This study compares the effects of feeding growing rats with increasing concentrations of casein (C) and wheat gluten (G), proteins that show different biological qualities, on the morphometrical and biomechanical properties of the femoral diaphysis.
Materials and methodsFemale rats were fed with one of ten diets containing different concentrations (5–30%) of C and G between the 30th and 90th days of life (Control=C-20%). Biomechanical structural properties of the right femur middiaphysis were estimated using a 3-point bending mechanical test with calculation of some indicators of bone material properties.
ResultsBody weight and length were affected by treatments, values being highest in rats fed the C-20% diet. G diets affected negatively both parameters. Changes in cross-sectional geometry (mid-diaphyseal cross-sectional and cortical areas, femoral volume, and rectangular moment of inertia) were positively related to the C content of the diet, while they were severely and negatively affected by G diets. Similar behaviors were observed in the bone structural properties (fracture load, yielding load, diaphyseal stiffness and elastic energy absorption). When values of strength and stiffness were normalized for body weight, the differences disappeared. The bone material quality indicators (elastic modulus, yielding stress, elastic energy absorption/volume) did not differ significantly among all studied groups. Femoral calcium concentration in ashes was not significantly different among groups.
ConclusionThe clear differences in strength and stiffness of bone beams induced by dietary protein concentration and quality seemed to be the result of an induced subnormal gain in bone structural properties as a consequence of a correlative subnormal gain in bone growth and mass, yet not in bone material properties.
Este estudio compara los efectos sobre las propiedades morfométricas y biomecánicas de la diáfisis femoral de ratas en edad de crecimiento de dos proteínas dietarias, caseína (C) y gluten de trigo (G), que muestran características biológicas diferentes, ofrecidas en concentraciones crecientes en las dietas utilizadas.
Material y métodosRatas hembras fueron alimentadas entre los días 30 y 90 de sus vidas con una de diez dietas que contenían concentraciones diferentes (5-30%) de C y G (Controles = C al 20%). Se estimaron las propiedades biomecánicas estructurales de la parte media de la diáfisis del fémur derecho mediante la prueba mecánica de flexión a tres puntos calculándose, además, algunos indicadores de las propiedades biomecánicas del material óseo.
ResultadosLos tratamientos afectaron al peso corporal y a la talla, con valores más elevados en aquellas ratas alimentadas con la dieta C al 20%. Las dietas conteniendo G afectaron en forma negativa a ambos parámetros. Los cambios en la geometría de la sección transversal (áreas de la sección transversal de la parte media de la diáfisis y cortical, volumen del fémur y momento rectangular de inercia) mostraron una correlación positiva con la concentración de C en las dietas, mientras que fueron severa y negativamente afectados por la presencia de G en las mismas. Se observaron comportamientos similares en las propiedades estructurales del hueso (carga o resistencia a fractura, punto de cesión, rigidez diafisaria y absorción de energía durante el período elástico). Cuando los valores de rigidez y resistencia diafisarias fueron normalizados por el peso corporal, desaparecieron las diferencias. Los indicadores de la calidad biomecánica del material óseo (módulo de elasticidad, estrés elástico límite, absorción elástica de energía/volumen óseo) no fueron estadísticamente diferentes entre los grupos estudiados. La concentración de calcio femoral no mostró diferencias entre grupos.
ConclusiónLas diferencias significativas de rigidez y resistencia entre las diáfisis femorales inducidas por la concentración y calidad de las proteínas dietarias parecieran ser el resultado de la ganancia subnormal de las mismas como consecuencia de una ganancia también subnormal del crecimiento y de la masa ósea, sin alteraciones de las propiedades materiales.
During evolution, the skeleton of vertebrates developed an important property, the resistance to deformation, and indirectly to fracture that was adapted to the physiological mechanical demands of the environment. The criterion for adequate support function is the formation and maintenance of sufficient quantity and quality of bone adequately distributed to support the body throughout life and to withstand ordinary stresses to which skeletal components are subjected.1
It is assumed that the mechanical properties of bones integrated as organs (structural properties) are directly related to both the amount (bone mass) and the architectural distribution of the mineralized tissue (geometric properties) and to the mechanical quality of bone material (material properties).2,3 The structural stiffness, measured as a load/deformation ratio, is usually kept high enough to withstand to everyday bone deformation to avoid damage, and hence fracture. The structural stiffness, and indirectly the strength of bones, is thought to be controlled by a “bone mechanostast”.4 This is a feedback mechanism that optimizes the bone design through a permanent re-distribution of the mineralized tissue.
Several factors have been recognized to play an important physiological role in determining bone stiffness and strength and its resistance to spontaneous and traumatic fractures. Both body weight and somatic muscles contractions could be considered as the most important “mechanical factors” in the determination of bone strength.5 However, other “nonmechanical factors” also exist that can modulate bone physiology, by either establishing or maintaining the mechanical competence of bones.
Several years ago, we reported the biomechanical repercussion of a severe protein restriction on the shaft of long bones and on cortical tissue in growing rats.6 A great reduction in growth-related gains in bone stiffness and strength with respect to well-nourished controls was found. The alterations described correlated with very low values of the cross-sectional bone mass indicators and moments of inertia and the calcium content of bone tissue. The additive effects of protein and energy deficiencies were also demonstrated.7 These findings were partially confirmed later by other investigators.8–10 In general, these later studies, which were performed in adult rats, demonstrated that isocaloric protein undernutrition decreased bone mineral mass and strength and/or negatively affected intrinsic tissue properties of bone. In these studies, the effects of protein restriction on bone biomechanics were thus isolated from the concomitant effects on body growth seen in young animals, which could also secondarily alter bone biomechanical properties.
We have recently estimated11 the biomechanical repercussion of protein restriction imposed to rats between the 26th and the 135th days of age on the mandible, a bone that are not influenced by body weight but by the mechanical loads generated during mastication. The clear differences in strength and stiffness of the bone seen between protein restricted and well-nourished animals seemed to be the result of an induced subnormal gain in bone structural properties as a consequence of a correlative subnormal gain in bone growth and mass, yet not in bone material properties.
It has been also repeatedly demonstrated that dietary protein restriction negatively affects skeletal development in young rats.12–17 It was also evident that the protein concentration in the diet is an important determinant of the body growth rate, as it is the quality of the protein given to experimental animals.18–21 As far as we know, comparison of biomechanical bone effects between dietary proteins having different biological values (BV) in growing rats is not available in the specialized literature. The present study was thus designed to compare the effects of feeding growing rats with increasing concentrations of two proteins showing different biological qualities on the biomechanical properties of femur diaphysis, demonstrated by mechanical assessment of diaphyseal stiffness and strength and calculation of some indicators of bone material properties.
Materials and methodsTen groups of 7 female Sprague-Dawley rats aged 30d and weighing about 58g at the start of the experiment were housed in stainless-steel cages under natural light-dark photoperiod and in a temperature controlled (23°C) room. Rats were fed freely with one of 10 diets containing two protein sources, casein (BV=77.0) and wheat gluten (BV=64.0). Casein was used at four different concentrations (5, 10, 15 and 20%=C-diets) whereas gluten was given at six concentrations (5, 10, 15, 20, 25 and 30%=G-diets). The diet containing 20% casein was considered as the “control” or “standard” diet. It has been previously shown to meet all necessary requirements to allow normal skeletal growth in the rat.21 All the diets were isocaloric and protein was included in the protein-free diet by substituting an equivalent amount of dextrin. The protein-free diet contained 7% corn oil, 88% dextrin, 1% vitamins (AIN Vitamin Mixture 76, MP Biomedicals, Ohio, USA), 3.5% minerals (AIN-76 Mineral Mixture), and 0.5% choline.
The experimental period lasted 60d. During this period, both body weight and length and food consumption were registered every week. At its end, final body weight and length were established. Body length was taken as the distance between nose and tip of tail. Rats were then sacrificed by ether overdose. The femurs were dissected, cleaned of adhering soft tissue, weighed in a Mettler scale and stored at −20°C wrapped in gauze soaked with Ringer's solution in sealed plastic bags, in accordance with Turner and Burr.22
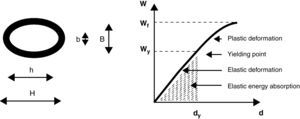
On the day of testing, each bone was thawed at room temperature before analysis. To assess cortical bone mechanical properties, the right femur was tested in 3-point bending23 (Fig. 1). Each bone was placed horizontally with the anterior side facing down on two transverse supports (L=13mm span) and central along its length. Load was applied perpendicularly to the long axis of the bone until fracture. The test machine (Instron model 4442, Instron Corp., Canton, MA, USA) was operated in stroke control at a constant rate of 5mm/min, which is useful for describing the static properties of the bone structure. For this biomechanical test, load/deformation (W/d) curves showing both the elastic (Hookean behavior) and the plastic (non-Hookean behavior) phases separated by the yielding point, enabled graphic determination of the main structural mechanical properties of bone shafts as beams24 which essentially measures the resistance to both deformation (stiffness) and fracture (strength) and the ability to absorb energy by deforming. They are: (a) STRUCTURAL PROPERTIES (whole-bone properties, as derived from the slope of the W/d curve in the linear region of the elastic behavior): (1) maximal elastic deflection (yield deflection dy, elastic limit, or load at the yielding point Wy) represents the value of the force at the upper extent of the linear region (yielding point) and indicates the appearance of the first microcracks that occur on the periosteal surface of the bone; (2) structural elastic stiffness (load/deflection relationship, diaphyseal stiffness, bone beam's rigidity, or slope of the linear phase of the W/d curve) represents the rigidity of the beam or the resistance to deformation; (3) elastic absorption of energy by the whole bone (the total energy absorbed by the specimen up to the yielding point) represents the energy necessary to initiate the first microcracks; and (4) structural strength (whole-bone strength, maximal supported load, ultimate load, load at fracture Wf) represents the value of the load at fracture and expresses directly the resistance of the whole bone to fracture, incorporating both the elastic and the plastic behaviors; (b) GEOMETRIC PROPERTIES (bone design characteristics): (1) bone length and diameters: the femur length was measured directly using a stereomicroscope (Stenu DV4 Stereo microscope, Carl Zeiss Microimaging, Gottinge, Germany) with an accuracy of ±100μm; (2) mid-diaphyseal cross-sectional area, CSA: using an Isomet low-speed diamond saw (Buehler, Lake Bluff, IL, USA) a 2-mm cross-section slide was cut from the fracture section to perform regularized micromorphometrical determinations of the vertical (load direction) and horizontal (right angle to load direction) outer (HB) and inner (hb) diameters of the elliptic-shaped fracture sections (Fig. 1). Measurements were taken with a digital caliper with the aid of a magnifier 40×. CSA was calculated by applying the equation: CSA=3.14 (HB−hb)/4mm2; (3) second moment of inertia of cortical bone (with reference to the anterior–posterior bending axis, xCSMI) as estimated by the equation: xCSMI=(3.14[B3H−b3h/64]). B=vertical outer diameter, H=horizontal outer diameter, b=vertical inner diameter, h=horizontal inner diameter. CSMI captures both bone mass and distribution on the cross section. The larger the xCSMI, the further the disposition of bone cortical mass from a given reference axis. As bones were tested in anterior–posterior bending, the selected reference axis was the “horizontal” diameter of the bone cross section; and (4) bone volume between supports (Lπ(HB−hb)); and (3) BONE MATERIAL PROPERTIES (intrinsic properties of the mineralized tissue), as calculated from structural and geometric properties. Thus, bone material properties were not directly determined by mechanical means: (1) Young's modulus of elasticity (bone material stiffness, intrinsic stiffness, stress–strain relationship) calculated by the formula: E=WyL3/48dy·Ix (Wy=load at the yielding point, L=distance between supports, dy=maximal elastic deflection, Ix=second moment of inertia of the cross-section in relation to the horizontal axis); (2) maximal elastic stress, which expresses the reacting force opposed by the deformed bone to the deforming load. It was calculated by the formula: σ=LBWy/8Ix (B=vertical outer diameter of the regularized fracture section); and (3) energy absorbing capacity (EAC, expressed per unit of bone tissue volume, EAC/vol).
The left femur of each animal was ashed at 600°C in a muffle furnace for 18h and the ash weight obtained. The bone ash was dissolved in 2-N HCl and calcium content determined by atomic energy absorption spectrophotometry.24
Results were summarized as means±SD and were considered statistically significant at the level of p<0.05. Comparisons between parameters were performed by one-way analysis of variance (ANOVA) and test of Student–Newman–Keuls by using GraphPad Prism Software (GraphPad Software Inc., San Diego, CA, USA).
The experiment was conducted in accordance with the principles outlined in the National Institute of Health Guide for the Care and Management of Laboratory Animals, and approved by the University of Buenos Aires Ethics Committee.
ResultsResults are presented graphically for easier interpretation. When statistically significant differences were encountered among groups, results are presented as percentage of the value corresponding to the group fed the diet containing 20% casein (control group), with the exception of both body weight and length. When values from different groups did not differ significantly, the actual values are shown.
- (a)
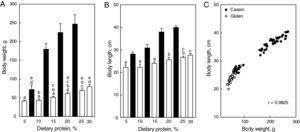
Morphometric: as expected, both body weight and body length were affected by dietary protein concentration and quality (Fig. 2A and B). Two observations were remarkable: (1) both parameters were highest in rats fed the diet containing 20% casein, and (2) they were significantly less in animals fed gluten at every level of protein concentration. However, the high correlation (r=0.9825, Fig. 2C) found between body length and body weight for all animals together in the same graph suggests that body growth was harmonic and not influenced by the protein content of the diet.
Figure 2.Final body weight (A) and body length (B) in female rats fed ad lib diets containing casein (black bars) or wheat gluten (white bars) as unique protein source between the 30th and 90th days of postnatal life. Each bar represents the mean±SD for 7 rats; equal letters on top of bars indicate p>0.05; (C) correlation between body length and body weight for all animals plotted together.
- (b)
Femur morphology: changes in cross-sectional geometry of the femur are presented in Fig. 2. Mid-diaphyseal cross sectional area (B) and cortical cross-sectional area (C), as well as femoral volume (D) and rectangular moment of inertia of the fracture section (A), were positively related to the casein content of the diet. These parameters were severely affected in animals fed gluten instead of casein at every dietary protein level. It is noteworthy that a high coefficient of determination (r2=0.8611) was found between xCSMI and the femur calcium mass of animals from all groups put together in the graph (data not shown).
- (c)
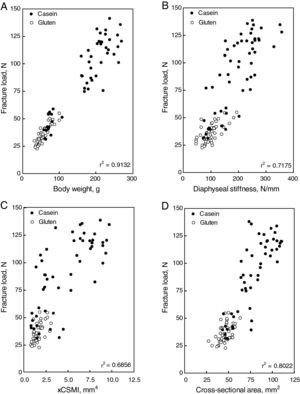
Structural properties: the diaphyseal structural properties of the femoral diaphysis are shown in Fig. 4. Fracture load (A), yielding load (C), diaphyseal stiffness (E) and elastic energy absorption (F) increased with the increment of casein in the diet. All parameters were reduced drastically in gluten fed rats. However, when fracture load was expressed in relation to body weight (B), no significant differences were found between casein and gluten fed animals. High correlations between fracture load and body weight (Fig. 5A), diaphyseal stiffness (Fig. 5B), cross-sectional area (Fig. 5C) and cross-sectional moment of inertia (Fig. 5D) were observed. The yielding load/fracture load ratio did not differ significantly among groups, indicating that the elastic and plastic components of the load/deformation curve was not altered neither by the concentration nor the quality of proteins in the diet.
Figure 4.Femoral structural properties [fracture load (A), fracture load in relation to body weight (B), yielding load (C), yielding load in relation to fracture load (D), diaphyseal stiffness (E), and elastic energy absorption (F)] in rats fed diets containing casein (black bars) or gluten (white bars); values are presented as percentage of the control value, which corresponds to the group fed the standard diet containing 20% casein and express mean±SD; equal letters on top of bars indicate p>0.05.
- (d)
Material properties: the bone material quality indicators, or pre-yield bending stiffness (elastic modulus, Fig. 6A), the yielding stress (Fig. 6B) and the elastic energy absorption/volume (Fig. 6C) did not differ significantly among all studied groups, as was the calcium concentration in ashes (Fig. 6D). Femoral calcium concentrations in ashes were not significantly different among groups. However, the femoral bone mass (or calcium mass) was significantly reduced in gluten fed animals. When the femoral bone mass was normalized for 100-g femur, no significant differences among groups were found.
Figure 6.Femoral bone material properties [elastic modulus (A), yielding stress (B), and elastic energy absorption (C)] and calcium concentration in bone ashes (D) in rats fed diets containing casein (black bars) or gluten (white bars); each bar represents the mean±SD for 7 animals; equal letters on top of bars indicate p>0.05.
The present investigation was designed to evaluate the effects of dietary protein concentration and quality on bone quality in female rats during days 30th and 90th of postnatal life. During this 60-day period, rats were actively growing, with the exception of the animals fed the diets containing the two lowest concentrations of gluten. Thus, the peak bone mass had not been reached at the end of the experimental period. The study was performed on the femur, a weight-bearing bone in the rat. The femoral mid-diaphysis in the rat is primarily composed by cortical tissue, whose primary function is strength and support. Therefore, the real purpose of the study was to evaluate the effects of concentration and quality of dietary proteins on cortical bone biomechanics.
The present study began with very young animals and the effects of treatments on bone mechanical competence were assessed when they were reaching adulthood.25 Therefore, the very-well known effects of dietary proteins on body growth should be separated from their possible direct effects on bone mechanical properties.
When compared with the standard group (C-20%), final body weight was lower in all other groups of rats fed either casein or gluten as dietary source of protein. These finding indicate that weight gain during the experimental period was a function of daily protein intake, which agrees with previously reported studies.18,20,21,26,27 Bozzini et al.21 found that body weight was maintained in rats fed a 5%-casein diet, while rapid gains of body weight were observed in animals placed on diets containing between 10 and 30% casein. The curve that relates body weight and dietary casein concentration showed a plateau at 20%-casein. This finding induced us to consider the C-20% group as the reference one in the present investigation. It has to be remembered that dietary protein concentration affects food intake and that food intake affects protein intake.18 However, when gross caloric intake is expressed as a function of the metabolic size of the animal (Wtkg0.75), no significant difference could be demonstrated between protein-deficient and well-nourished rats.15
Animals were actively growing during the entire experimental period, condition that explains the marked differences in final body weight and length that were evident among groups: the different growth retardation effect of both the quantity and the quality of the dietary studied proteins. In general, body growth was directly proportional to the protein concentration of the diet, independently of the protein quality, until a maximum was reached. However, body growth in rats fed the low quality protein was always less than in those fed the high quality protein. The high correlation found between body length and body weight of all animals put together indicates that neither the quantity nor the quality of dietary proteins affects the harmony of growth.
Both the final weight and the length of the femur were undoubtedly affected by growth retardation, as was the bone volume. The differences in cross-sectional area (CSA) and cross-sectional moment of inertia (xCSMI) indicate that the size of the bone, in terms of the diaphyseal cross sections, was significantly affected by subnormal body growth.
These alterations were paralleled by a weakening of bone beams strength (Fig. 3A, and stiffness Fig. 3C), which was highly dependent on both quantity and quality of dietary proteins. Fig. 4B shows a high positive correlation between both parameters when data from all animals were plotted together in the same graph. The other structural mechanical properties were also adversely affected by the used diets. The body weight of the animals is one of the most important factors which influence bone ability to develop or to resist stress. A positive linear correlation between the load at fracture of the femur and the body weight of animals was established (Fig. 4A). Therefore, it appears that bone mass, and consequently the structural bone strength, grew up following the normal proportionality with body weight in all animals. When the load at fracture was normalized by body weight, the differences between groups disappeared, which support this hypothesis (Fig. 3B). In other words, growth retardation induced by the quantity or the quality of dietary proteins made animals to have smaller bones. Therefore, the load at fracture normalized per body weight was not different from that of similarly sized control rats.
Femoral morphometric properties [xCSMI (A), cross-sectional area (B), cortical cross-sectional area (C), and volume (D)] in rats fed diets containing casein (black bars) or gluten (white bars); values are presented as percentage of the control value, which corresponds to the group fed the standard diet containing 20% casein and express mean±SD; equal letters on top of bars indicate p>0.05.
The above discussion suggests that the impaired performance of diaphyseal shafts induced by changes in concentration and/or quality of dietary proteins is the result of changes in the amount of cortical bone mass (Fig. 6C) and cortical bone volume (Fig. 6D). However, the spatial distribution of the cortical bone mass could be an additional factor (Fig. 6A). This possibility is supported by the high positive correlation found between the strength of bone beams and their sectional moment of inertia in control and experimental rats (Fig. 4C). However, the high coefficient of determination (r2=0.8611) found between the xCSMI and the bone calcium content for all groups together in the same graph suggests that there were no direct effects of dietary protein quantity and/or quality on the distribution of bone mineralized tissue within the diaphyseal cross sections. Therefore, the affected variable was only bone mass (normally related to body weight), not bone mass distribution. The lower values of the xCSMI (which captures both, bone mass and distribution) may only reflect the much lesser amount of bone mass in the cross-sections, and not necessarily the distribution of those small amounts of mass in the experimental animals.
The large differences in diaphyseal strength between groups contrasted with the maintenance of normality of the elastic modulus (Fig. 5A), yielding stress (Fig. 5B), and elastic energy absorption/volume (Fig. 5C), all indicative of intrinsic properties of bone material, which depends on its constitution but not on its amount or spatial distribution, further suggest that the adverse effects evoked by treatments may have been only quantitative in nature. The finding that those effects disappear when parameters indicative of bone extrinsic mechanical properties are normalized for body mass gives support to this concept. The natural stimuli for the bone mechanostat would be the strain of bone tissue, sensed by osteocytes that are induced by both gravitational forces and contractions of regional muscles.4 Therefore, on a weight-bearing bone as the femur, different body weights will produce different loads and strains.
The variations in dietary protein concentration and/or quality given to animals in the present study induced significant changes in the femoral calcium mass although they did not alter femoral calcium concentration in ashes (Fig. 5D). The last finding could explain the normal rigidity of femoral bone material (modulus of elasticity) found in the experimental animals. Material properties of bone tissue are usually thought to depend on many factors, calcium content being one of the main determinants.28
In conclusion, we have described a number of alterations in both morphometrical and biomechanical variables in rat femoral shafts resulting from feeding growing rats with diets containing different concentrations of two proteins (casein and wheat gluten) having different biological qualities. Both the concentration and the quality of the tested proteins affected the body growth rate (weight gain) of the animals. The femur, as a part of the skeleton that grows in direct relation with body growth, was thus similarly affected. Both geometric (diaphyseal cross-sectional area and mineralized cortical area, and cross-sectional moment of inertia) and structural (stiffness and strength) of the femoral shaft correlated positively with the body weight of the animals. Neither concentration nor quality of the tested proteins affected the material properties (elastic modulus, mineralization) of the bone tissue. Therefore, it is suggested that the concentration of either casein or wheat gluten in the diet affect the mechanical competence (resistance to fracture) of the rat femoral shaft indirectly, through their effects on body growth and body weight in the absence of changes in the quality of the bone mineralized material.
Conflict of interestThe authors have no conflict of interest to declare.
This work was supported by Research Grants from the University of Buenos Aires (UBACYT O-002 and O-005) and National Research Council (CONICET PIP 5501).
RMA and CEB are Career Investigators from CONICET. The valuable technical assistance of Graciela Champin is greatly acknowledged.



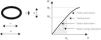





![Femoral structural properties [fracture load (A), fracture load in relation to body weight (B), yielding load (C), yielding load in relation to fracture load (D), diaphyseal stiffness (E), and elastic energy absorption (F)] in rats fed diets containing casein (black bars) or gluten (white bars); values are presented as percentage of the control value, which corresponds to the group fed the standard diet containing 20% casein and express mean±SD; equal letters on top of bars indicate p>0.05. Femoral structural properties [fracture load (A), fracture load in relation to body weight (B), yielding load (C), yielding load in relation to fracture load (D), diaphyseal stiffness (E), and elastic energy absorption (F)] in rats fed diets containing casein (black bars) or gluten (white bars); values are presented as percentage of the control value, which corresponds to the group fed the standard diet containing 20% casein and express mean±SD; equal letters on top of bars indicate p>0.05.](https://static.elsevier.es/multimedia/15750922/0000005900000001/v1_201305082256/S157509221100324X/v1_201305082256/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Femoral bone material properties [elastic modulus (A), yielding stress (B), and elastic energy absorption (C)] and calcium concentration in bone ashes (D) in rats fed diets containing casein (black bars) or gluten (white bars); each bar represents the mean±SD for 7 animals; equal letters on top of bars indicate p>0.05. Femoral bone material properties [elastic modulus (A), yielding stress (B), and elastic energy absorption (C)] and calcium concentration in bone ashes (D) in rats fed diets containing casein (black bars) or gluten (white bars); each bar represents the mean±SD for 7 animals; equal letters on top of bars indicate p>0.05.](https://static.elsevier.es/multimedia/15750922/0000005900000001/v1_201305082256/S157509221100324X/v1_201305082256/en/main.assets/thumbnail/gr6.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Femoral morphometric properties [xCSMI (A), cross-sectional area (B), cortical cross-sectional area (C), and volume (D)] in rats fed diets containing casein (black bars) or gluten (white bars); values are presented as percentage of the control value, which corresponds to the group fed the standard diet containing 20% casein and express mean±SD; equal letters on top of bars indicate p>0.05. Femoral morphometric properties [xCSMI (A), cross-sectional area (B), cortical cross-sectional area (C), and volume (D)] in rats fed diets containing casein (black bars) or gluten (white bars); values are presented as percentage of the control value, which corresponds to the group fed the standard diet containing 20% casein and express mean±SD; equal letters on top of bars indicate p>0.05.](https://static.elsevier.es/multimedia/15750922/0000005900000001/v1_201305082256/S157509221100324X/v1_201305082256/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

