EUS-guided biliary drainage is a new tool for palliation of distal obstructive biliary lesions. In patients with distal biliary obstruction (pancreatic mass or papillary cancer) the creation of an EUS-guided access between the duodenal bulb and the distal common biliary duct is an effective method to relieve jaundice, with low morbidity and mortality. This technique is called choledochoduodenostomy and is reviewed in this article. EUS-guided biliary drainage should only be performed under strict research projects and only by very experienced pancreato-biliary endoscopists.
El drenaje biliar guiado por ultrasonido endoscópico (DBUSE) es una nueva herramienta para el tratamento paliativo de las obstrucciones biliares distales. En pacientes con lesiones pancreáticas o de la papila de Vater, la creación de un acceso guiado por DB-USE entre el bulbo duodenal y el colédoco es un método efectivo para mejorar la icterícia, que de acuerdo a los reportes existentes tiene una baja morbilidad y mortalidad. Esta técnica es conocida como coledocoduodenostomía. El presente trabajo es una revisión de la literatura médica, respecto al tema. El DB-USE actualmente debe hacerse bajo protocolos estrictos y solamente por endoscopistas muy experimentados.
Introduction
Endoscopic biliary stenting at ERCP is a well-established therapy for both benign and malignant biliary obstruction.1-3 To overcome ERCP failures and improve outcomes over those afforded by more invasive alternatives such as percutaneous transhepatic biliary drainage (PTBD) and/or surgery- EUS-guided ductal access techniques paired with standard ERCP drainage techniques have been developed in the last decade. This hybrid procedure has been given a variety of names, but the more encompassing one is endosonographic cholangiopancreatography (ESCP).4 Based on the combination of the three possible access routes (intrahepatic bile duct, extrahepatic bile duct and pancreatic duct) with the three possible drainage routes (transmural, transpapillary antegrade and transpapillary retrograde), ESCP admits nine variant approaches, six for the bile duct and three for the pancreatic duct.5,6 The six ESCP variant approaches to bile duct drainage are also referred collectively as EUS-guided biliary drainage (EUSBD). This review will focus on the EUSBD technique that provides transmural drainage from an extrahepatic bile duct access route, and is most commonly termed EUS-guided choledochoduodenostomy (EUS-CDS). Transmural intrahepatic EUSBD (hepaticogastrostomy) is discussed below, whereas transpapillary EUSBD (antegrade and rendezvous) will be also covered.
Rationale
As stated above, EUSBD is divided by the access route employed into EUS-guided intrahepatic bile duct drainage, where the intrahepatic bile duct is punctured from a transesophageal, transgastric or transjejunal approach, and EUS-guided extrahepatic bile duct drainage, where the common bile duct (CBD) is punctured from a transduodenal or transgastric approach (usually from the distal antrum). The overall rationale for EUS-CD is shared by the alternative EUSBD techniques, and it is threefold: 1) logistic advantage (it can be performed in the same session as the originally failed ERCP without further delay); 2) physiologic advantage (it provides immediate internal biliary drainage without the need for external drains); and 3) anatomic advantage (it can be tailored to the individual patient's anatomy; the precise imaging afforded by EUS resulting in a potentially less invasive procedure than PTBD).
In addition to the underlying common rationale for EUSBD implicit in EUS-CDS, there is a specific rationale for it. The CBD is more easily imaged under EUS than the intrahepatic bile ducts, in contrast to what happens under transabdominal US. This means that it can be imaged and accessed under EUS without added risks even in patients with minimal or no bile duct dilation. In those patients with dilated bile ducts, the CBD is a much more obvious target for puncture than the intrahepatic ducts. This results in faster, smoother access without repeated puncture attempts, thereby minimizing risks. The retroperitoneal location of the CBD makes it also an attractive access site for patients with ascites, in whom fluid around the liver makes transhepatic access (whether percutaneous or transgastric under EUS) more difficult and hazardous.
Besides the advantages of extrahepatic access over intrahepatic access, the specific rationale for EUSCDS is also derived from the transmural drainage route, as opposed to transpapillary EUSBD (antegrade or rendezvous). As explained in more detail, antegrade stent insertion from an extrahepatic access site is challenging and has only been reported in two exceptional cases.7,8 The real choice between transmural and transpapillary drainage after extrahepatic bile duct access under EUS therefore lies between EUS-CDS and rendezvous. Proponents of rendezvous argue that it may be less invasive than EUSCDS, since transmural intervention is usually limited to puncture and guidewire passage, then drainage is accomplished retrogradelly via ERCP without the need for puncture tract dilation.9 However, EUSBD rendezvous carries a 20% failure rate -even in expert centers- because guidewire passage across the stricture and the papilla is often unsuccessful. The needle allows virtually no interplay with the guidewire, which can not be manipulated across the stricture through a needle in the same way as it can be done at ERCP using flexible catheters. EUSBD needle-rendezvous (that is, without creating a fistula to allow passage into the bile duct through the puncture tract of flexible devices to help manipulate the guidewire antegradely) may require repeat punctures with different angles or trying different types of guidewires, often resulting in a prolonged, labor-intensive procedure. The second part of rendezvous following antegrade guidewire passage involves scope exchange and guidewire retrieval, and it is also cumbersome and plagued with problems. In summary, the advantages of EUS-CDS over transpapillary rendezvous are its higher success rate and relative simplicity, which appear to make it a more reproducible approach, despite being perhaps more invasive. Nonetheless, both EUSBD variant approaches can be considered complementary inasmuch as these procedures are used in a heterogeneous patient population. As we will discuss below, some indications are better suited for EUSCDS, whereas in other cases EUSBD rendezvous is clearly advantageous. Similarly, even if rendezvous is the intended drainage technique, EUS-CDS can be used as a second line approach to salvage the significant proportion of failed rendezvous cases.10,11 This open-ended approach to EUSBD (i.e. inclusive of both rendezvous and EUS-CDS) results in comparatively higher success rates than that of EUSBD series limiting their approach to just rendezvous.9
Technical data, discussion of possible therapies and recommendations for type of stent used and proposed endoscopic techniques
a. Indication
In common with other EUSBD techniques, EUSCDS should only be considered in patients with confirmed (not just suspected) biliary obstruction after failed ERCP despite maximal attempts by experienced operators. General patient, operator and equipment requirements are the same as for other EUSBD techniques. However, EUS-CDS has specific anatomic requirements differing from other EUSBD alternatives. The first anatomic requirement is distal biliary obstruction. In other words, EUS-CDS is not suitable for proximal (hilar) biliary obstruction, where intrahepatic EUSBD approaches are clearly required. The second anatomic requirement is the ability to image the CBD under EUS. Since the CBD is typically imaged from the distal stomach or the duodenal bulb, this is difficult to impossible in patients with prior gastrectomy and gastrojejunostomy (e,g, Roux-en-Y).12
Finally, as with most other EUSBD approaches, EUS-CDS is predominantly used in patients with malignant biliary obstruction. But whereas alternative approaches such as rendezvous might be considered appropriate after failed cannulation in patients with documented benign causes of biliary obstruction (eg, CBD stones or papillary stenosis), EUS-CDS is less adequate in these settings, where biliary drainage is usually accomplished by means of sphincterotomy (with or without stone removal) as opposed to stenting.
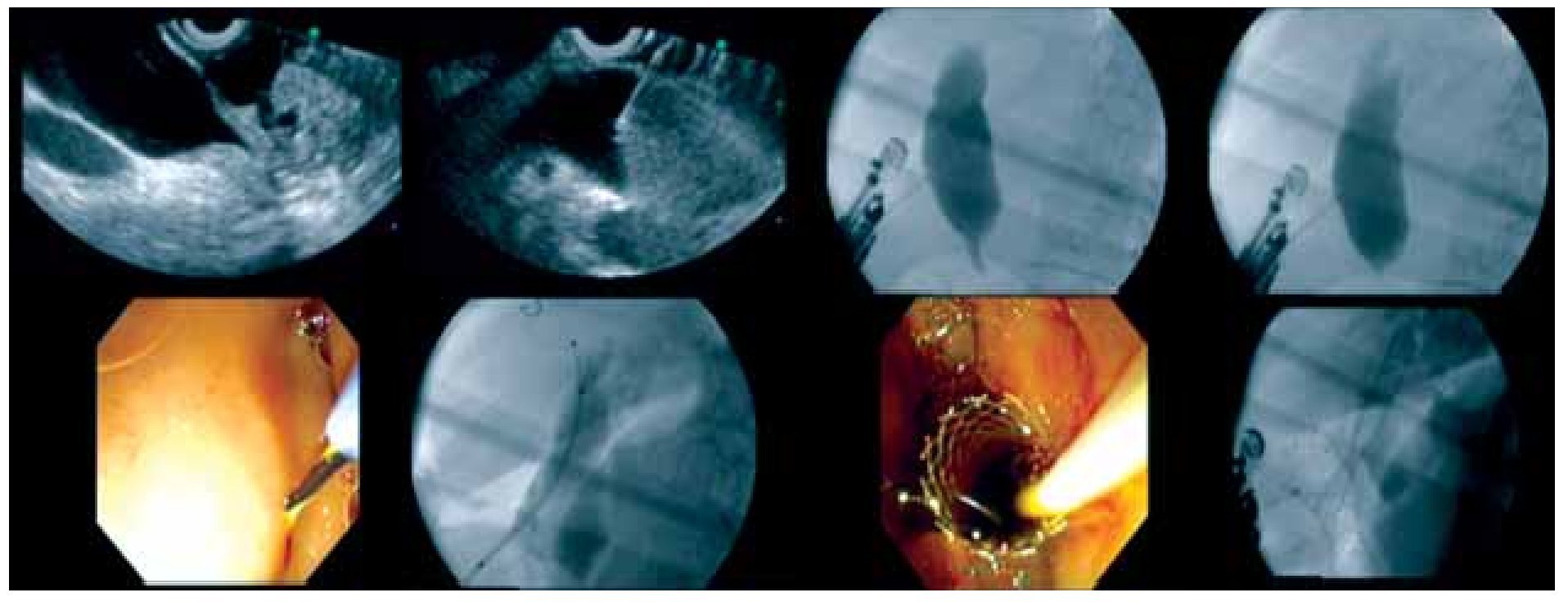
b. Procedure (Figure 1)
○ Figure 1. Technical sequence of the procedure of EUS-guided choledochoduodenostomy. Clockwise image sequence show: EUS images of dilated CBD above the stenotic tumor, needle into to the CBD, cholangiography, and passage of guide wire, needle-knife creating fistulization in the bulb, deployment of the biliary and, in this case, a duodenal stent.
As stated above, puncture of the CBD from the duodenum (EUS-CDS) is the most common approach. A similar approach from the stomach (EUS-choledochogastrostomy or EUS-choledochoantrostomy) may also be used in selected instances depending on the patient's anatomy (see below). The CBD is visualized from the duodenal bulb by using a curved linear array echoendoscope in a long or a short scope position. The direction of the needle in the long scope position is toward the hilar (proximal) bile duct. The direction of the needle in the short scope position is toward the lower (distal) bile duct. The correlation between scope position and needle orientation is not always straightforward. Anatomic distortion may make necessary additional fine adjustments involving torque of the echoendoscope shaft and/or the control wheels. The orientation of the needle can be checked with fluoroscopy before the puncture is actually carried out. It is relevant to do so, because an upward needle orientation makes EUS-CDS easier, since it tends to decrease the angle for transmural stent advancement over the guidewire into the bile duct. Conversely, a downward needle orientation is sought when rendezvous is intended as the initial drainage choice.
Two types of needle devices are available for access. Conducting flexible needles (Precut needle knife), commonly used at ERCP for pre-cut and pseudocyst drainage, using electrocautery (EndoCut ICC200®, ERBE GmbH, Tübingen, Germany). A special needle-knife (Zimmon papillotome®, Cook Endoscopy, Winston-Salem, NC), used also for precut, produces axial cutting with a alightly thicker wire than other needle-knives, has a wire extending 2 mm beyond the tip of the catheter. So called Cystotomes (Cook Endoscopy®, Winston-Salem, NC; Endoflex®, Tubingen, Germany, available in 6, 8.5 and 10Fr diameters), traditionally used for pseudocyst drainage, have a blunt, round metal tip that transmits electrical current and produces a larger orifice than needle knives. Cystotomes are also slightly stiffer than needle-knifes and produce a larger burn on the duodenal and CBD walls. This larger, round controlled perforation reduces the need for dilatation before stent insertion. Cystotomes are therefore particularly useful in cases where resistance to the advancement of flexible devices over the wire into the duct is met. Thinner caliber cystotomes (6Fr) are preferable to larger caliber ones (10Fr). Needle-knives, on the other hand, being more flexible, can be used free-hand under EUS as the initial access device. There are also non-conducting stiff cutting needles, commonly used for EUS-guided fine needle aspiration (EUS-FNA). EUS-FNA needles are available in several calibers. The two most commonly used are the large 19-gauge needle and the thin 22-gauge needle (EchoTip®, Cook Endoscopy). A specific needle for EUSBD has recently been developed by Cook (19 gauge HD Access needle). Whatever the needle choice, it is inserted transduodenally into the bile duct under EUS visualization. To confirm needle ductal access, the stylet is removed and bile is aspirated. If there is a bile return, contrast medium is injected into the bile duct for cholangiography, then, a 450 cm long, 0.035-inch, 0.021-inch, or 0.018-inch guidewire is inserted through the outer sheath and its position is confirmed fluoroscopically. We will comment below on differential guidewire features. If there is no return of bile or a bloody aspirate, the needle is removed, flushed with saline inside the gastrointestinal lumen to prevent clogging, and a repeat puncture attempted. Nonetheless, the problem of a needle appearing inside the duct under EUS but actually on a different plane, usually occurs only when accessing very small ducts, which is hardly ever the case during EUS-CDS. After guidewire access into the bile duct, some dilation of the puncture track is usually necessary, using either a dilating biliary catheter (Soehendra biliary dilator®, Cook Endoscopy), a papillary balloon dilator (Maxpass®, Olympus medical systems, Tokyo, Japan) or both sequentially (axial dilator first, then balloon dilator). This is aimed at dilating the duodenocholedochal fistula to facilitate stent insertion. The need for dilation is maximal when no cautery is used for initial entry under EUS, a stiffer (metal) or larger caliber plastic (10Fr) stent is intended, and when the distance to the CBD or the resistance felt during the initial advancement of the needle are greater. Finally, a 5Fr to 10Fr biliary pig-tail or straight plastic stent or a fully covered self-expandable metal stent (SEMS) (Zeon Medical Co. Ltd®, Tokyo, Japan) is inserted through the choledochoduodenostomy site into the CBD. Care should be taken to monitor by fluoroscopy the intraductal placement of the proximal end of the stent and to monitor by endoscopy the intraduodenal (or intragastric) position of the distal (closer to the scope) end of the stent. This latter aspect is of particular relevance when using SEMS. SEMS tend to foreshorten upon full expansion, which takes place a few hours after the procedure. Early SEMS dislodgment may be caused by foreshortening towards the CBD beyond the GI wall. To prevent this serious complication an adequate length of SEMS (15-20 mm) should be left inside the GI lumen. This is longer than what is customarily done when placing SEMS transpapillary at ERCP. Additional anchorage techniques to prevent dislodgment are forceful balloon dilation of the SEMS up to 8-10 mm after initial deployment, or the use of a coaxial double pig-tail trhough the SEMS, as reported for pseudocyst drainage using transmural SEMS.13
Despite the seemingly simple sequence of duct imaging and puncture under EUS, guidewire advancement and track dilation under fluoroscopy, and eventually stent insertion and deployment under combined fluoroscopic and endoscopic monitoring, EUS-CDS is an invasive, complex procedure. Knowledge of the full array of needle devices, guidewires, dilators and stents as well as about the subtle variations in scope position (gastric or duodenal), scope orientation (upward and downward), and stent anchoring techniques is highly recommended to increase success rates and minimize complications. Operator confidence with specific devices also plays a role. Some authors feel that access without cautery is less prone to complications. These authors favor initial non-conducting needle access and then use cautery only selectively after failed mechanical dilation over the guidewire of the puncture tract.6,14 Mechanical dilation without cautery requires a stiffer 0.035-inch guidewire for support, which in turn involves the use of a 19-gauge EUS-FNA needle. Other authors find the stiffer 19-gauge EUS-FNA needles cumbersome to use in the relatively long position of the echoendoscope in the duodenum, and resort to either initial direct needle-knife access under EUS15 or needle-knife access under a thinner 0.018 guidewire passed into the CBD after puncture with a 22-gauge EUSFNA needle.16 Finally, some other authors resort to both needle-knife and EUS-FNA needle access.17 These procedural variations as reported in the literature are discussed next.
Literature findings based on the perspective of evidence-based medicine5-27
EUS-guided choledochoduodenostomy was first reported by Giovannini et al.18 Some authors exchanged the echoendoscope over a catheter-protected guidewire for a duodenoscope, through which the stent was eventually inserted. As detailed earlier, the puncture needles available are conducting needles and nonconducting needles. About half and half have been used in published reports. This is in contrast to what is reported for intrahepatic EUSBD, where nonconducting needle access is clearly preferred. The reason why cautery access (conducting needle) is favored during EUS-CDS is probably fourfold. Firstly, for EUS-CDS the echoendoscope is in a longer, curved position in the duodenum in comparison with the shorter distance to the subcardial region from where intrahepatic access is typically gained. This long position increases friction between the stent delivery system and the endoscope working channel, which impairs the transmission of the pushing force, thereby making transmural stent insertion more difficult. Secondly, the thicker, fibrous wall of the CBD is harder to penetrate mechanically than the relatively soft liver parenchyma (except in cases with underlying cirrhosis) and the wall of smaller bile ducts. Thirdly, the tendency to create a space by pushing until the bile duct wall yields, is greater between the duodenal wall and the CBD than between the gastric wall and the liver. Finally, the CBD is larger and has the nearest vessels at a greater distance than the intrahepatic bile ducts (where vessels run closely in parallel), which offers some protection against severe bleeding, a feared complication of cautery access.
In most reported cases, a plastic stent has been placed. However, recently, the use SEMS is increasingly been reported.14 The success rate for the 61 cases reported to date is as high as 95%, with excellent results in all successfully drained patients (100% per-protocol clinical response rate). There were some cases where stent insertion was too difficult and a nasobiliary drainage tube was placed instead.17,24 Another interesting variation on EUS-CDS is illustrated by a few cases where the extrahepatic bile duct was punctured from the stomach rather than the standard transduodenal approach.14,20 Although only 6 cases were reported, all were successful.
Expected complications and treatment options
Complications can be divided into procedure-related complications and stent-related complications. Definitions of procedural complications are not well standardized. Most are related to bile (or just air) leakage into the retroperitoneum (with transduodenal access) or the peritoneum (with transgastric access to the CBD), with or without added infection. The severity ranges from a self-limiting condition that resolves within 48-72 hours with conservative measures, to full-blown peritonitis requiring emergency surgery. Most reported complications are mild. The need for emergency surgery is exceedingly rare. Other interventional measures that may be required in the event of complications, such as percutaneous drainage, are however not uncommon.
Peri-procedural leakage of bile into the abdominal cavity is most likely due to poor drainage. Poor drainage can be caused by factors such as too large a fistula, early stent clogging, and inappropriate positioning of the stent (including foreshortening of SEMS).
Late stent-related complications, that is, once a mature fistula is formed, are similar to those seen with transpapillary stents placed at ERCP, namely, migration and stent occlusion. Stent migration or occlusionare managed in the same way as in stents placed at ERCP, by inserting a new stent. The technique for repeat stent placement differs from what is commonly done at ERCP. If a clogged plastic stent is in place across the fistula, a guidewire is advanced through the stent and the stent is grasped with a snare passed over-the-wire and removed over it. This somewhat more complex maneuver is aimed at keeping guidewire access to the duct after stent removal. After plastic stent removal, a SEMS may be placed using a duodenoscope. If clogging of a SEMS occurs, the debris occluding its lumen may be cleaned up. But just cleaning is probably not long-lasting in this setting. A new coaxial stent needs to be placed inside the clogged one, either a plastic stent, or a SEMS, the so called stent-in-stent approach.
Distal stent migration into the GI tract lumen with a mature fistula only involves repeat biliary drainage, since migrated stents usually pass out spontaneously. Repeat biliary drainage may be attempted in several ways. The mostsimple one is placing a new stent through the same fistula, if it is still visible. If the fistula cannot be identified endoscopically, either repeat EUS-CDS through a new puncture site or PTBD are required. If proximal stent migration to the retroperitoneum or the peritoneum occurs, recovery of the stent as well as emergency surgery should be considered. This serious complication, however, has not yet been reported for EUS-CDS. Finally, even if the less serious distal migration occurs but the fistula is still immature (a fibrous track not yet formed), this may cause bile leakage into the abdomen. In the event of stent migration and leakage with an immature fistula, repeat EUS-guided biliary drainage (perhaps using a SEMS), or PTBD need to be considered. Surgery should also be considered depending on the patient's condition.
Conclusion
Although the current data is still limited, EUS-guided extrahepatic transmural bile duct drainage has a high potential as an alternative biliary decompression procedure in cases of failed ERCP. It is a complex, invasive procedure that requires careful patient selection and an experienced operator supported by a well-trained team in a multidisciplinary setting. Specific patient's anatomic factors favor EUS-CDS over complementary EUSBD approaches. Although multicenter trials aimed at standardizing the technique for performing EUS-guided extrahepatic transmural bile duct drainage would be desirable, the relatively few patient candidates for it and the wide spectrum of technical variations reported to date make this endeavor difficult to accomplish in the near future. Detailed prospective studies with homogeneous inclusion criteria and careful follow-up and dedicated hands-on training models will probably be more effective in advancing this burgeoning field of interventional endoscopy.
Correspondence:
Everson L.A. Artifon.
University of Sao Paulo (USP).
Rua Guimaraes Passos, 260/121.
Vila mariana. Sao Paulo, Brazil.
Z.P. 040030.000.
E-mail: eartifon@hotmail.com