The International Task Force on Obesity (IOTF) and the World Health Organization (WHO) have defined obesity as a 21st century epidemic. In countries with economies in transition and even in some urban areas in developing countries, progressive increase in obesity has been reported to be an emerging problem in recent years. Its causes include lifestyle changing, particularly consumption of high-calorie food, as well as an increasingly sedentary lifestyle. However, the genetic origin of obesity is well known and currently proven. Obesity usually results from interaction of certain gene polymorphisms with environment. Moreover, only a small number of cases of obesity (5%) result from mutations in specific genes (monogenic obesity), causing in some cases Mendelian syndromes with a very low incidence in the population. One hundred and thirty genes related to obesity have been reported, some of which are involved in coding of peptide transmitting hunger and satiety signals, while others are involved in adipocyte growth and differentiation processes, and still others are involved in regulation of energy expenditure. In addition, obesity is a chronic inflammatory state. In this regard, altered expression of genes related to insulin metabolism and adipose tissue inflammation is a basic process which may explain the etiology of obesity.
El grupo internacional de trabajo en obesidad (IOTF) y la Organización Mundial de la Salud (OMS) han definido la obesidad como la epidemia del siglo XXI. En los países con economías en transición e incluso en determinadas áreas urbanas en los países en desarrollo, el aumento progresivo de la obesidad se ha descrito como un problema emergente en los últimos años. Entre sus causas se encuentran cambios en los hábitos de vida, especialmente por el consumo de alimentos de gran contenido calórico, junto con un cada vez mayor sedentarismo. Sin embargo, el origen genético de la obesidad es un hecho bien conocido y demostrado en la actualidad. Generalmente, la obesidad resulta de la interacción de determinados polimorfismos génicos con el medio ambiente. Por otra parte, solo un pequeño número de casos de obesidad (5%) resulta de la existencia de mutaciones en genes concretos (obesidad monogénica), originando en algunos casos síndromes mendelianos de muy escasa incidencia entre la población. Por el momento se han descrito 130 genes relacionados con la obesidad, genes algunos de ellos implicados en la codificación de péptidos transmisores de las señales de hambre y saciedad, otros implicados en los procesos de crecimiento y diferenciación de los adipocitos y genes implicados en la regulación del gasto energético. Asimismo, la obesidad constituye un estado de inflamación crónico. En este sentido, alteraciones de la expresión de genes relacionados con el metabolismo de la insulina y la inflamación del tejido adiposo son procesos básicos que explican la etiología de la obesidad.
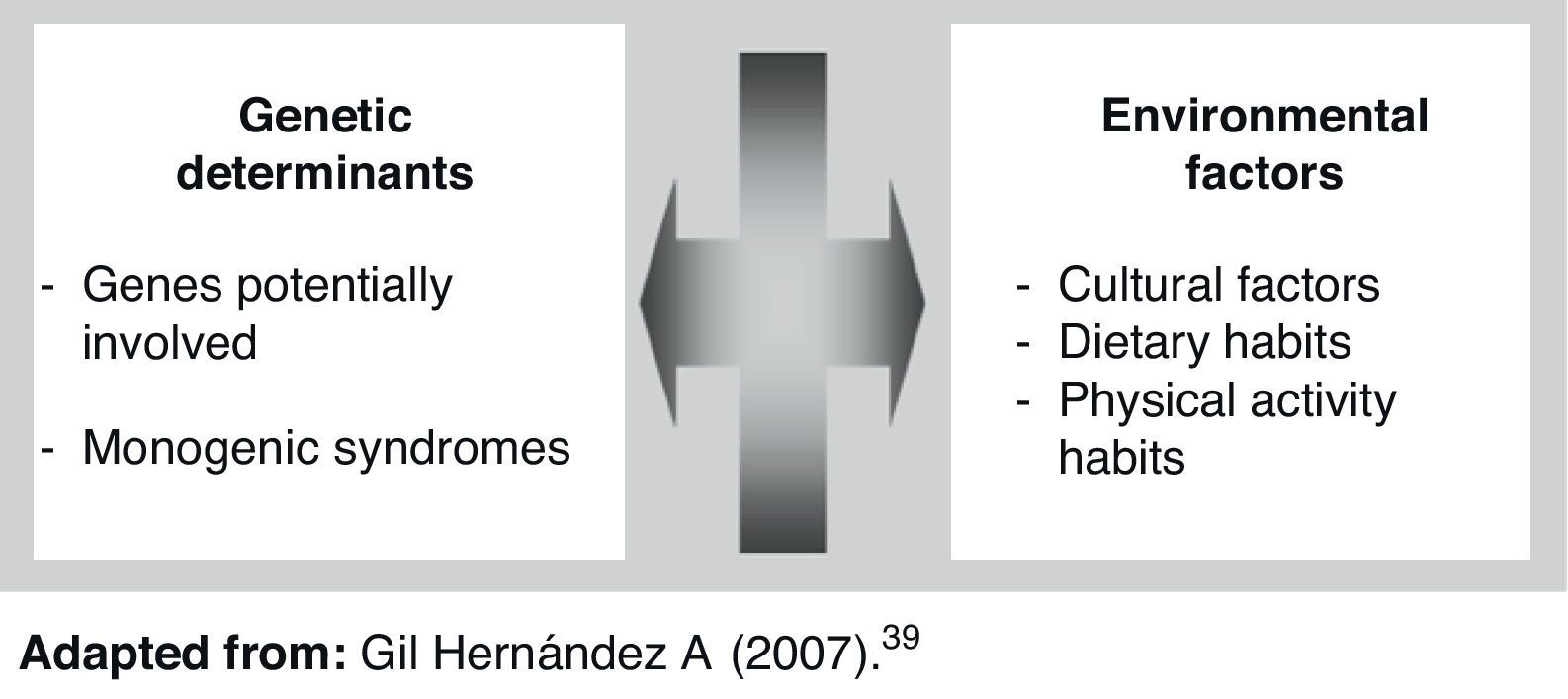
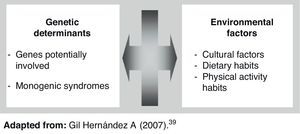
Obesity is a complex and multifactorial chronic disease characterized by excess body fat accumulation. Obesity represents one of the endocrine-metabolic conditions most significant for public health, and is the starting point of a significant number of diseases including type 2 diabetes mellitus, cardiovascular diseases, metabolic syndrome, and some types of cancer.1–6 In addition, despite the well known impact of environmental factors in the development of obesity,2 many studies have reported the significance of the genetic component in this condition.7–10 In this regard, data from a large meta-analysis demonstrated that approximately 50–70% of changes in body mass index (BMI) are attributable to genetic differences inherent to each subject.11 Based on this, environmental interaction in subjects with obesity susceptibility genes is currently considered to be the main cause of obesity.12 In only 5% of cases is obesity due to the existence of monogenic changes or to low incidence syndromes.13
The purpose of this review was to provide some epidemiological data, as well as an updated review of the current understanding of genetics of human obesity, discussing the results of some recently published studies.
Prevalence of obesityAn alarming increase in the prevalence of obesity in the past two decades to pandemic levels, both in terms of the number of obese subjects and the degree of obesity, has led to the World Health Organisation (WHO) calling it the “21st century epidemic”.
Obesity is the most common nutritional disorder in developed countries. It is estimated that there are more than one billion obese people worldwide. In the United States, 65% of the adult population and 15% of children are overweight, and what causes even more concern is that no trend towards a decrease in these figures is envisaged.14
According to the International Obesity Task Force (IOFT), obesity is more prevalent in Spain as compared to North European countries such as Sweden, Denmark, the Netherlands, or France, but less prevalent as compared to the United Kingdom or the United States.14 Thus, prevalence of obesity has been estimated at 14.5% in the Spanish population aged 25–60 years, and is significantly higher in females (15.75%) as compared to males (13.9%). Only 0.5% of the Spanish population has morbid obesity, which is also more common in females. Thirty-nine percent of the population, 45% of males and 32% of females, is overweight.14
On the other hand, the prevalence of obesity in children has increased alarmingly in both developed and developing countries. In the Spanish infantile and juvenile population (2–24 years of age), the prevalence rates of obesity and overweight were estimated at 13.9% and 12.4% respectively based on data from the ENKID study (1998–2000).15 As regards sex, obesity was more prevalent in males (15.6%) as compared to females (12.0%). In southern Spain, and specifically in the province of Granada, an epidemiological study conducted by González et al.16 on a population of 977 school children and adolescents found prevalence rates of overweight of 23.01% in girls and 20.81% in boys. Prevalence rates of obesity were 12.70% in girls and 4.98% in boys. In addition, this study estimated prevalence rates of systolic arterial hypertension of 9% in males and 5% in females. These values are far higher than those reported by the ENKID study in 2000, thus showing an increasing trend over time.
Genes potentially involved in obesity developmentObesity is a complex condition because it results from the interaction of multiple genes with the environment.17 Genes involved in the etiology of obesity include genes encoding peptides targeted to transmit hunger and satiety signals, genes involved in adipocyte growth and differentiation, and genes involved in energy expenditure control.18 In the seventh review of the human obesity map, using data collected up to October 2000, 47 cases of monogenic obesity, 24 cases of Mendelian changes, and 115 different loci susceptible to being involved in polygenic obesity were reported. The obesity map thus suggests that all chromosomes, except for chromosome Y, have genes involved in obesity occurrence and development.19 There is now sufficiently solid scientific evidence, provided by 222 studies conducted on genes and obesity, for us to be able to say that there are 71 genes which are potential inducers of the occurrence of obesity.20 Of these, 15 genes are closely associated with body fat volume.21 It is therefore natural to think that there is not a single type of obesity, but several types with similar phenotypes.22
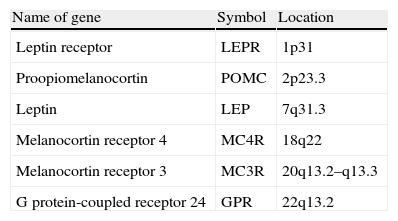
It is currently also accepted that mutations in genes implicated in the coding and synthesis of proteins involved in appetite regulation are responsible for pathological changes associated with the development of obesity.23 Gene mutations which, in turn, have been found to cause monogenic obesity include mutation in leptin gene, leptin receptor (LEPR), carboxypeptidase E, orexigenic agouti protein, the prohormone convertase 1 (involved in the processing of insulin and proopiomelanocortin, POMC), and POMC 6–8 itself.24 Forms of obesity resulting from mutations in the gene encoding for melanocortin receptors 3 and 4 (MC3R and MCR4) have also been reported25 (Table 1).
Main genes involved in development of monogenic obesity.
| Name of gene | Symbol | Location |
| Leptin receptor | LEPR | 1p31 |
| Proopiomelanocortin | POMC | 2p23.3 |
| Leptin | LEP | 7q31.3 |
| Melanocortin receptor 4 | MC4R | 18q22 |
| Melanocortin receptor 3 | MC3R | 20q13.2–q13.3 |
| G protein-coupled receptor 24 | GPR | 22q13.2 |
Adapted from: Gil Hernández.39
Another gene widely studied because of its potential implication in the development of obesity at early ages is the FTO gene.26,27 This gene is considered to induce progressive weight gain in subjects in whom it is overexpressed.28 Its expression is usually greater in the hypothalamic areas involved in the feeding process.29 Thus, expression of this gene has been shown to be modified under conditions of acute food deprivation. Wardle et al. showed a close relationship between the sensation of satiety reported by obese subjects and the degree of expression of the gene.30 In this regard, patients carrying two risk alleles had a lower satiety response (Fig. 1).
The number of genes potentially involved in the development of human obesity continues to increase. The most recent review of the obesity gene map, covering up to October 2005, reported that more than 600 genes and chromosomal regions were involved in obesity.8 Mutations in 11 genes and 50 loci have been directly related to some of the already reported syndromes.
On the other hand, mutations in some human genes responsible for the occurrence of pleiotropic effects associated with morbid obesity conditions such as clinical manifestation have been known since the 1980s.31 One such condition is the Prader-Willi syndrome, an autosomal dominant disorder. Seventy percent of patients with this syndrome have abnormalities in several genes located, in turn, in chromosome 15 of the father.32 Clinically, this syndrome is characterized in children by the occurrence of obesity, muscle hypotony, mental retardation, hypogonadism, cryptorchidism, and low height associated with small hands and feet. In some cases, the syndrome is usually associated with the presence of noninsulin-dependent diabetes mellitus, as well as ketogenesis and hyperglycemia. This syndrome represents one of the most prevalent examples of dysmorphic obesity in humans.
The Alström-Hallgren syndrome, of an autosomal recessive nature, is characterized by the occurrence of neurosensory deafness and diabetes mellitus, but without polydactyly or mental retardation. In this syndrome, obesity usually occurs from two years of age, with weight often increasing to values 100% higher than normal values for age and sex.33 Another characteristic feature is the occurrence of skin changes, mainly acanthosis nigricans, arising from the chronic association between diabetes mellitus and a marked insulin resistance.34
The Cohen syndrome is an autosomal recessive disorder characterized by the presence in children of hypotonia, mental and pubertal retardation, gothic palate, a characteristic facies (prominent incisor teeth, elevated nasal root, small jaw), and obesity from five years of age.35
In the Carpenter syndrome, patients develop craniosynostosis, exophthalmos, syndactyly, brachymesophalangy, and gothic palate.36
Finally, the Bardet-Bield syndrome, transmitted as an autosomal recessive disorder, has four known different variants depending on the affected genes. Mutated genes include BBS1, located in chromosome 11, BBS2 located in chromosome 16, BBS3 located in chromosome 3, and BBS4 located in chromosome 15.37 This unique chromosome distribution of the involved genes accounts for the phenotypic variability of the syndrome. Clinically, pediatric patients will experience retinitis pigmentosa, mental retardation, hypogonadism, and some finger abnormalities reported by Mykytyn et al.38 Interactions between many of these genes involved and of these genes with the environment may lead to a phenotypic expression of obesity.
ConclusionsCurrently available studies and data clearly show the significance and involvement of the genetic component in the development of obesity. It should be noted, however, that genetic changes leading to the development of obese phenotypes tend to overexpress as the result of their interaction with environmental factors. Future management of obesity conditions with a genetic component will therefore necessarily include control of the genes involved in food intake and metabolic processes. At any rate, what is evident today is the complexity of the condition and the need for further research on the etiology and probable genetic nature of obesity.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please, cite this article as: González Jiménez E. Genes y obesidad: una relación de causa-consecuencia. Endocrinol Nutr. 2011;58:492–6.