Changes in water metabolism and regulation of vasopressin (AVP) or antidiuretic hormone (ADH) are common complications of pituitary surgery. The scarcity of studies comparing different treatment and monitoring strategies for these disorders and the lack of prior clinical guidelines makes it difficult to provide recommendations following a methodology based on grades of evidence. This study reviews the pathophysiology of diabetes insipidus and inappropriate ADH secretion after pituitary surgery, and is intended to serve as a guide for their diagnosis, differential diagnosis, treatment, and monitoring.
Las alteraciones en el metabolismo del agua y en la regulación de la vasopresina (AVP) u hormona antidiurética (ADH) son complicaciones frecuentes de la cirugía hipofisaria. La escasez de estudios que comparen diversas estrategias de tratamiento y monitorización de estos trastornos, así como la falta de guías clínicas previas, hace difícil hacer recomendaciones siguiendo la metodología basada en grados de evidencia. Este trabajo revisa la fisiopatología de la diabetes insípida y la secreción inadecuada de ADH en el postoperatorio de la cirugía hipofisaria, y pretende servir de guía en su diagnóstico, diagnóstico diferencial, tratamiento y monitorización.
Antidiuretic hormone (ADH) or vasopressin (AVP) is a nonapeptide synthesized at the supraoptic and paraventricular nuclei. After being stored as a prehormone in intracytoplasmic granules, ADH is transported along axons in the median eminence to the neurohypophysis, where it is converted into the native hormone. Once released together with neurophysin ii, it is bound to specific V2 receptors located in the basal portion of collecting tubule cells. This interaction results in displacement to the apical membrane of aquaporin 2 preformed in the cytoplasm and in passive water reabsorption.
AVP is mainly regulated by plasma osmolality (Osmp) and effective circulating volume. Sodium is the predominant cation in the extracellular compartment, and is therefore the main determinant of plasma osmolality. Small changes in serum sodium levels modify ACP secretion.
Hypothalamic osmoreceptors maintain Osmp in a range from 280 to 290mOsm/kg by modifying AVP secretion which is maximally inhibited at values below 280mOs/kg. Above 290mOsm/kg, both AVP secretion and thirst osmoreceptors are maximally stimulated.1
These guidelines review the pathophysiology and clinical management of water metabolism disorders occurring after pituitary surgery. No prior clinical guidelines are available on the management of water and electrolyte disorders after pituitary surgery, and no studies have been reported comparing different treatment and monitoring strategies. These guidelines do not therefore use a methodology based on grades of evidence, but provide an updated review of the subject, making suggestions for action based on the various sources consulted.
Diabetes insipidus after pituitary surgeryDiabetes insipidus (DI) occurs as a consequence of decreased or absent AVP. DI is characterized by polydipsia and polyuria with voiding of diluted or hypotonic urine. If water excretion exceeds water supply, natremia and Osmp are increased, which may cause hypovolemia and hypotension in extreme situations.
During pituitary surgery, some of the secretion and/or release levels of AVP may be affected (hypothalamus, pituitary stalk, or neurohypophysis), causing DI.
EpidemiologyTransient DI is a very common perioperative complication of pituitary surgery, but its incidence depends on the degree of clinical suspicion and the diagnostic criteria used. Hensen et al. noted polyuria in the early postoperative period in 31% of 1571 patients undergoing surgery for pituitary adenoma,2 and Nemergut et al. in 18.3% of 881 patients operated on for various sellar lesions.3 In a prospective, controlled study conducted on 52 patients who underwent surgery for pituitary adenoma, 38% experienced isolated DI and 15.7% combined DI (DI followed by hyponatremia).4 Persistent DI is however uncommon (0.25% and 2%).2,3
Factors apparently increasing the risk of DI include:
Patterns which depend on the course of the disease. PathophysiologyTransient diabetes insipidusThis usually occurs 24–48h after surgery and resolves in the following 10 days.4 It is caused by the transient dysfunction of AVP-secreting neurons resulting from trauma induced by surgical manipulation or by decreased blood supply to the stalk and posterior pituitary gland. The condition resolves when the neurons recover their normal function.6,7
Permanent diabetes insipidusThis only occurs when more than 80–90% of AVP-secreting hypothalamic neurons degenerate.7 Degeneration is the more likely, the higher the stalk lesion.2,6,7
Triphasic patternThis is relatively uncommon (1.1% of operated patients).2 It is characterized by three consecutive phases: DI lasting 5–7 days, followed by a second phase of variable duration (2–14 days) of inappropriate antidiuresis (SIADH) due to uncontrolled AVP release from the degenerated nerve endings in the posterior pituitary gland, with a trend to hyponatremia and hypo-osmolality.6 The third phase consists of the recurrence of DI, which is often permanent,8,9 once AVP deposits have depleted and the number of neurons able to synthesize it is insufficient.
Biphasic patternThe occurrence of the first two phases only is more common than the triphasic pattern (3.4% in the Hensen et al. study and 15.7% in the prospective Kristof et al. study2,4).
Inappropriate antidiuretic hormone secretion aloneNot preceded or continued by DI.
Clinical signsThe sudden occurrence of polyuria and polydipsia is characteristic. It is traditionally described as a craving for drinking cold water. If fluids are not adequately replaced by the oral or parenteral route, volume depletion and hyperosmolality may occur, leading to the development of neurological symptoms such as irritability, lethargy, confusion, and coma.10
DiagnosisA diagnosis of DI after pituitary surgery should be considered in the presence of a high urine output, usually >40–50mL/kg/day in adults (normally 3–3.5L/day) or >100mL/kg/day in children, and typically >2.5mL/kg/h of hypotonic urine. A urine volume greater than 200–300mL per hour for 2–3 consecutive hours is a reasonable marker for suspecting this diagnosis.6 Since most patients are awake in the postoperative period, maintain their thirst mechanisms intact, and have access to fluid, volume depletion, hypernatremia, and hyperosmolality are relatively uncommon, although they may be seen in children, the elderly, or patients with impaired consciousness or who restrict fluid intake.
Laboratory data should show the presence of hypotonic polyuria: urinary osmolality (Osmu)<100mOsm/kg (or specific density<1005) or inappropriately low in relation to plasma osmolality. Hyperosmolality and hypernatremia support the diagnosis.
A suggestive clinical picture with a consistent Osmp/u ratio is sufficient to make the diagnosis. Water restriction for a few hours is only occasionally required to confirm the diagnosis, and a prolonged water restriction test is needed even more rarely. The hypertonic saline test is not performed, except in clinical studies7 (Table 1).
Diagnosis of DI after pituitary surgery.
| Signs and symptoms |
| Polyuria, polydipsia, thirst, typically occurring within 24–48h of surgery/h |
| Usual urine output 4–18L/day of diluted urine (>40–50mL/kg/24h; >2.5mL/kg/h; or >250mL/h×2 consecutive hours) |
| Significant hypovolemia (rare in alert situation and with a preserved thirst mechanism) |
| Laboratory data |
| Normal or increased natremia |
| Normal or increased serum osmolality |
| Urinary osmolality<100 or lower than plasma osmolality |
Assess for diabetes mellitus, diuretic treatment, and acromegaly, and differentiate from polyuria based on greater perioperative fluid provision.
Modified from Dumont et al.6
The lack of typical hyperintensity in the neurohypophysis in MRI is not relevant in the postoperative period after pituitary surgery.
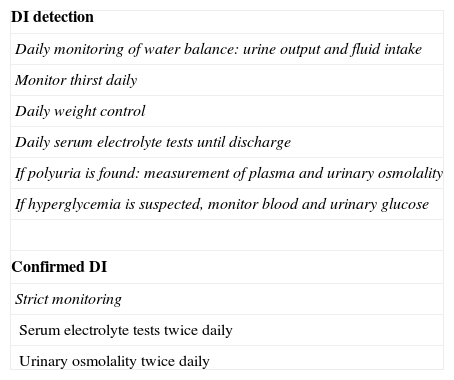
Because of the high frequency of DI in the postoperative period, water balance should be assessed daily, with urine output and water intake (fluid ingested, serum therapy, and water contained in medication) being monitored. Daily weight control and questioning about thirst are also recommended. The measurement of serum electrolyte levels is advised a few hours (6–8h) after surgery and daily thereafter until discharge.10 If polyuria is found, Osmp and Osmu should be measured. If DI is confirmed, such measurements should ideally be made twice daily9 (Table 2).
DI monitoring after pituitary surgery.
| DI detection |
| Daily monitoring of water balance: urine output and fluid intake |
| Monitor thirst daily |
| Daily weight control |
| Daily serum electrolyte tests until discharge |
| If polyuria is found: measurement of plasma and urinary osmolality |
| If hyperglycemia is suspected, monitor blood and urinary glucose |
| Confirmed DI |
| Strict monitoring |
| Serum electrolyte tests twice daily |
| Urinary osmolality twice daily |
The presence of polyuria after pituitary surgery may be due to causes other than DI:
- -
Osmotic polyuria: because of the presence of glycosuria in patients with hyperglycemia, Osmu may be falsely increased by glycosuria, and sodium levels may be artefactually lower in the presence of hyperglycemia.6
- -
Postoperative excretion of excess intravenous fluids administered perioperatively: this is very common. Typically, natremia is normal or low, and there is no increase in thirst.6 A careful review of water balance should provide a diagnosis.
- -
Acromegaly: increased urine output may occur after adenoma resection.8
The objective of treatment is to ensure the restoration and/or maintenance of osmotic homeostasis. Treatment should be individualized for each patient and depend on DI severity and duration.
DI is mild and transient in most cases and requires no specific treatment. If the patient is conscious and has the sensation of thirst preserved, thirst itself is the best guide to the need for water intake, and only stricter monitoring is recommended.
If the patient is unable to replace urinary losses by the oral route due to intolerance of oral intake or inadequate perception of thirst, water balance may be maintained by intravenous serum therapy. Water deficiency in liters may be estimated using the following formula: 0.6×body weight (kg)×([serum Na/140]−1). It is recommended that serum electrolyte levels be measured every 6–8h to verify that water provision is adequate.7
Drug treatment is reserved for cases where polyuria is excessive and uncomfortable for patients, particularly during the night, or if hypernatremia occurs.9 Desmopressin (DDAVP), an AVP analog which interacts with V2 receptors only and has therefore no pressor action, is the drug of choice. Desmopressin rapidly decreases urine output, and its effect lasts 6–12h. It may be administered by the subcutaneous route (dose of 1–2μg), widely used in the early postoperative period, intranasally (10μg) or orally (0.1mg).5 Very occasionally, desmopressin is administered as a continuous intravenous infusion, but only in adults.7
Since postoperative DI is usually short-lasting, a single dose of desmopressin is usually sufficient.8,10 The correction of DI should be assessed by monitoring urine output, serum levels, and urinary osmolality. This allows for deciding whether additional doses are required, if polyuria persists, taking into account that excess treatment may lead to water retention and hyponatremia and that DI may be followed by hyponatremia from SIADH.9
In patients discharged on desmopressin, weekly monitoring for 3–4 weeks is advised, the dosage being adjusted depending on DI severity. Periodic drug discontinuation allows for assessing whether or not it is still required8 (Table 3).
Treatment of DI after pituitary surgery.
| Drug treatment |
| Subcutaneous or intravenous desmopressin. Starting dose, 1–2μg |
| Repeat the dose when urine output is between 200 and 250mL/h for ≥2h with osmolality<200mOsm/kg or urinary specific gravity<1005 |
| Indications: patient unable to maintain oral fluid intake, urine output higher than fluid intake, hypernatremia |
| Maintenance of water balance |
| Recommend patients to drink depending on thirst |
| If patients are able to maintain plasma osmolality and normal serum sodium levels, administer supplemental intravenous hypotonic solutions (5% glucose solution, followed by 0.45% glucose solution) |
| Replacement therapy of the hypothalamic-pituitary-adrenal axis |
| Administer stress doses of glucocorticoids (hydrocortisone 50–100mg IV every 8h), gradually switching to an oral maintenance dose (15–30mg/d). This dose should be continued until anterior hypophysis function may be fully assessed |
| Monitoring of resolution of transient DI |
| Check for the disappearance of polyuria and the normalization of plasma osmolality and serum electrolyte levels |
| A positive water balance>2L suggests inappropriate antidiuresis |
| If this occurs, in addition to discontinuing desmopressin, water intake should be restricted to maintain serum sodium at normal levels |
DI may occur concomitantly with impaired thirst when surgery damages the hypothalamic osmoreceptors that regulate thirst and AVP secretion. This is highly uncommon, but its management is difficult and it causes high morbidity and mortality. The tumor most commonly causing this condition is craniopharyngioma.
Sites secreting AVP are sometimes intact (they respond with AVP release to a drug-induced hypotensive stimulus) and AVP is continuously released in small amounts irrespective of natremia and plasma osmolality. When the hypothalamic lesion is more extensive, the sense of thirst is lost and no AVP secretion occurs in response to any stimulus, so increasing the risk of dehydration and severe hypernatremia.11
When the sense of thirst is absent or inadequate, patients are at risk of experiencing dehydration and hypernatremia. By contrast, excess fluid intake when AVP is not suppressed, or during treatment with AVP analogs, may promote water intoxication and hyponatremia. Patients fluctuate between these two situations which are otherwise difficult to differentiate because both of them may cause drowsiness, weakness, irritability, nausea, vomiting, seizures, and coma.12,13 Patients often have moderate and well tolerated chronic hypernatremia for long time periods. Venous thrombosis, favored by such chronic hypernatremia, is not infrequent.11
Other symptoms due to hypothalamic involvement may also occur, such as obesity, sleep apnea, seizures, and thermoregulatory dysfunction.11
DI is usually permanent, but thirst impairment may improve or even disappear, usually in the nine months following surgery, suggesting that thirst osmoreceptors, unlike AVP-secreting sites, maintain some capacity for recovery. However, recovery more than one year after surgery is exceptional.14
DiagnosisA diagnosis of adipsia/hypodipsia may easily be made when a patient with hypernatremia or hyperosmolality denies having thirst and/or does not drink spontaneously or even shows an aversion to fluids.13 Thirst may be estimated more objectively using validated visual analog scales.15
Different tests may be performed to verify the diagnosis or to categorize the type and degree of involvement:
- -
A hypertonic saline test (0.05mL/kg/min for 2h or until an Osmp of 300mOsmol/kg is reached) to measure AVP release and thirst in response to an increase in Osmp. Natremia, Osmp, and AVP are monitored every 30min, and natriuria and Osmu every hour. Blood pressure and thirst sensation are also recorded using a visual analog scale.11,14
- -
A water overload test (30mL/kg of water by mouth over 15min) to assess the suppression of AVP release in response to a hypotonic stimulus in the following 4h.13
- -
A test of AVP release upon non-osmotic stimulation using drug-induced hypotension.11,13
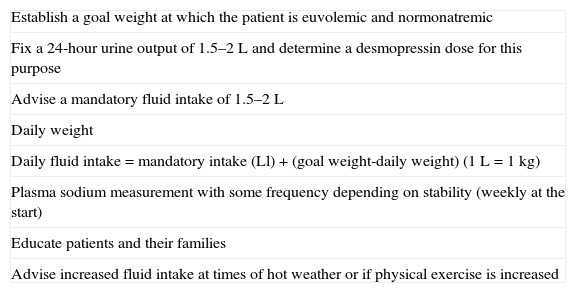
The management of these cases is usually complex and requires the training and collaboration of patients and their relatives. If the correction of hypernatremia or hyponatremia by changing DDAVP doses is attempted, sharp fluctuations between these two situations may easily occur. The most widely accepted approach is to maintain a fixed DDAVP dose and to establish a mandatory fluid intake of approximately 1.5L, which is increased or decreased based on daily changes in weight11,13 (Table 4). Special attention is required in conditions in which insensible water loss may be increased, such as high temperatures, intercurrent diseases, etc.
Treatment of DI with adipsia (based on 11,13).
| Establish a goal weight at which the patient is euvolemic and normonatremic |
| Fix a 24-hour urine output of 1.5–2L and determine a desmopressin dose for this purpose |
| Advise a mandatory fluid intake of 1.5–2L |
| Daily weight |
| Daily fluid intake=mandatory intake (Ll)+(goal weight-daily weight) (1L=1kg) |
| Plasma sodium measurement with some frequency depending on stability (weekly at the start) |
| Educate patients and their families |
| Advise increased fluid intake at times of hot weather or if physical exercise is increased |
Antithrombotic prophylaxis with low molecular weight heparin may be advised, at least during episodes of hypernatremic dehydration.6,11
Inappropriate antidiuretic hormone secretion after pituitary surgeryEpidemiologyInappropriate antidiuretic hormone secretion (SIADH) is a relatively common complication of transsphenoidal surgery. Hyponatremia of any cause occurs in 13–35% of patients undergoing transsphenoidal surgery, and is symptomatic in 2–7%.2,4,16–22 The reason for the above variability is that it is often overlooked because its symptoms are usually mild and non-specific and may also be seen in patients with normal or low sodium levels.16 Some of these surgical series attempted to better estimate the incidence of SIADH, by differentiating it from other conditions associated with hyponatremia. However, they reported only slightly lower percentages.18,23
Some studies reported female sex22 or Cushing's disease2,16 to be predisposing factors, but most studies published did not find age, sex, or tumor type or size to be associated with a statistically significant excess risk.17,18,21,22 By contrast, the presence of postoperative DI did increase the risk of subsequent SIADH in some series.2,4,18,22
Pathophysiology. Patterns of inappropriate antidiuretic hormone secretionSIADH after pituitary surgery is attributed to the uncontrolled release of AVP by degenerating AVP-secreting hypothalamic neurons whose cell bodies or axons forming the hypothalamic-pituitary stalk have been damaged during surgery. SIADH may also be secondary to other complications of surgery such as meningitis, intracranial bleeding, etc. Relative AVP excess leads to concentrated urine, with decreased urine volume and inappropriately high Osmu, and when patients continue drinking and receiving intravenous fluids, to hyponatremia and hypo-osmolality. Renal sodium handling remains unchanged thanks to the adequate functioning of the renin–angiotensin–aldosterone axis, atrial natriuretic peptide, and other natriuretic factors. Any plasma volume expansion therefore causes renal excretion of sodium and water, which maintains a euvolemic state.10
In rare instances, SIADH is part of the previously discussed classical triphasic response to damage of the hypothalamic-pituitary stalk.
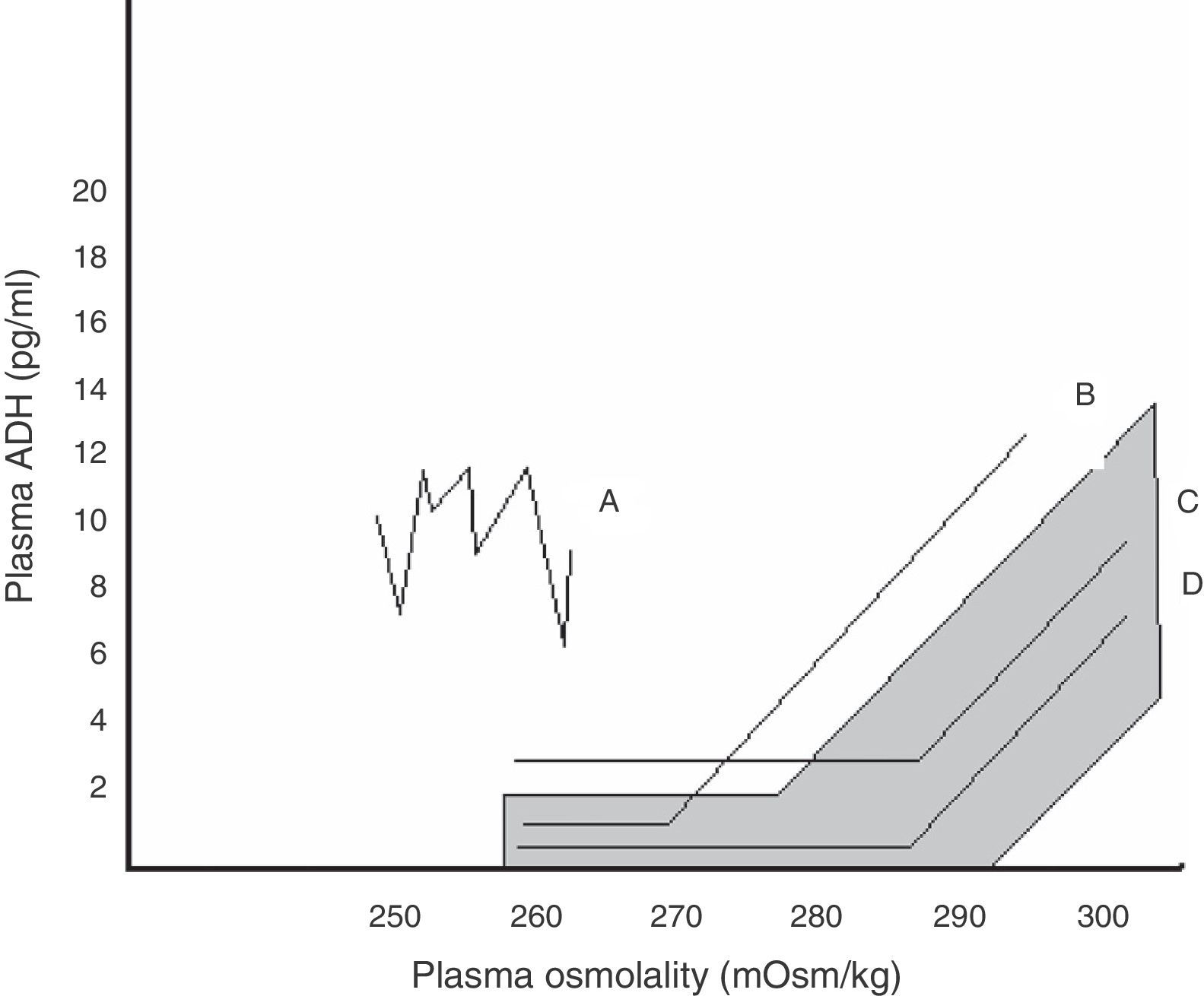
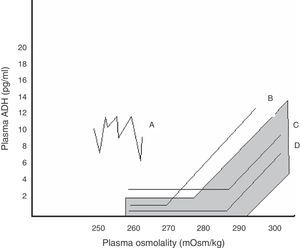
Within SIADH, several response patterns of AVP to changes in plasma osmolality have been described. These patterns are depicted in Fig. 1. No correspondence has been found between these patterns and the different causes underlying SIADH, and no studies have assessed which pattern predominates after pituitary surgery.24
Relationship between plasma AVP and plasma osmolality in the different patterns of SIADH. The shaded area corresponds to normal values.
Pattern A. Erratic AVP secretion, the most common. Pattern B. Reset osmostat. AVP adequately responds to changes in plasma osmolality, but with a curve displaced to the right from normality. Pattern C. AVP is adequate for plasma osmolality in its normal and high values, but is not suppressed at low plasma osmolality values, perpetuating hypo-osmolality. Pattern D. Normal AVP secretion. It is hypothesized that hypo-osmolality is due to increased sensitivity to AVP effects.
SIADH usually occurs between four and 10 days after surgery. SIADH causes no or mild symptoms in most cases and is usually diagnosed by hyponatremia found in postoperative control tests. Symptoms are due to plasma hypo-osmolality and the resultant cell edema, and their severity depends on hypernatremia intensity and the rate of occurrence. Hyponatremia, which is sometimes associated with serious complications or death from brain edema, may be well tolerated if the body, and specifically the central nervous system cells, have had time to adapt by releasing other osmoles, thus maintaining Osmp similar to the extracellular to prevent edema.24,25 Other associated metabolic changes (hypoxia, acidosis, hypercalcemia) may increase the natremia threshold associated with symptom occurrence.
Its presentation is similar to that of other water and electrolyte and acid–base balance disorders. The most common symptoms include headache, irritability, concentration difficulties, weakness, and nausea. These symptoms are quite non-specific, particularly in patients recently operated on, and are therefore often overlooked, hence the importance of routine electrolyte monitoring after pituitary surgery to diagnose hyponatremia before it becomes more severe. Moderate hyponatremias may also cause vomiting, which may in turn aggravate hyponatremia, disorientation, confusion, restlessness, muscle cramps, and anorexia. In more severe cases, impaired consciousness that may end in coma, seizures, signs of extrapyramidal involvement, brainstem herniation, and death may occur. An absence of focal neurological signs makes it possible to differentiate hyponatremia from other neurosurgical complications.
Hyponatremia from any cause is known to be associated with greater mortality and an increased incidence of falls and longer mean hospital stay, with the risk being the greater, the lower the sodium level, particularly when no action is taken to correct it.24,25 Although no specific studies in patients undergoing pituitary surgery are available, these risks are assumed to be similar to those in other clinical settings.
Diagnosis and differential diagnosisSIADH should be suspected in all patients who have symptoms suggesting hyponatremia in the days following pituitary surgery. However, laboratory control tests should also be performed in asymptomatic patients.10 Since hyponatremia and hypernatremia cause no or non-specific symptoms in most cases and their management is easier if they are mild, electrolyte testing in blood may be recommended 6–8h after surgery, and if levels remain within the normal range, daily thereafter until discharge.
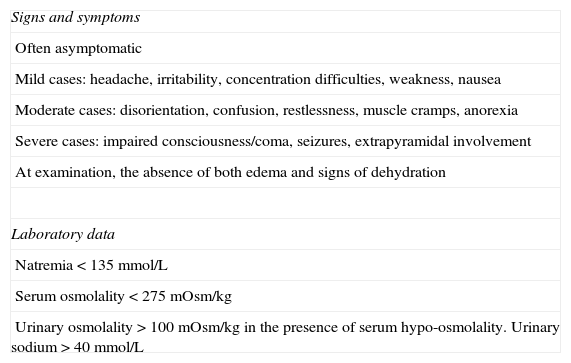
SIADH may be diagnosed in a patient with hyponatremia (serum sodium level <135mmol/L) and plasma osmolality (osmolality <275mOsm/kg), inappropriately concentrated urine in relation to plasma osmolality (>100mOsm/kg), usually with elevated sodium urinary levels (>40mmol/L), with normal sodium intake, in the presence of normal extracellular volume, and once renal failure and glucocorticoid and thyroid hormone deficiencies have been ruled out.26,27 A physical examination is essential to determine if extracellular volume is normal. It should show no edema or other signs of water overload, nor skin and mucosal dehydration, orthostatic hypotension, tachycardia, or other signs of hypovolemia. The measurement of central venous pressure is not necessary, but helps to confirm an euvolemic state when available. Other biochemical findings supporting a diagnosis of SIADH include decreased serum levels of urea, uric acid, and hemoglobin, all of them related to the hemodilution that occurs (Table 5).
Diagnosis of SIADH after pituitary surgery.
| Signs and symptoms |
| Often asymptomatic |
| Mild cases: headache, irritability, concentration difficulties, weakness, nausea |
| Moderate cases: disorientation, confusion, restlessness, muscle cramps, anorexia |
| Severe cases: impaired consciousness/coma, seizures, extrapyramidal involvement |
| At examination, the absence of both edema and signs of dehydration |
| Laboratory data |
| Natremia<135mmol/L |
| Serum osmolality<275mOsm/kg |
| Urinary osmolality>100mOsm/kg in the presence of serum hypo-osmolality. Urinary sodium>40mmol/L |
All of this in the presence of normal effective circulating volume and once renal failure, adrenal failure, and hypothyroidism have been ruled out. Assess for diabetes mellitus, diuretic treatment, and acromegaly, and differentiate from polyuria based on greater perioperative fluid provision.
Differential diagnosis should include other causes of hyponatremia in the setting of pituitary surgery (Table 6). In the first few hours after anesthesia, the most common cause is the excess administration of intravenous fluids. It is associated with hypotonic polyuria, which should not be confused with DI (in which high or normal-high natremia is found).
Causes of hyponatremia after pituitary surgery.
| Hypotonic fluid overload during surgery |
| SIADH |
| Cerebral salt-wasting syndrome |
| Secondary adrenal insufficiency |
| Central hypothyroidism |
| Desmopressin intoxication |
| Other drugs: diuretics, antipsychotics, SSRIs, tricyclic antidepressants, NSAIDs, etc. |
| Hyperglycemia |
NSAIDs: non-steroidal anti-inflammatory drugs; SSRIs: serotonin selective reuptake inhibitors; SIADH: syndrome of inappropriate antidiuretic hormone secretion.
Secondary adrenal insufficiency also usually appears in the first few days. This is a known complication of both pituitary tumors and their surgery, and hyponatremia is one of its usual clinical signs and is associated with hypotension, decreased extracellular volume, malaise, weakness, and nausea. In addition, cortisol is a physiological inhibitor of AVP secretion, and its deficiency therefore promotes the occurrence of SIADH.5,10 However, the widespread prophylactic use of high-dose glucocorticoids before surgery makes this complication uncommon. A dramatic improvement after corticoid administration also supports diagnosis, even in the presence of relatively normal serum cortisol levels, which may reflect relative adrenal insufficiency in a stress situation.
Secondary or central hypothyroidism is another potential cause of hyponatremia. This is usually an undiagnosed or inadequately treated preoperative deficiency. Extracellular volume is usually normal. Inadequate plasma AVP levels are also found in these cases. Their cause is not exactly known, but may include decreased AVP clearance, osmostat readjustment, or the baroreceptor-mediated stimulation of AVP secretion in response to decreased cardiac output.5,10
Hyperglycemia causes the downregulation of serum sodium levels to maintain a normal Osmp, which is associated with polyuria due to osmotic diuresis induced by glycosuria. Thus, unlike in SIADH, this hyponatremia is not associated with hypo-osmolality.
Drugs should also be considered as a potential cause. DDVAP is the drug most commonly responsible for hyponatremia. If patients do not decrease fluid intake or, what is more common, continue to receive intravenous fluids, they develop a clinical condition similar to “water intoxication” which is virtually identical to that occurring in SIADH. In addition, as previously noted, patients with preoperative DI are more prone to develop SIADH. Because of this, caution should be taken when DDAVP is prescribed, and it should not be forgotten that DI is often transient and may be managed with adequate fluid replacement, with no need for drug treatment. Other drugs that may promote hyponatremia include diuretics, antipsychotics, serotonin selective reuptake inhibitors, tricyclic antidepressants, sedatives, and non-steroidal anti-inflammatory drugs.8,27
The condition most similar to SIADH and the one that raises the most doubt is cerebral salt-wasting syndrome. The pathophysiology of this syndrome has still to be fully elucidated. It is assumed that some natriuretic factor released by the central nervous system in response to surgical aggression stimulates urine output and natriuresis and suppresses aldosterone secretion even in a context of hypovolemia and hyponatremia.25,28 Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), synthesized in significant amounts in the hypothalamus, have been proposed as candidates, but the results of their measurement in affected patients are conflicting. This condition shares many characteristics with SIADH, because it is also characterized by hyponatremia and hypo-osmolality and inadequately concentrated urine, inadequate natriuria, and normal thyroid and adrenal function. Urinary volume may be greater as compared to SIADH, but there is too much overlapping of both conditions for this to be of help in differential diagnosis. Plasma AVP levels may be low in response to hypo-osmolality, but may also be somewhat elevated, stimulated by hypovolemia, and are, therefore, of no diagnostic value either. The main difference is that excess natriuresis is associated with decreased extracellular volume, which leads to decreases in body weight and central venous pressure and hemoconcentration (higher levels of urea, uric acid, hemoglobin, potassium, etc.). In addition, plasma aldosterone is decreased. Adequate differentiation between this condition and SIADH is important because the treatment of salt-wasting syndrome involves adequate water and salt replacement, and even the use of fludrocortisone has been suggested, while the initial approach in SIADH is precisely water restriction.12,25,26,29
To sum up, there are several factors that may guide diagnosis when hyponatremia occurs after pituitary surgery. If the time of occurrence is considered, iatrogenic overload of hypotonic fluids, secondary adrenal insufficiency, or AVP release due to no-osmotic stimuli such as pain or nausea are more common in the first few days, while SIDAH or cerebral salt-wasting syndrome are more likely to occur one week after surgery or later.5,8,10,28 Based on extracellular volume, SIADH or hypothyroidism should be considered in euvolemic patients, while the presence of signs of hypovolemia should suggest adrenal insufficiency, hyperglycemia, or salt-wasting syndrome.
TreatmentAlthough no studies comparing different treatment and monitoring strategies in SIADH are available, the current approach is relatively similar in the different hospitals, as is shown by the various publications on the subject, and is widely accepted.30,31
Before specific treatment for SIADH is started, other causes contributing to hyponatremia or SIADH should be ruled out or corrected to the extent possible.5,8,10 When doubt exists as to whether the patient has SIADH or salt-wasting syndrome, an infusion of physiological saline (e.g. 1000mL) and a re-evaluation of the patient is recommended: if natremia clearly improves, the patient very likely has volume and solute depletion, but if the patient has SIADH, he/she will most likely excrete the excess water and sodium received but osmolality will not be modified, or will even worsen if urinary osmolality is very high.32
The first decision to be taken in any patient with hyponatremia is the rate at which it should be corrected. For this decision, both the risks of hyponatremia and those of its rapid correction, mainly osmotic demyelination syndrome (ODS), should be weighed. In the event of acute hyponatremia, which is arbitrarily defined as that lasting less than 48hours, the risk of brain edema and brainstem herniation is great, because the brain has no time to adapt and eliminate intracellular solutes, and the risk of myelinolysis is small, even with rapid correction of hyponatremia. By contrast, in chronic hyponatremia (>48h), the neurological signs will be less marked and the risk associated with rapid correction will be much greater.
ODS is the most serious complication of rapid correction. ODS consists of myelin degeneration in the central nervous system mainly affecting the brainstem, the basal ganglia, the corpus callosum, and the hippocampus. It is associated with high morbidity and mortality, because it may cause permanent neurological sequelae. The clinical signs usually include dysarthria, spastic paraparesis or tetraparesis or pseudobulbar paralysis, which occur after initial neurological improvement due to improved hyponatremia. The risk of myelin degeneration mainly depends on the natremia correction rate, even if natremia remains at lower than normal levels, but also on the severity and duration of pre-existing hyponatremia. Alcoholic or malnourished patients or those with hypokalemia are more prone to suffer ODS. Diagnosis may be confirmed by MRI, although the characteristic images may take days or even a few weeks to appear.
In order to decide the correction rate, it is necessary to take into consideration whether there have been factors contributing to hyponatremia which are reversible (adrenal insufficiency after treatment, the discontinuation of treatment with desmopressin or diuretics, etc.), because once they are removed, natremia will tend to improve spontaneously and water diuresis will occur.
In asymptomatic patients with mild hyponatremia, above 125mmol/L, a restriction of fluid to less than 1000mL and a liberalization of salt intake, usually low in hospital diets, is normally sufficient. The main difficulty in applying this treatment is that patients continue to be thirsty despite hypo-osmolality, and are sometimes unable to comply with the restriction.28,33 If a patient with moderate hyponatremia (120–125mmol/L) has no or minimal symptoms, chronic hyponatremia should be assumed and slowly corrected. A somewhat more aggressive (less than 500mL daily) water restriction (WR) or an initial infusion of 3% hypertonic saline (HS) to increase natremia at a rate of 0.5mmol/L/h is recommended. This same approach is also advised in some cases with more intense hyponatremia, in patients with only a few symptoms in whom long-lasting hyponatremia is suspected, and in those in whom the correction rate should be even slower. If patients have symptoms, e.g. progressively impaired consciousness or seizures, or an acute onset of hyponatremia, an infusion of HS is recommended at an initial rate of 1mmol/L/h for the first few hours. If the administration of saline causes volume overload, a concomitant administration of furosemide is recommended.
For practical purposes, the following formula may be used to predict the increase in natremia that will occur in response to the infusion of a given amount of saline:
Expected increase in natremia (mmol/L) (after the administration of 1000mL of saline)=(Infused sodium [mmol/L]−serum sodium [mmol/L])/Total body water (L)+1.
To calculate total body water, female weight (kg) should be multiplied by 0.5, and male weight by 0.6.
Regardless of the correction rate selected, the discontinuation of acute treatment is recommended when: (1) patient symptoms resolve; (2) safe natremia is achieved (usually 120mg/dL); or (3) a total magnitude of correction of 10–12mmol/L in 24h or 18mmol/L in 48h is reached.28,31,33,34 Initial control laboratory tests are recommended at 2–4h. The Furst formula (Na+u+K+u/Na+p) is helpful for taking decisions in mild to moderate cases because it makes it possible to predict the effectiveness is of WR: if the result of the formula is >1, the patient is not excreting free water by the renal route, and is unlikely to improve with WR.35
Repeat monitoring of natremia at 12–24h is suggested in mild cases, while in severe cases monitoring is advised 2h after treatment start and every 4h thereafter until the previously discussed goals are achieved and patients may be switched to less aggressive treatment (RH+salt provision or a vasopressin antagonist).
Although various drugs (demeclocycline, lithium), or urea, are used for the treatment of SIADH, none of them have been assessed in controlled clinical trials after transsphenoidal surgery. All of them will probably be displaced by non-peptide antagonists of the V2 receptor of AVP or vaptans, which act as pure aquaretic drugs that promote free water release without affecting solutes, unlike diuretics, which promote the removal of both. Tolvaptan, indicated for hyponatremia associated with SIADH, is marketed in Spain. Although tolvaptan has not been specifically tested in the context of pituitary surgery, the results of clinical trials suggest that it is effective and safe for the correction of dilutional hyponatremia.34,36 No evidence suggesting that tolvaptan decreases the morbidity and mortality associated with hyponatremia is available, and no use of the drug in severe hyponatremia has been reported (i.e. in no patients with sodium levels <115mmol/L). Its effect is considerably faster than that of WR, significantly increasing natremia in 24–48h. The administration of tolvaptan should be started at the hospital in order to monitor the correction rate and adjust the dosage, with initial controls every 6h. The most common adverse effect is a sensation of thirst and a dry mouth. The starting dose is 15mg once daily, but may be increased by up to 60mg/day. The prescription of tolvaptan is advised in mild patients after the failure of WR or in moderate cases as an alternative to hypertonic saline or, after this, once a safe natremia level has been achieved.26,33,37 It may also be used in chronic SIADH, but this complication is highly uncommon after transsphenoidal surgery.
The early discharge of patients undergoing transsphenoidal surgery, three or four days after the procedure, is increasingly common. The peak incidence of SIADH occurs after the first week, and the condition, therefore, often becomes manifest when patients are already at home. It is therefore advisable to recommend at discharge a moderate restriction in fluid, but not salt, intake, except in patients with DI, and to inform patients about the potential symptoms associated with hyponatremia, so that they are encouraged to consult their physician if such symptoms occur. A good but insufficiently widespread practice is to perform control laboratory tests 7–10 days after surgery at local healthcare centers, with patients being referred to the hospital if the results are abnormal.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Lamas C, del Pozo C, Villabona C, en representación del Grupo de Neuroendocrinología de la SEEN. Guía clínica de manejo de la diabetes insípida y del síndrome de secreción inapropiada de hormona antidiurética en el postoperatorio de la cirugía hipofisaria. Endocrinol Nutr. 2014;61:e15–e24.