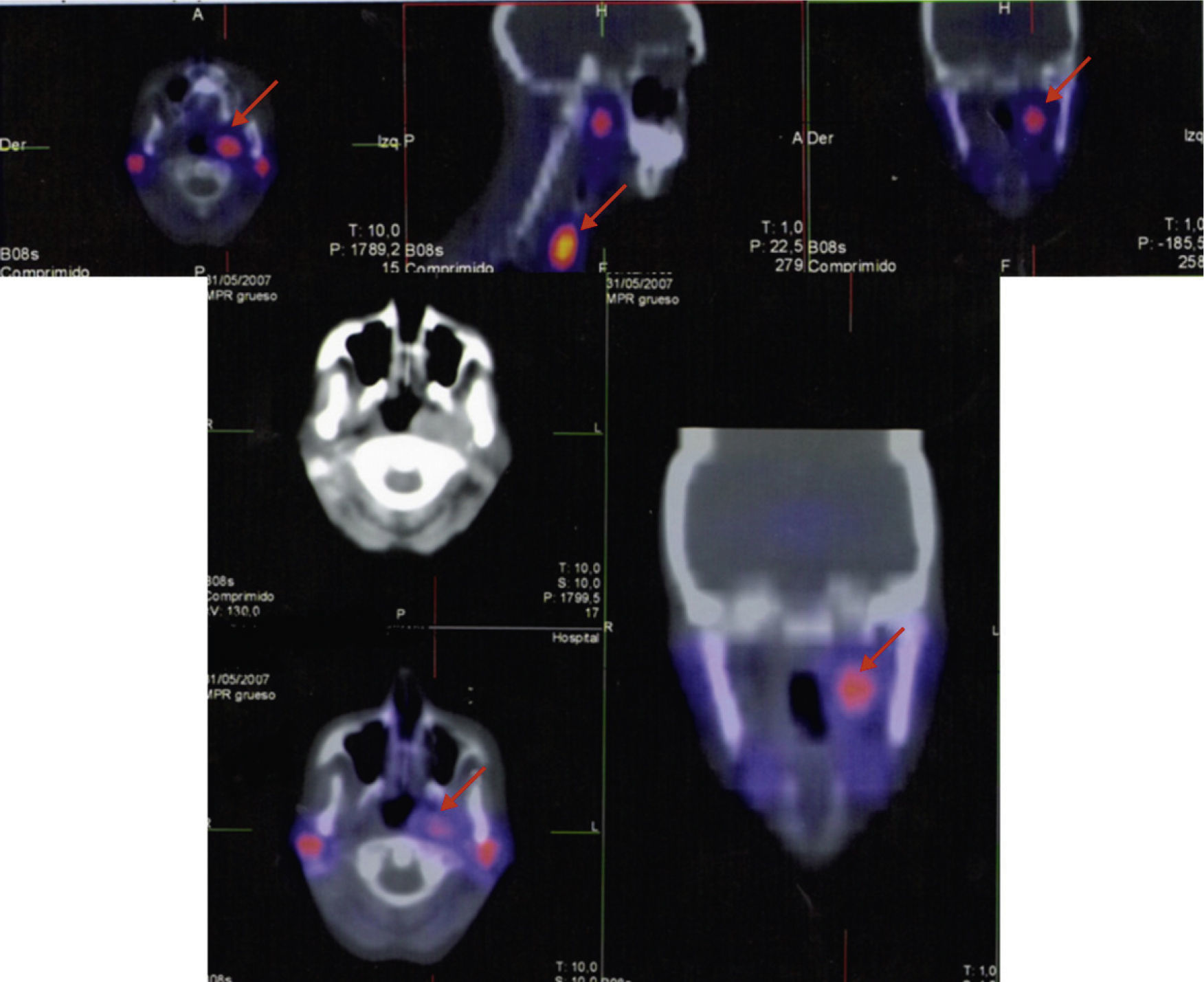
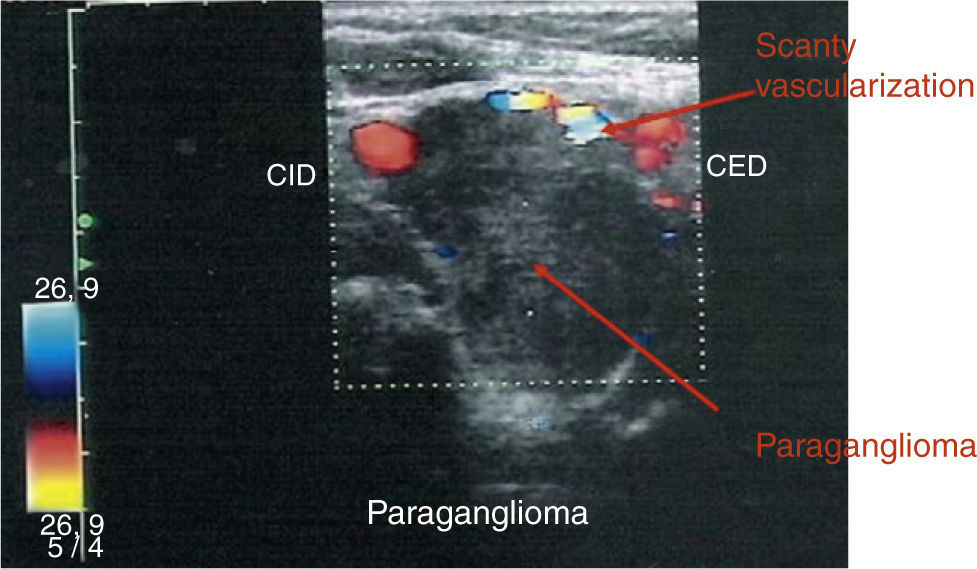
A 50-year-old woman was referred to our hospital for evaluation of a mass on the right side of the neck. She had a past medical history of multiple interventions of cosmetic surgery in her face and breasts. She also had a macroprolactinoma treated with transsphenoidal surgery in our clinic in 1993 because of intolerance to bromocriptine. The surgery confirmed the diagnosis of lactotroph adenoma but amenorrhea-galactorrhea syndrome and prolactinoma persisted after surgery. Three years later she came back to our hospital to be operated on a neck mass on the left side. The histology reported a glomic tumor. In 1996 she was treated with cabergoline and the amenorrhea-galactorrhea syndrome disappeared. She did not reappear until July 2007, when she came complaining about a large growth of a nodule on the right side of the neck. A cervical ultrasound scan revealed a nodule on the right side of 29mm of maximum diameter and the octreotide scan and the single-photon emission computed tomography (SPECT) (Fig. 1) showed the existence of three masses: one was adjacent to the phaynx on the left side, the second one was also on the left side, adjacent to the thyroid and the third one was located in the right carotid bifurcation and all of them were suggestive of paraganglioma (PGL). Besides, the scintigraphy with radiotracer-labeled metaiodobenzyl-guanidine (MIBG), serum and urine catecholamine and metanephrines levels was negative. The pituitary magnetic resonance imaging (MRI) did not demonstrate remaining prolactinoma. Her family medical history was studied after the diagnosis of multiple PGLs. The patient reported that a paternal uncle had recently had an operation on a carotid PG and another paternal aunt had also a cervical tumor. We initiated study for familial PGL. Therefore, we recommended the patient to be operated on but she refused, so she started a treatment with lanreotide 120mg/28 days in the meantime. The color-Doppler imaging (Fig. 2) control after 7 months of somatostatin analogs treatment showed a stabilization of tumor size, as well as an important decrease of the vascularity. Finally, the patient agreed to be operated on and a surgery excision of the right neck mass was performed without complications, with a histological diagnosis of PGL (inmunohistochemistry: chromogranin A, synaptophysin and S-100 protein positive and Ki-67: 5%). After surgery the somatostatin analogs treatment was stopped and the octreotide scan, SPECT (Fig. 1) and color-Doppler imaging 6 and 12 months after surgery showed a total surgical excision of the right neck PGL and no changes were observed in the other nodules.
Spect before surgery (upper): three masses; one was adjacent to the pharynx on the left side, the second one was also on the left side adjacent to the thyroid and the third mass was located in the right carotid bifurcation; all of them were suggestive of paraganglioma (arrow). Spect after surgery (lower): only one mass adjacent to pharynx on the left side (arrow).
Currently, the patient is asymptomatic. The genetic study was positive to mutation in succinate dehydrogenase (SDH) subunit D (P81L exon 3). Consequently, her brother was also studied for the mutation with a positive result. In addition, a cervical scan of her brother revealed a cervical PGL.
Head and neck PGLs are mostly benign, with less than 10% thought to be malignant, slow growing tumors, with an average increase on size of 1mm per year.1 PGLs occasionally are associated with genetic multisystemic disorders as Von Hippel-Lindau disease, multiple endocrine neoplasia type 2 (MEN 2), neurofibromatosis type 1, the recently known MEN type 4-Carney's triad (PGL, gastrointestinal stromal tumors and pulmonary chondromas) and Carney-Stratakis syndrome (PGL and gastrointestinal stromal tumors). In addition, the PGLs can form a part of an association with other tumors such as kidney cancer, parathyroid adenoma, thyroid carcinoma, gastrointestinal stromal tumors and astrocytoma.1
Familial PGL is a genetically heterogeneous entity. Inactivating mutations of the mitochondrial complex II SDH enzyme subunits B (SDHB) and C (SHDC) and D (SDHD) are responsible for 70% of familial cases. The SDH, with its four sub units A, B, C and D plays an important role in the Krebs cycle and, as part of the mitochondrial complex II, in the aerobic electron transport of the respiratory chain. Mitochondrial complex II is thought to function as a tumor suppressor because when it is defective, it results in the overexpression of several hypoxia-induced gene that are believed to result in proliferation of paraganglia. The transmission of all PGL is by autosomal-dominant gene and patients with SDHD gene mutation, like our patient, have a parent-of-origin-dependent (maternal transmission does not cause tumor development). The SDHD mutation conferred 50% penetrance by 31 years of age and 86% by 50 years of age.1 Multifocal PGL are significantly more frequent in SDHD patients compared with SDHB and SDHC mutation. Malignant PGL are rarely seen in SDHD, which are more frequent in SDHB mutations.1
The best known association of SDH germline mutation with other tumors is the Carney-Stratakis syndrome, which includes the association of PGL, pheochromocytomas and gastrointestinal stromal tumors; however, they are not associated with pituitary tumors, as in our patient.2–4 It is the first time, as far as we know, that a case of an association of prolactinoma and multiple PGLs is described in literature. Twenty-five cases have been described in the literature of the association of pituitary adenomas with pheochromocytomas, but only one with extradrenal location, three with prolactinoma and none with both tumors.2
There have been different hypotheses in literature to explain the coexistence of a pituitary adenoma and pheochromocytoma. One is purely that of a coincidence given the infrequent association of PGL and prolactinoma in the same patient. Some authors have suggested the possibility of a new variant of MEN syndromes. This attractive theory is based on the common origin of both pituitary adenomas and pheochromocytomas/paragangliomas from the neural crest. The occurrence of these tumors in the same person has been speculated to be related to aberrant neural crest development.2–4 Nevertheless, the absence of an autosomal dominant pattern of transmission in families of the report index cases makes such a variant of MEN syndrome unlikely.2 Our case shows the coincidence in the same patient of two infrequent tumors like macroprolactinoma and multiple PGLs, with a mutation in SDHD with a paternal family transmission.
The gold standard for diagnosis of PGL is arteriography, which demonstrates a patognomonic tumor blush as well as the feeding vessels of the tumor but the best functional imaging for the diagnosis of PGLs is also a matter of debate.5 In a recent study that studied the functional imaging of SDHx-related head and neck paragangliomas, compared 18F-fluorodihydroxyphenylalanine, 18F-fluorodopamine ((18)F-FDOPA), 18F-fluoro-2-deoxy-d-glucose PET, 123I-metaiodobenzylguanidine scintigraphy and 111In-pentetreotide scintigraphy concluded that (18)F-FDOPA PET proved to be the most efficacious functional imaging modality in the localization of SDHx-related head and neck paragangliomas.6
The treatment of choice for PGLs is surgical resection, though preoperative chemo-embolization, sterotactic radiosurgery, chemotherapy, radiolabeled octreotide derivates and somatostatin analogs7–10 have been mentioned in literature. Because PGLs are close to important vessels and nerves, there is risk of morbidity and mortality. Surgery may be complicated by extensive bleeding and the most frequent complications include stroke (9–20%) and cranial nerve injury (11–49%).5 Preoperative embolization is performed to reduce blood supply to the tumor but it may elicit an inflammatory response in the tumor and complicate the dissection.5
Treatment with lanreotide 120mg/28 days for 7 months did not result in a reduction in tumor size but a notable decrease of vascularity was reported by color-Doppler imaging. Only a few papers have focused on somatostatin analog efficacy on volume of head and neck paragangliomas with a high variability in tumor response. Unfortunately, the percentage of shrinkage observed is not significant8–10 and studies evaluating the effects on the vascularization of the somatostatin analogs are lacking.
In our case the treatment with lanreotide 120mg/28 days for 7 months did not produce a significant shrinkage of PGLs but reduced vascularity markedly. We suggest that somatostatin analogs could be used before the intervention of PGLs to reduce the risk of morbidity and mortality of surgery.
Please, cite this article as: Varsavsky M, et al. Coexistence of a pituitary macroadenoma and multicentric paraganglioma: A strange coincidence. Endocrinol Nutr. 2013;60:154–6.