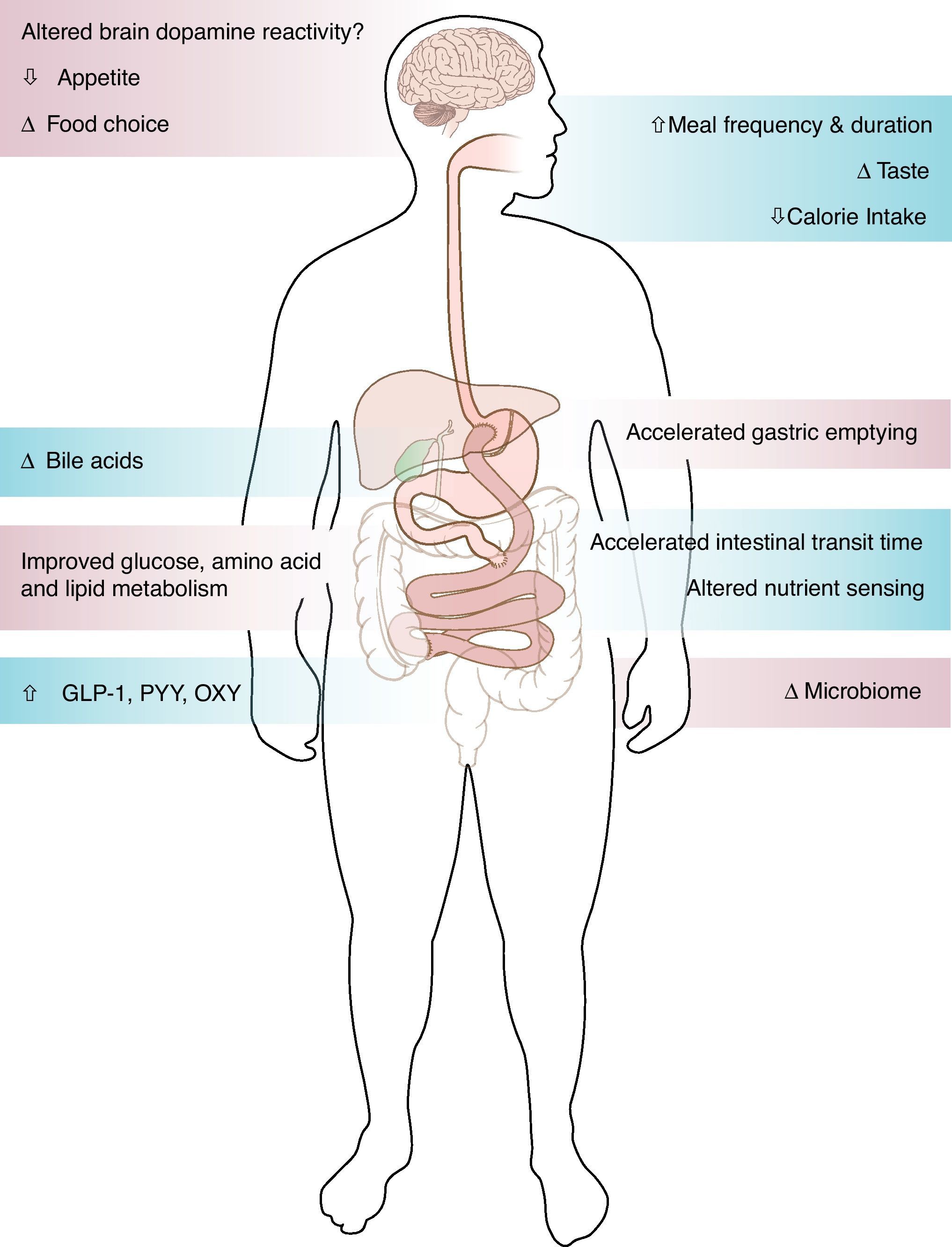
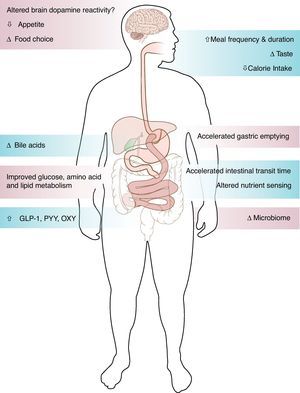
Studies of patients going into diabetes remission after gastric bypass surgery have demonstrated the important role of the gut in glucose control. The improvement of type 2 diabetes after gastric bypass surgery occurs via weight dependent and weight independent mechanisms. The rapid improvement of glucose levels within days after the surgery, in relation to change of meal pattern, rapid nutrient transit, enhanced incretin release and improved incretin effect on insulin secretion, suggest mechanisms independent of weight loss. Alternatively, insulin sensitivity improves over time as a function of weight loss. The role of bile acids and microbiome in the metabolic improvement after bariatric surgery remains to be determined. While most patients after bariatric surgery experienced sustained weight loss and improved metabolism, small scale studies have shown weight regain and diabetes relapse, the mechanisms of which remain unknown.
Estudios de pacientes que experimentaban remisión de la diabetes tras cirugía de derivación gástrica han demostrado la importante función del intestino en el control de la glucemia. La mejoría de la diabetes tipo 2 después de cirugía de derivación gástrica se produce por mecanismos dependientes e independientes del peso. La rápida mejoría de las concentraciones de glucosa días después de la cirugía, relacionada con el cambio del patrón alimenticio, el rápido tránsito de nutrientes, el aumento de la liberación de incretinas y el mejor efecto de las incretinas sobre la secreción de insulina, indica mecanismos independientes de la pérdida de peso. También es posible que mejore con el tiempo la sensibilidad a la insulina en función de la pérdida de peso. Resta por determinar el papel de los ácidos biliares y el microbioma en la mejoría metabólica tras la cirugía bariátrica. Mientras que la mayoría de los pacientes experimentaban después de la cirugía bariátrica una pérdida de peso mantenida y una mejoría del metabolismo, estudios a pequeña escala han mostrado recuperación del peso y recidiva de la diabetes, cuyos mecanismos siguen desconociéndose.
Surgical weight loss, the only efficient long-term weight loss treatment for severe obesity, results in remarkable improvement in blood glucose levels in 50–80% of patients with type 2 diabetes (T2DM).1,2 All bariatric surgeries, either purely restrictive, such as adjustable gastric banding (AGB), or with some element of gut-ectomy or bypassing, such as biliopancreatic diversion (BPD), vertical sleeve gastrectomy (VSG) or Roux-en-Y gastric bypass (GBP), induce weight loss and improve diabetes.1,2 Although the improvement of T2DM is solely and proportionally related to weight loss after AGB,2 the rapidity of the improvement of glucose concentrations after bypass surgeries, before significant weight loss has occurred, suggests alternate mechanisms related to biochemical and/or hormonal changes. GBP is a very complex surgery resulting in anatomical and neuroendocrine changes (Fig. 1). Among other endocrine and metabolic changes, the enhanced post-prandial release of the gut hormone incretins after GBP3–5 and their resulting effect on insulin or glucagon secretion are thought to be mediators of the greater improvement of glucose levels after GBP, as compared to diet4 or AGB.6
Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) are gastrointestinal hormones secreted respectively from the neuroendocrine K cells and L cells.7–9 The physiological effect of the incretins is responsible for ∼50% of post-prandial insulin secretion.10–12 The incretin effect is described as the differential insulin response after oral glucose compared to an equivalent dose of intravenous glucose.11 In addition to its glucose-dependent insulinotropic effect, GLP-1 delays gastric emptying,13 decreases appetite and promotes weight loss,13,14 inhibits glucagon,15 and may improve insulin sensitivity.16 GLP-1 and GIP are rapidly inactivated by the enzyme dipeptidyl peptidase 4 (DPP-4). The incretin effect on insulin secretion is blunted in patients with T2DM.17 GLP-1 mimetics and DPP-4 inhibitors are efficient anti-diabetic agents.18
Enhanced incretin release and effect after GBPThe enhanced post-prandial circulating incretin concentrations after bypass surgeries was first reported in the early 1980s, at a time when no commercial assays were available. GLP-1 consistently increased after jejunoileal bypass, BPD and GBP.19–21 More recent reports confirm a significant increase in GLP-1 levels by a factor of 5 to 10 after GBP in response to a meal5,22 or to oral glucose.3 This also occurs after VSG.23 The effect of bypass surgeries on changes in GIP levels is less consistent with either elevated or decreased levels.19,21,24–26 We reported an increase of GIP levels at one month,3 1 year27 and 2 years28 after GBP in morbidly obese patients with T2DM. In addition to the enhanced circulating post-prandial incretin concentrations, the incretin effect on insulin secretion, blunted in patients with diabetes, normalized to the levels of non-diabetic controls as early as one month3 and up to 2 years28,29 after GBP. An elegant study by Kindel et al. in the Goto-Kakizaki (GK) rats showed that the improved glucose tolerance after duodenojenunal bypass is reversed by the administration of the GLP-1 receptor antagonist exendin 9–39.30 This proof-of-concept study provides direct evidence that improvement of glucose tolerance following a GBP-like surgery is mediated, at least in part, by enhanced GLP-1 action.30 A similar experiment in humans showed that exendin 9–39 not only decreased post-prandial insulin release31, but also corrected the hypoglycemia32 in patients with neuroglycopenia after GBP.
The change of incretins after GBP is independent of weight lossOne month after GBP there is a significant increase of post-prandial GLP-1 and of the incretin effect on insulin. An equivalent weight loss by calorie restriction alone4 or with AGB,6 did not change the incretin levels or effect. Patients after GBP were able to discontinue their diabetes medications at the time of surgery with normalization of their blood glucose levels. Patients after diet alone improved their glucose levels, but did not always normalize them, and remained on some medications, although with lower dosage. Surgical or diet-induced weight loss resulted in a significant and similar decrease of fasting glucose and fasting insulin. A change of the pattern of insulin response to oral glucose with recovery of the early phase insulin secretion, and the improvement in incretin levels and effect were, however, observed only after GBP, not after diet. These data suggest an effect of GBP, independent of weight loss, on glucose homeostasis. Other clinical studies after various types of bypass surgeries with or without ileal transposition in patients with body mass index (BMI) less than 35kg/m2 suggest that diabetes may improve without weight loss.33
Long-term changes after bypass: the incretins are still up but the variance increasesThe enhanced incretin response observed early after GBP persists over time. Cross sectional data from Naslund et al. showed persistent elevated post-prandial GLP-1 and GIP levels 20 years after duodenal jejunal bypass compared to obese non-operated controls.21 Our own data show enhanced GLP-1 and GIP response to oral glucose up to six years after GBP in patients with diabetes remission, with normal incretin effect (Laferrère et al., unpublished). Parameters such as incretin levels and effect, early phase insulin release during the oral glucose tolerance test, and the insulinogenic index all improve rapidly after GBP, without further change overtime in spite of continuous weight loss. On the contrary, other outcome variables such as fasting glucose, fasting insulin, leptin or adiponectin,27 and insulin sensitivity34 continue to improve as a function of weight loss in the first year after GBP. This suggests that some changes occur as a result of the surgery, independent of weight loss, while other changes are clearly weight loss related.
We recently observed that the prolonged enhanced GLP-1 response 2 years after GBP was associated with increased variance of the GLP-1 response, in 15 patients with T2DM.28 This suggests that, in addition to the foregut bypass and the accelerated intestinal transit time, other mechanisms of enhanced GLP-1 release develop over time, and may develop differentially from one patient to another. This may explain the increase in the variance of the incretin response over time. Although the mechanism of the increased variance of GLP-1 release over time after GBP is unknown, one can speculate that some element of gut adaptation may occur, in relation to changes in the microbiome35 or bile acids.36
The exaggerated incretin response observed early after GBP, accompanied by improved post-prandial insulin and glucose levels seems to be responsible for the later development of hyperinsulinemic hypoglycemia with or without nesidioblastosis.37–40 The administration of exendin 9–39, a potent GLP-1 receptor antagonist, to a selected group of individuals, improved the post-prandial hypoglycemia after GBP.32 Although GLP-1 has been shown to preserve human islet in vitro41 and prevent β-cell apoptosis in rodents,42 there is no human data to suggest that GLP-1 increases β-cell mass after bypass in humans. An elegant study by McLaughlin et al.43 demonstrated that hyperinsulinemic hypoglycemia post GBP may not be due to the dysfunction of the β-cell, but rather to the accelerated mode of nutrient delivery to the lower intestine. Administering a meal per mouth via the alimentary limb generated rapid release of GLP-1 and insulin, resulting in hypoglycemia. Administering the same meal via a gastrostomy in the gastric remnant connected via the pylorus to the duodenal limb, triggered neither the exaggerated insulin response nor hypoglycemia.43
Mechanisms of enhanced incretin release after GBP: not just the bypassGBP consists of the creation of a small gastric pouch of about 30cm3, anastomosed directly to the distal part of the jejunum (alimentary limb). The gastric remnant, including the pylorus, the duodenum and part of the jejunum is therefore shunted from nutrients (biliopancreatic limb) and reattached to the ileum to allow gastrointestinal and pancreatic juice to be mixed with nutrients in the distal intestine (common limb).
Studies in the GK rat suggest that the exclusion of the upper gut (foregut hypothesis), rather than weight loss, benefits glucose tolerance.44,45 Rats after gastrojejunal bypass have better glucose tolerance than sham-operated pair-fed control animals with equivalent body weight, or rats with gastrojejunal anastomosis.44 Recent trials with endoluminal sleeves in animals and in humans also support the foregut hypothesis.46
The hindgut hypothesis, based on studies after ileal interposition,47–49 suggests that the rapid stimulation of the distal ileum by nutrients is responsible for increased GLP-1 and beneficial effect on glucose tolerance, similarly to data obtained with ileal transposition in rodents.50,51 After GBP, the accelerated emptying of the gastric pouch is faster for liquids and is related to the rapid release of GLP-1.22,52 The study by McLaughlin43 also seems to highlight the importance of the rapid emptying on the exaggerated GLP-1 response after GBP. VSG, a procedure that removes most of the gastric pouch, leaving in place the lesser curvature and the pylorus, without discontinuity of the intestine, not only induces weight loss and type 2 diabetes remission similarly to GBP,53,54 but has gut endocrine effects with elevation of GLP-1 release.23,55 This enhanced gut peptide release after VSG, likely in relation in part to accelerated gastric emptying, refutes the hypothesis that the shunting of the duodenum is an important element in diabetes remission. It is likely that the accelerated nutrient transit time and the rapid exposure of the distal ileum to undigested nutrients (hindgut hypothesis)50,51 mostly contribute to the enhanced incretin response to nutrients after GBP.
Gastric emptying/CHO absorptionGastric emptying for liquid is accelerated after GBP as measured by scintigraphic method56,57 or indirectly by acetaminophen58 or d-xylose59 methods. We recently have shown the absence of significant carbohydrate malabsorption by d-xylose after GBP, in patients with BMI <50kg/m2.59 However, these data may not apply to more obese patients with BMI >50kg/m2, who may get a variant of the GBP with a longer bypassed limb.
Meal durationMeal patterns change drastically in rodents and in humans after bariatric surgery, with smaller meal size, increased meal duration and increased number of meals.60 In our cohort, the time it took subjects to drink 200ml of non-carbonated glucose solution increased from 2min before surgery to 10min at 1 month, and remained elevated at 5min at 1 year (Laferrère et al., unpublished). Although unlikely to be a major contributor, it is important that studies of incretin concentrations after bariatric surgery control for the duration of meal ingestion. A recent study of healthy lean individuals showed that eating ice cream over 20′ rather than 5′ results in a significant raise of Peptide YY3–36 (PYY) and GLP-1 over the 4hours following the ice cream ingestion.61
Dipeptidyl peptidase-4 activityWe hypothesized that a decrease in the activity of dipeptidyl peptidase-4 (DPP-4), the enzyme that inactivates incretins, may explain the rise in incretin levels and effect post-GBP. Fasting plasma DPP-4 activity was measured after 10kg equivalent weight loss by GBP (n=16) or by caloric restriction (n=14) in obese patients with T2DM. DPP-4 activity decreased by 11.6% after GBP (p=0.01), but not after caloric restriction. The decrease in fasting plasma DPP-4 activity after GBP, which may occur by a mechanism independent of weight loss, did not relate to the increased peak GLP-1 and GIP response to oral glucose. Whether the change in DPP-4 activity contributes to improved diabetes control after GBP therefore remains to be determined.62
Bile acidsBile acids have been shown to stimulate GLP-1 release in vitro63 and to be important regulators of glucose metabolism, in addition to their effect on lipid digestion.64 Some elements of the entero-hepatic cycle of bile acids likely change after bypass procedures. Fasting bile acids levels have been shown to be elevated after GBP in a cross sectional study36 and 3 months after malabsorptive bariatric procedure.65 Bile acids after bypass are related to peak GLP-136 and to fasting GIP levels.65 We recently reported a strong association between bile acids and GLP-1 up to 2 years after GBP in patients with T2DM.66
Not just the incretinsIn addition to GLP-1, other products of the neuroendocrine L cells, PYY67 and oxyntomodulin,68 show an enhanced post-prandial release after GBP, but not after diet. Both PYY and oxyntomodulin play a role in food intake control. The changes of the gastric hormone ghrelin after GBP are more complex than initially described.69 Although circulating ghrelin concentrations decreased initially after GBP, they increased in proportion to weight loss one year after this surgery.27 The changes of these gut hormones could favor satiety and/or appetite control after GBP.
The gut peptides are not the only factors contributing to weight loss and diabetes improvement after GBP surgery. The adipose tissue cytokine changes occur according to weight loss: adiponectin increases and leptin decreases with weight loss after GBP.27 As β-cell function improves, the abnormal elevation proinsulin and amylin concentrations decrease in patients with diabetes after GBP.27
The gut microbiome has recently emerged as an important player in obesity, metabolism and inflammation. Obesity is associated with changes in the relative abundance of the two dominant bacterial divisions, the bacteroidetes and the firmicutes, and possibly change in the gut microbial–host metabolic cross-talk.35,70 The effect of GBP surgery on the host metabolic–microbial cross-talk augments our understanding of the metabolic phenotype of bariatric procedures. In addition, it helps define the possible physiological link between microbial metabolic activities and mammalian regulation of lipid and glucose metabolism, energy balance and inflammation.71
In addition to glucose metabolism, lipid72 and amino acid metabolism73 are ameliorated after GBP surgery. It has been known for a long time that branched-chain amino acids (BCAA) and related metabolites are linked to insulin resistance and diabetes,74 and can cause metabolic dysfunction.75 A recent epidemiological study showed that BCAA and aromatic amino acids predict the risk for T2DM.76 Applied metabolomic profiling shows that BCAA and their acyl carnitin derivatives are more responsive to surgical than dietary-induced weight loss.73 The reduction of circulating BCAA and the aromatic amino acids phenylalanine (Phe) and tyrosine (Tyr) was greater in a GBP group of patients compared to a matched group of patients who lost an equal amount of weight by diet.73 This greater reduction in BCAA, Phe and Tyr was linked to better improvement in glycemic control and greater improvement of insulin secretion in the GBP group. Future studies will characterize the metabolic pathways involved in the specific metabolic signature of GBP surgery, and how it is related to changes in gut hormones, β-cell function and changes in insulin sensitivity.
Long-term effect of GBP on diabetes: not so rosy after all?Most patients experience considerable improvement of co-morbidities and quality of life, and reduction of mortality after bariatric surgery.77,78 The change of gut peptides, glucose, lipid and protein metabolism favor and/or explain some of the clinical improvement. Yet, for some patients, the benefits are of short duration. Small scale, long-term studies show significant weight regain and/or type 2 diabetes relapse in a significant percentage of patients.79,80 Other patients develop invalidating postprandial hypoglycemia that necessitates drastic nutritional and pharmacological interventions and in rare cases, pancreatectomy.37 Finally, protein, mineral and vitamin malnutrition are frequent long-term after bypass surgeries and data are lacking as to what type and how much of supplementation should be provided, although guidelines have been issued.81 Research needs to be done to identify behavioral, social, biological, endocrine, neuronal and genetic markers of success, and/or of weight regain and diabetes relapse after bariatric surgery.
ConclusionThe magnitude of weight loss (∼40%) and its persistence (years) in most patients are considered the major contributors to glucose control after GBP. However, the data clearly show beneficial changes of incretin levels and improved insulin secretion profile and decreased post-prandial glucose, occurring rapidly after GBP, and for the most part independent of weight loss. Recently identified change in circulating amino acids, in bile acids and in gut microbiome after GBP, open new avenues for clinical investigation. The gut might turn out to play a predominant role in normal and impaired metabolism and energy balance. Understanding the mechanisms of the change in metabolism after GBP should help define the role of the gut in the physiopathology of T2DM, and help discover new therapeutic surgical or medical intervention, and possibly prevent type 2 diabetes relapse after the surgery.
FundingDr Laferrère received funding from the American Diabetes AssociationCR-7-05 CR-18, NIH NIDDK R01-DK67561, 1 UL1 RR024156-02, ORC DK-26687, DERC DK-63068-05.
Conflict of interestThe author has not declared any conflicts of interest.
We thank Phoebe Bunker for her help in preparing this manuscript and Marie Rossetti for help with the figures.