The aim of this paper is to review the main aspects related to high bone density (HBD) as well as to discuss the physiologic mechanisms involved in bone health. There are still no well-defined criteria for identification of individuals with HBD and there are few studies on the topic. Most studies demonstrate that overweight, male gender, black ethnic background, physical activity, calcium and fluoride intake and use of medications such as statins and thiazide diuretics play a relevant and positive role on bone mineral density. Moreover, it is known that individuals with certain diseases such as obesity, diabetes, estrogen receptor-positive breast or endometrial cancer have greater bone density than healthy individuals, as well as athletes having higher bone density than non-athletes does not necessarily mean that they have healthy bones. A better understanding of risk and protective factors may help in the management of patients with bone frailty and have applicability in the treatment and in the prevention of osteoporosis, especially intervening on non-modifiable risk factors.
El objetivo de este artículo es revisar los aspectos principales relacionados con la Densidad Ósea Elevada (DOE) y analizar los mecanismos fisiológicos implicados en la salud ósea. No existen aún criterios bien definidos que sirvan para identificar a los individuos con DOE, y los estudios sobre el tema son escasos. La mayoría de los estudios demuestran que el exceso de peso, el sexo masculino, la raza negra, la actividad física, la ingesta de calcio y flúor y el uso de medicamentos como las estatinas y los diuréticos tiazídicos desempeñan un papel relevante y positivo en la Densidad Mineral Ósea (DMO). Además, se ha observado que los individuos con enfermedades tales como obesidad, diabetes, cáncer de mama positivo para receptores de estrógenos o cáncer del endometrio tienen mayor DMO que los individuos sanos; del mimo modo, se observa una mayor DMO en atletas frente a los que no lo son, sin que ello necesariamente sea sinónimo de salud ósea. Un mejor entendimiento de los factores de riesgo y de protección podría ayudar a mejorar el tratamiento de los pacientes con fragilidad ósea e incidir en la prevención de la osteoporosis, especialmente en los factores de riesgo no modificables.
The high prevalence of osteoporosis and fractures due to bone frailty worldwide underscores the importance of seeking new prevention and treatment strategies,1 such as the identification of factors involved in the increase of bone mineral density (BMD) for possible applicability in high risk populations. Few studies have addressed the prevalence of high BMD or the main clinical and laboratory aspects of individuals with high BMD. Most of these studies indicate that the following factors seem to have a positive influence on bone density: anthropometric factors (weight and body mass index [BMI] above 30kg/m2),2 demographic factors (male gender, black ethnic background),3 genetic factors (LRP 5 mutations),4 healthy lifestyle habits (regular physical activity,5 calcium intake above 1500mg/day)6 and use of medications such as statin7 and thiazide diuretics.8 Moreover, individuals with certain diseases as obesity, estrogen receptor-positive breast or endometrial cancer9,10 and type II diabetes11 have greater BMD than that of healthy individuals and among healthy subjects as athletes have greater bone density than non athletes.12 Based on the items addressed in the studies cited above, the aim of this paper is to review and discuss the aspects related to high BMD and to bone health.
High bone mineral densityThe occurrence of high bone density (HBD) has been described in the literature in the last ten years. However, yet there are no well-defined criteria for adequate and precise identification of HBD. In 1994, the World Health Organization defined criteria to characterize normal and low bone density (osteopenia and osteoporosis).13 However, there is currently no consensus on the cutoff point for the definition of individuals with high BMD.
A cohort study involving more than 1800 women14 suggests that the cutoff point for high BMD is 1.209g/cm2 for the femoral neck and 1.228g/cm2 for the lumbar spine (upper quartiles). Assessing more than ninety six thousand bone densitometry exams, Gregson et al.15 defined Z-score equal to or greater than 3.5 standard deviations for spine and/or hip for the characterization of such individuals; based on this criterion, only 169 individuals with HBD were found (approximately 0.2% of the overall sample). Beginning with the premise that a T-score less than or equal to −2 standard deviations indicates low bone density and so a greater risk for fractures, it is reasonable to assume that a T-score above +2 standard deviations in the absence of fractures or diseases known to affect bone quality to define HBD.
However, there is no reference in these definitions regarding the relation between high bone mass and bone health reflected by a lower rate of stress or bone frailty fractures and better bone quality and strength. In other words, it is not known if higher BMD is truly associated with bone healthy.
The main factors associated to HBD are unknown.4 Genetic, mechanical, environmental, nutritional and endocrine factors are among the determinants related to the acquisition peak bone mass, to bone health and potential factors associated with HBD are discussed below.
Genetic factorsGenetic factors account for 75–80% of the variation in bone mass peak.16 An increase in bone mass may be caused by rare (often hereditary) osteochondrodysplasias and a variety of dietetic, metabolic, endocrine, hematological, infectious and neoplastic disorders.17
Low-density lipoprotein receptor-related protein 5 (LRP5) gene mutations and some other mutations are among the most studied genetic factors.
LRP5 is involved in the Wnt canonical signaling pathway, in which it acts as a coreceptor and regulator of the intracellular signaling of β-catenin. Expressed in various tissues, LRP5 is considered a key protein for the physiology of bone tissue as well as in different pathological processes that include bone formation or neoformation.18 Mutations in the LRP5 gene are responsible for bone abnormalities, such as high bone mineral density and osteoporosis-pseudoglioma syndrom.4
Genetic mutations and gene polymorphisms may cause either positive or negative phenotype modifications in bone tissue. Thus, it is of clinical importance to know whether an excess in expression or gain in function of a particular gene, such as VDR, could be associated to high BMD or to a lesser risk of fractures.
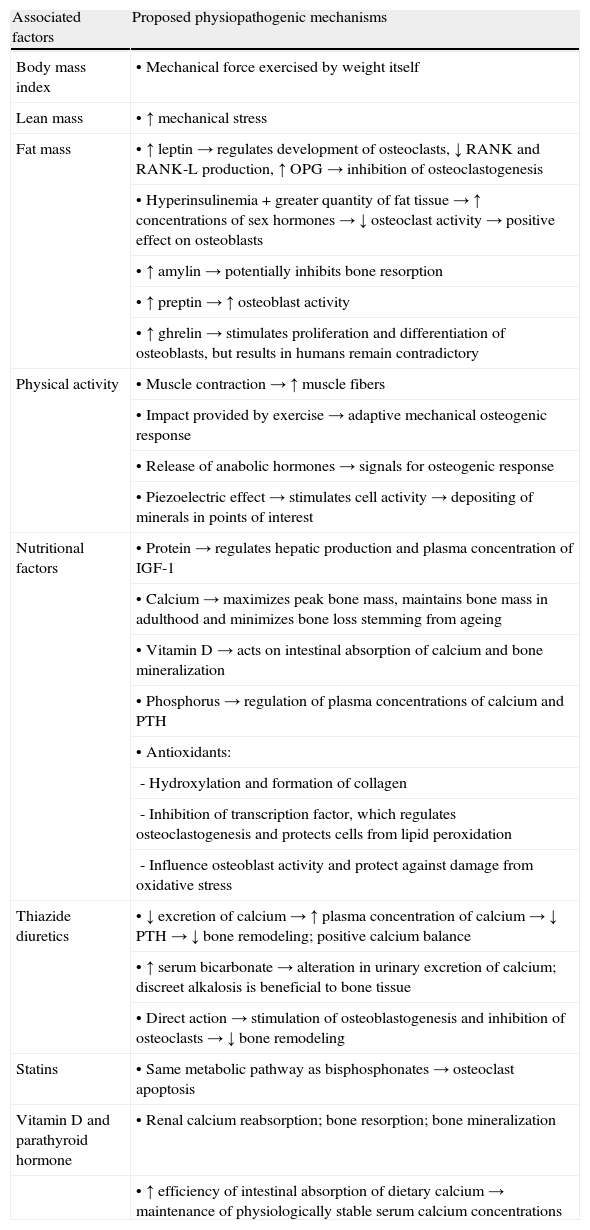
Non-genetic factorsNon-genetic factors account for 20–25% of peak bone mass and may change over time. The main modifiable factors and physiopathogenic mechanisms suggested for health and increase in bone mineral density are displayed in Table 1.
Modifiable risk and protection factors and physiopathogenic mechanisms proposed to explain the association between high bone density and bone health.
| Associated factors | Proposed physiopathogenic mechanisms |
| Body mass index | • Mechanical force exercised by weight itself |
| Lean mass | • ↑ mechanical stress |
| Fat mass | • ↑ leptin→regulates development of osteoclasts, ↓ RANK and RANK-L production, ↑ OPG→inhibition of osteoclastogenesis |
| • Hyperinsulinemia + greater quantity of fat tissue→↑ concentrations of sex hormones → ↓ osteoclast activity→positive effect on osteoblasts | |
| • ↑ amylin→potentially inhibits bone resorption | |
| • ↑ preptin→↑ osteoblast activity | |
| • ↑ ghrelin→stimulates proliferation and differentiation of osteoblasts, but results in humans remain contradictory | |
| Physical activity | • Muscle contraction→↑ muscle fibers |
| • Impact provided by exercise→adaptive mechanical osteogenic response | |
| • Release of anabolic hormones→signals for osteogenic response | |
| • Piezoelectric effect→stimulates cell activity→depositing of minerals in points of interest | |
| Nutritional factors | • Protein→regulates hepatic production and plasma concentration of IGF-1 |
| • Calcium→maximizes peak bone mass, maintains bone mass in adulthood and minimizes bone loss stemming from ageing | |
| • Vitamin D→acts on intestinal absorption of calcium and bone mineralization | |
| • Phosphorus→regulation of plasma concentrations of calcium and PTH | |
| • Antioxidants: | |
| - Hydroxylation and formation of collagen | |
| - Inhibition of transcription factor, which regulates osteoclastogenesis and protects cells from lipid peroxidation | |
| - Influence osteoblast activity and protect against damage from oxidative stress | |
| Thiazide diuretics | • ↓ excretion of calcium→↑ plasma concentration of calcium→↓ PTH→↓ bone remodeling; positive calcium balance |
| • ↑ serum bicarbonate→alteration in urinary excretion of calcium; discreet alkalosis is beneficial to bone tissue | |
| • Direct action→stimulation of osteoblastogenesis and inhibition of osteoclasts→↓ bone remodeling | |
| Statins | • Same metabolic pathway as bisphosphonates→osteoclast apoptosis |
| Vitamin D and parathyroid hormone | • Renal calcium reabsorption; bone resorption; bone mineralization |
| • ↑ efficiency of intestinal absorption of dietary calcium→maintenance of physiologically stable serum calcium concentrations |
RANK: receptor activator of nuclear factor Кβ; RANK-L: receptor activator of nuclear Кβ ligand; OPG: osteoprotegerin; IGF-1: insulin-like growth factor; PTH: parathyroid hormone.
Details on the positive influence of these factors on bone tissue are provided below.
Anthropometrics and body compositionThe vast majority of studies include body weight as a positive factor for bone density with 10–20% of the variation in bone density related with high BMI.19 In a cohort study carried out with more the sixteen thousand women over 50 years of age, Morin and Leslie20 found that the proportion of women with high BMD increased significantly with BMI and that low BMI was associated to a greater risk of low bone density and osteoporotic fractures.21 With regard to body composition, an increase in lean mass in respect to fat mass leads to an increase in muscle strength and stimulates bone formation (osteoblasts and osteocytes) through mechanical stress.22 According to most authors, the higher proportion of lean mass is the main determinant of bone density.22,23 and adipose mass contributes in a less important manner.
Regarding the role of fat tissue on bone cells, it is speculated that one of the main implicated physiopathogenic mechanisms is an increase in serum concentrations of leptin, which is a hormone secreted by adipocytes that directly inhibits osteoclastogenesis via the RANK/RANKL/OPG system. Leptin also acts indirectly through the hypothalamus, especially by decreasing in bone formation rate. Moreover, in obese individuals, there is an increase in the secretion of pancreatic hormones (insulin, preptin and amylin), which have a positive effect on bone tissue. Hyperinsulinemia associated to a greater amount of fat tissue results in several abnormalities, such as increased ovarian production of estrogen and reduced hepatic synthesis of sex-hormone binding proteins. Amylin is secreted together with insulin and inhibits bone resorption, while preptin increases osteoblast activity, especially in obese individuals.10 Ghrelin is mostly synthesized by cells of the gastric fundus and is able to stimulate the proliferation and the differentiation of osteoblasts25 as well as to activate osteoclastogenesis during periods of fasting in animal models. These results are contradictory in humans, where ghrelin levels are being closely related to BMD in adolescent women, but the same may not occur in older men and women.10
Is important to stand out the fat mass contributed in a less important manner than lean mass to bone formation, since it is frequently associated with other metabolic disorders such as diabetes, cardiovascular diseases and some neoplasms.
Ethnic backgroundAfrican American women have greater bone mass compared with Caucasian women.24 Greater renal resorption of calcium and resistance to PTH is one of the main mechanisms proposed for this finding.25 Moreover, the higher lean mass observed in African American, especially related to muscle modulation by traction (biomechanical force), might be associated with lower risk of fractures in both genders.26
Physical activityThe benefits of physical activity on bone mass are widely recognized, particularly the effect on muscle contraction (recruitment and activation of muscle fibers)5 with the impact on bone cells, which favors periosteal apposition (adaptive mechanical response) and release of osteoanabolic hormones, such as growth hormone, insulin-like growth factor (IGF-1), estrogen and PTH.27 Besides, strain-related adaptation is essential for normal bone development and regulation of strength in relation to exercise.
It is well known that the mechanical loads can stimulate responses from osteocytes and bone lining cells. Osteocytes probably do not respond directly to mechanical strain (deformation) of bone tissue, but respond indirectly to extracellular fluid flow caused by loading. Osteocytes exposed to fluid shear stress release several messengers, including prostaglandins and nitric oxide.28 Osteoblasts also secrete these substances, as well as they express several growth factors. Mechanical stimuli can also affect osteoclasts, but this effect appears to be indirect. Loading increases hydrostatic pressure within the bone marrow, which may induce a decrease in osteoclast differentiation through marrow stromal cells participation. When exposed to strain, preosteoblastic marrow stromal cells reduce expression of RANK-L, which in turn decreases osteoclast number.29 Consequently, cells of osteoblastic lineage appear to be mediators of the suppressive effects of mechanical stimuli on bone resorption.30
Mechanical stress also improves bone strength by influencing collagen alignment as new bone is being formed. Cortical bone tissue located in regions subject to predominantly tensile stresses has a higher percentage of collagen fibers aligned along the bone's long axis.
In regions of predominant compressive stresses, collagen fibers are more likely to be aligned transverse to the long axis.31
These aspects have been demonstrated along with an increase in osteocalcin and N-terminal propeptide of type 1 procollagen (PINP)32 and greater bone density in high-performance athletes (resistance and impact activities),12 although with contradictory results depending on the type of training used on women in the pre-menopause and post-menopause periods.33
In a review article, Wallace and Cumming34 demonstrate the positive effects of physical activity (exercises with and without impact – running and weight lifting, respectively) on vertebral bone density. Aquatic activities, however, such as swimming and water aerobics, do not offer consistent benefits to bone health.35
The American College Sports of Medicine (ACSM) suggests that an increase in bone density occurs in adults only with high-intensity aerobic and resistance exercises. The frequency varies in accordance with the modality. The ACSM recommends three to five sessions of impact exercises a week and two to three sessions of resistance exercises, with each session lasting 30–60min.36
Nutritional factorsWhile there is evidence regarding the association between bone mass and inadequacy of micro and macronutrients, there are few studies that relate dietary intake to HBD.
ProteinProtein is a primary component of bone tissue and has an osteoanabolic effect, mainly in individuals with adequate calcium intake. Dietary protein regulates the hepatic production and plasma concentrations of the growth hormone and IGF-1, which are responsible for differentiation, maturation and recruitment of osteoblasts.37 While diets with protein restriction may reduce bone density,37 protein-rich diets have shown conflicting results. Hunt et al.38 found that an increase in IGF-1 following the ingestion of proteins had a beneficial effect on bone metabolism. However, it should be pointed out that increase in protein intake also led to a greater kidney excretion of calcium and could therefore have a negative effect on bone density,39 which may be minimized when there is adequate calcium and vitamin D intake.40
Calcium and vitamin DCalcium is the most abundant mineral in the organism and nearly 99% is found in the bones and teeth. Calcium is involved in biological functions, such as muscle contraction, mitosis, blood coagulation, mineral reserves and homeostasis, synapses and structural support.41 In bone tissue, calcium is extremely important for maximizing peak bone mass and maintaining it throughout adult life and for minimizing bone loss during ageing.42
Data obtained by dietary survey in regions of the State of São Paulo, Brazil, demonstrated that mean calcium intake was low for age and gender (483mg/day for women and 410mg/day for men).43 This low intake was also demonstrated in the Brazilian Osteoporosis Study (BRAZOS), which describes a mean per capita intake of 400mg/day,44 with statistically significant differences between regions of the country, genders and age groups. According to Pereira et al.,45 the main reasons for this low calcium intake are the high cost of the main food sources as well as regional, cultural and dietary habits.
Moreover, the bioavailability of calcium is dependent on exogenous factors, such as phytates, oxalates, tannins and sodium, which play a negative role in absorption, and on endogenous factors, such as age, physiological conditions and hormonal regulation. On the other hand, oligosaccharides may maximize calcium absorption, as these molecules increase intestinal fermentation and the production of short-chain fatty acids able to acidify the medium and to stimulate the calcium absorption.45 It is also important to highlight the role of vitamin D in increasing absorption of this mineral.46 However, no study has yet demonstrated the relation between dietary calcium and high BMD.
PhosphorusPhosphorus is essential for bone formation and highly related to concentrations of calcium and PTH. A high phosphorus intake, together with low calcium intake, may alter mineral and bone metabolism due to increase in the secretion of PTH47 and greater gene expression of RANK/RANKL with a consequent stimulus of osteoclastogenesis.48 Pinheiro et al.44 found that each 100g of phosphorus ingested increased the risk of fracture by 9%. However, diets with phosphorus restriction do not promote increase of bone mineral density. Indeed, they may cause a negative phosphorus balance, thereby leading to greater bone loss. Thus, adequate intake of phosphorus and calcium is essential to bone mineralization.
AntioxidantsAntioxidants from the diet, such as selenium, vitamin C and vitamin E, are capable of reducing the adverse effects of reactive oxygen species (ROS) on cell physiology.49
Formation of ROS is an unavoidable outcome of life in an oxygen-rich environment and occurs primarily in the mitochondria from the escape of electrons passing through the electron transport chain during aerobic metabolism.50 ROS are also generated during fatty acid oxidation or in response to external stimuli, such as inflammatory cytokines, growth factors, environmental toxins, chemotherapeutics, UV light, or ionizing radiation and is associated with ageing, diabetes, atherosclerosis and osteoporosis.51
Several in vitro studies and animal models have demonstrated that oxidative stress has an important impact on the differentiation and function of osteoclasts52,53 as well as the pathogenesis of bone loss.54
Osteoporotic women have low plasma concentrations of vitamin E,53 which together with low intake of this antioxidant may increase the risk of fractures among smokers. In contrast, the high consumption of fruits and vegetables has a positive effect on bone mass,55 although no studies have demonstrated its association with high BMD.
DiseasesOne could assume that higher BMD could be related to greater bone strength and lower fracture risk and, thus, better bone health. However, several diseases are associated with BMD values above normal limits, as some estrogen receptor-dependent cancers and other genetic diseases such as osteopetrosis, osteosclerosis and van Buchem disease. Studies have demonstrated a significant association between high BMD and estrogen receptor-positive breast and endometrial cancer.9,56 Assessing more than 1500 elderly women. Ganry et al.,56 found that those with femoral neck bone density above 0.769g/cm2 had a two-fold greater risk of breast cancer. The main mechanisms involved are greater serum concentrations and estrogen's gene polymorphism.57
There is also an association of HBD with obesity, due to the mechanical strength exercised by weight itself as well as the release of hormones from fat tissue (peripheral conversion of female hormones,10 low concentrations of sex hormone-binding globulin58 and hyperinsulinemia or peripheral resistance to insulin11). Generally, diabetic patients have higher BMD than nondiabetic one. However, they also have higher prevalence of fractures.59 Some explanations suggest higher risk of falling and poor bone quality due to accumulation of advanced glycation end products (AGEs) on bone tissue.60–62 This is a clear situation where high BMD does not prevent fractures. Moreover, structural alterations, such as osteoarthritis and disco-arthrosis of the spine or hip, can artificially increase bone density in these skeletal sites. This aspect should be considered in interpreting high BMD, especially in elderly individuals and patients with chronic and degenerative inflammatory joint diseases.63
DrugsSeveral drugs have harmful effects on bone density, such as glucocorticosteroids, aromatase inhibitors, GnRH analogues, heparin and anticonvulvants. On the other hand, some medications are related to potential positive effect, such as thiazide diuretics and statins.
Thiazide diuretics are used for treating hypertension, idiopathic hypercalciuria and other conditions.64 Randomized clinical trials have demonstrated higher bone density64 and lower fractures rate8 in current users versus non-users. These medications promote positive balance of calcium.65 Besides, these drugs can also directly stimulate osteoblasts, regardless of their effects on the kidneys.66
Statins have shown conflicting results67 primarily due to the pharmacokinetic and pharmacodynamic differences between them, such as hydrophilic (simvastatin and lovastatin) or lipophilic (pravastatin, atorvastatin and fluvastatin) characteristics.68 Studying post-menopausal women with low bone density, Tanriverdi et al. have reported the synergic densitometric effect of atorvastatin when associated with bisphosphonates.7
However, Rejnmark found no significant differences in bone density of 82 postmenopausal women after 12 months follow-up.69 Bone density is apparently unaltered by the use of simvastatin, pravastatin and atorvastatin.70 Among those in which an important increase in bone density was found, there are reports of a longer administration of atorvastatin.71 However, data on the anti-fracture ability of these drugs remain inconsistent,68 although they have been shown to decrease biochemical bone markers.72,73
Simvastatin may enhance osteoblastogenesis through the stimulus of the production of bone morphogenetic protein (BMP-2)74 and reduce osteoclastogenesis through inhibited production of farnesyl-diphosphate synthase.75
Hormone profileHormonal alterations play an important role on mineral and bone metabolism.46
Hormones related to obesity, such as leptin and pancreatic hormones, were discussed earlier.
Vitamin D and parathyroid hormone are discussed below.
Vitamin D and parathyroid hormoneVitamin D and parathyroid hormone (PTH) are fundamental regulators of mineral and bone metabolism, especially with regard to intestinal absorption and renal resorption of calcium as well as bone formation and resorption.46 Dietary vitamin D intake and adequate exposure to sunlight are necessary for the maintenance of plasma concentration of 25(OH)D.3 There are few food sources that contain vitamin D and in spite of what occurs in other countries there is no dietary fortification in Brazil. Environmental (latitude, season of the year, ozone layer and cloud cover) and personal (skin type, clothing and use of sun block) factors are involved in the serum level of vitamin D and bone health. Deficiency of vitamin D causes secondary hyperparathyroidism and higher bone loss.76
Sex hormonesGonadotropin-releasing hormone (GnRH) is secreted by the hypothalamus and stimulates the secretion of the follicle-stimulating hormone (FSH) and luteinizing hormone (LH) in the anterior pituitary. In women, these hormones stimulate the ovarian production of estrogen and progesterone and inhibin which, in turn, regulates secretion of GnRH, FSH and LH through a feedback mechanism.77 The loss of this mechanism, such as in menopause (hypoestrogenism), increases the plasma concentrations of FSH and LH, which have a negative effect on bone tissue.78
Several authors have demonstrated that a serum increase in FSH and reduction in inhibin lead to bone loss in perimenopausal women.77 In contrast, a reduction in FSH is closely related to a gain in bone mass.79 Assessing 699 healthy Chinese women between 20 and 82 years of age (464 in the pre-menopause period), Xu et al.77 found that an increase in FSH and LH of 10 UI/L was associated to a 5.5% and 4.4% reduction in spine BMD, respectively. FSH has recently been reported to regulate bone metabolism through the direct and indirect stimulations of osteoclasts.80,81 It also stimulates the production of TNF-α, with a consequent greater activation of osteoclastogenesis.82 The role of LH in bone tissue remains unknown, but it is speculated that it may induce an increase in androgens.81
Lipid profileEpidemiological studies investigate the association between low bone density and higher cardiovascular mortality, concomitant medication, associated diseases and traditional risk factors.83,84 Although results are contradictory, several authors speculate the negative association between bone mass and hypercholesterolemia.83–85In vitro studies have shown that products from the oxidation of lipids and lipoproteins inhibit the differentiation and function of osteoblasts85,86 probably mediated by the mevalonate pathway.87
Conclusions and implicationsConsidering the lack of available information on associated protective factors, further studies are needed in order to allow a better understanding of the clinical aspects and physiopathogenic mechanisms associated with higher BMD and bone health. A better understanding of these factors could be an important strategy for the implementation of prevention and treatment measures for patients with bone frailty especially related to osteoporotic fractures.
Sources of fundingConselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), process number 470862/2009-2.
Conflict of interestThe authors have no conflict of interest to declare.