter (during the 7 days before or after catheter withdrawal). We considered as a criterion of validity of the data provided by the MALDI-TOF those who were ≥1.7.
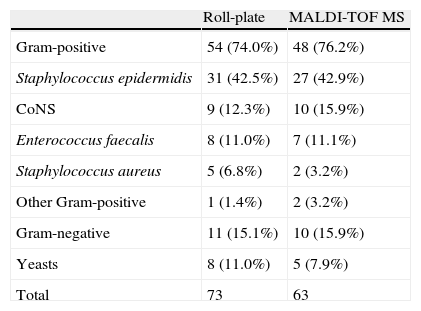
ResultsWe collected 182 intravascular catheters, as follows: central venous catheters, 122 (67.0%); peripherally inserted central venous catheters, 45 (24.7%); totally implantable venous access ports, 12 (6.6%), and peritoneal dialysis catheters, 3 (1.6%). Of the total, 43 (23.6%) were from patients from whom blood samples had not been taken for culture. The mean (SD) time for sending catheter tips after blood sampling was 2.44 (2.285) days. The overall colonization rate detected by the roll-plate technique and MALDI-TOF MS was 31.9% and 32.4%, respectively. Most colonized catheters detected by MALDI-TOF MS (98.4%) were associated with high scores (≥ 1.5). Gram staining of catheter tips was only positive in 13/182 cases (7.1%), although the results of Gram staining correlated with conventional culture and MALDI-TOF MS in 100% and 76.9% of cases, respectively. Conventional cultures were polymicrobial in 13/58 cases (22.4%), whereas MALDI-TOF MS only detected 1 catheter with polymicrobial colonization. The distribution of microorganisms is detailed in Table 1. We recorded 61 episodes (33.5%) of bloodstream infection, 33 of which (54.1%) were confirmed as C-RBSI. The validity values of MALDI-TOF-MS for the identification of colonization and C-RBSI are detailed in Figure 1. The mean (SD) time to identification of the microorganism was significantly lower using MALDI-TOF MS than using the roll-plate technique: 1.73 (1.16) vs. 3.60 (2.17) days (p<0.001), respectively.
Distribution of microorganisms detected by MALDI-TOF MS and roll-plate technique.
| Roll-plate | MALDI-TOF MS | |
| Gram-positive | 54 (74.0%) | 48 (76.2%) |
| Staphylococcus epidermidis | 31 (42.5%) | 27 (42.9%) |
| CoNS | 9 (12.3%) | 10 (15.9%) |
| Enterococcus faecalis | 8 (11.0%) | 7 (11.1%) |
| Staphylococcus aureus | 5 (6.8%) | 2 (3.2%) |
| Other Gram-positive | 1 (1.4%) | 2 (3.2%) |
| Gram-negative | 11 (15.1%) | 10 (15.9%) |
| Yeasts | 8 (11.0%) | 5 (7.9%) |
| Total | 73 | 63 |
CoNS, coagulase-negative staphylococci; MALDI-TOF MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.
Validity values of MALDI-TOF MS for the identification of colonization and C-RBSI.
Colonization
S, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value; MALDI-TOF MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; C-RBSI, catheter-related bloodstream infection.
A rapid diagnosis means better patient outcome and cost savings. To this end, it was recently demonstrated that MALDI-TOF improves the appropriateness of antibiotic treatment of bacteremia.7 Moreover, alternatives such as molecular techniques have proven very useful, particularly in the diagnosis of C-RBSI.8,9 A recent study by our group showed that 16S universal PCR was a good alternative diagnostic tool for ruling out port-related bloodstream infections.10 In the present study, we aimed to evaluate the yield of MALDI-TOF MS performed on all intravascular catheters received in the microbiology laboratory. Our data showed that, despite its low sensitivity for the prediction of catheter colonization, MALDI-TOF MS had a 92.6% negative predictive value for C-RBSI. Moreover, it demonstrated to be faster than conventional culture, which means that target therapy would be early performed and the patient management will be improved. One of the main limitations of the study was long period used in the pre-analytical procedure. Hence, future researches are needed to improve this step. Besides, although there was a low number of S. aureus and yeasts, MALDI-TOF could only detect 40% and 62.5% of these microorganisms, respectively. Therefore, negative results of MALDI-TOF should be interpreted with caution in patients with S. aureus or Candida spp. bloodstream infections.
MALDI-TOF MS could be an alternative diagnostic tool for ruling out C-RBSI. However, although it was faster than conventional culture, further research is needed to improve the pre-analytical process.
Financial supportThis study was supported by Ministerio de Sanidad y Consumo (Instituto de Salud Carlos III), Fundación Mutua Madrileña and Fundación para la Investigación Biomédica del Hospital Gregorio Marañón (FIBHGM) (CM09/00028).
We thank Thomas O’Boyle for his help in the preparation of the manuscript.
We thank the members of the GEIDI study group for their contribution to the work: José Eugenio Guerrero, Milagros Sancho, Braulio de la Calle, Carlos Sotillo, Guiomar Sánchez, Esther Bermejo López, Lorenzo Fernández Quero, Ana Lajara, Isabel Frías, Carmen Heras, María Jesús Pérez, José Maria Barrio, Alejandro Garrido Sanchez, Patricia Muñoz, Marta Rodríguez-Créixems, Mar Santos, Eduardo Verde, Fernando González García, Emilia Bastida, Maite López Gil, Teresa Blanco, Cristina Cuerda, Laura Frías, José María Tellado, Antonio Echenagusia, Fernando Camúñez, Gracia Rodríguez Rosales, Gonzalo Simó, Mikel Echenagusia, Sonia Zamorano Caballero, Ana Barrientos Guerrero, Abilio Calderón Martín, Carmen Flores Sánchez, Mª Jesús Ruano Santa Engracia, Esperanza Arranz García, Mª Ana Luna Caballero, Mar San Segundo Sánchez, Amelia V Fernández Alonso, Mª Nieves Moro Tejedor.