Osteogenesis imperfecta (OI) is an inherited disorder that causes low mineral density and bone fragility. Previous studies have shown the efficacy of bisphosphonates to increase bone mineral density (BMD). This study assessed changes over time in BMD and biochemical markers of bone metabolism in adult patients with osteogenesis imperfecta treated with intravenous zoledronic acid and the safety of this treatment.
Patients and methodsA prospective, observational study in patients with OI, osteoporosis or osteopenia (T score<−2) who were administered zoledronic acid infusions (4mg IV) every 6 months for three years and annually thereafter. Densitometry was performed annually. Acute changes in complete blood count and calcium, phosphate, and creatinine levels, as well as side effects of the infusion, were recorded 24 and 48h after treatment. Calcium, phosphate, parathyroid hormone (iPTH), 25OH-vitamin D and bone turnover markers (bone alkaline phosphatase, ß-crosslaps and urinary deoxypyridinoline) were measured at baseline and every 12 months.
Adverse events and new fractures were recorded.
ResultsTwenty patients (6 men and 14 women) were treated. Median follow-up time was five years. Calcium levels and platelet counts significantly decreased 24 and 48h after the first infusion, and the red blood cell count decreased at 24h. These changes were not clinically relevant. Seven patients experienced a flu-like episode after the first dose. Treatment induced significant increases in BMD in the lumbar spine (6.7%) after 12 months of follow-up (0.791±0.178 vs. 0.791±0.140g/cm2, p=.003) and at three (5.7%) and five years (9%) of follow-up. Femoral neck BMD significantly increased after 3 years (11.1%): 0.648±0.148 vs. 0.720±0.138g/cm2; p=.01. In total hip, increase in BMD (10.1%) was significant after three years of treatment (0.706±0.118 vs. 0.720±0.138, p=.01). There were no significant differences in calcium and 25OH-vitamin D levels during follow-up, phosphorus significantly decreased after one year, and iPTH increased at three years. ß-Crosslaps decreased after one year of treatment. Only one patient sustained new fractures.
ConclusionsZoledronic acid is a convenient, safe, and effective treatment that increases BMD in adult patients with OI.
La osteogénesis imperfecta (OI) es una enfermedad genética que cursa con baja densidad mineral y fragilidad ósea. Varios trabajos han demostrado la eficacia de los bisfosfonatos para mejorar la densidad mineral ósea (DMO). El objetivo de este estudio es evaluar la evolución de la DMO y parámetros bioquímicos de metabolismo óseo, en pacientes adultos con OI tratados con ácido zoledrónico intravenoso (iv) durante un periodo medio de 5 años, así como valorar la seguridad de dicho tratamiento.
Pacientes y métodosEstudio prospectivo, observacional en pacientes adultos con OI con osteoporosis u osteopenia, con T-score<−2, a los que se administró ácido zoledrónico (4mg iv) cada 6 meses durante 3 años y posteriormente de forma anual. Se registraron a las 24 y 48h los cambios agudos en calcio, fósforo, creatinina y hemograma así como los efectos secundarios tras la infusión. Se realizó densitometría basal y cada año. Se determinaron basal y anualmente calcio, fósforo, paratohormona (PTHi), 25OH-vitamina D y marcadores de remodelado óseo (fosfatasa alcalina ósea, ß-cross-lap y deoxipiridolina en orina).
Se registraron las nuevas fracturas.
ResultadosSe trataron 20 pacientes, 6 hombres y 14 mujeres con una mediana de seguimiento de 5 años. Los niveles de calcio y las plaquetas disminuyeron significativamente a las 24 y 48h tras la primera infusión. El recuento de hematíes disminuyó a las 24h. Estos cambios no fueron clínicamente relevantes. Siete pacientes presentaron un cuadro pseudogripal tras la primera dosis. La DMO medida en columna lumbar mostró un aumento significativo (6,7%) a los 12 meses de seguimiento (0,741±0,178 vs. 0,791±0,140g/cm2; p=0,003) así como a los tres (5,7%) y 5 años (9%) de seguimento. En cuello femoral se evidenció incremento significativo de la DMO a los 3 años (11,1%): 0,648±0,148 vs. 0,720±0,138g/cm2; p=0,01. En cadera total el incremento (10,1%) resultó significativo a los 3 años de tratamiento (0,706±0,118 vs. 0,720±0,138; p=0,01). No se evidenciaron diferencias significativas en los niveles de calcio y 25OH-vitamina D largo del seguimiento, el fósforo disminuyó significativamente al año y PTHi aumentó a los 3 años. ß-cross-lap disminuyó al año de tratamiento. Solo un paciente ha presentado nuevas fracturas.
ConclusionesEl ácido zoledrónico es un tratamiento cómodo, seguro y eficaz para mejorar la DMO en pacientes adultos con OI.
Osteogenesis imperfecta (OI) is an infrequent disorder that is highly heterogeneous from the clinical and genetic perspectives. It is caused by the mutation of genes involved in the formation of type 1 collagen, the main structural protein of skin and bone. The disease affects one in every 15,000–20,000 live births, and although bone fragility is the main and most common characteristic, several types of OI have been reported based on their clinical, radiological and genetic characteristics.1–3 Osteogenesis imperfecta is generally caused by heterozygous mutations of the genes encoding for chains α1 and α2 of procollagen type 1 (genes COL1A1 and COL1A2), and exhibits an autosomal dominant hereditary trait, though other genes are also known to be implicated.1–4
The high incidence of progressive bone pain, fractures and deformities leads to the consideration of drug therapy, though the treatment of choice in these cases has not been clearly established. Since most of these patients have osteoporosis or low bone mineral density (BMD), with increased bone remodeling, it seems logical to consider antiresorptive drug therapy. Bisphosphonates have been the drugs most commonly used to date, and several studies have shown their favorable effects upon BMD, bone remodeling parameters, and even histomorphometric variables when administered by either the oral or the intravenous (i.v.) route. These drugs are therefore now considered the treatment of choice in adult patients with OI and low BMD, though conclusive data on their fracture-preventing effects are lacking.5–8
The objective of this study was to assess the acute and long-term effects (evolution of BMD and biochemical parameters of bone remodeling and metabolism) of treatment with zoledronic acid in adult patients diagnosed with OI, as well as its safety.
Patients and methodsA prospective observational study was made involving adult patients diagnosed with OI (types I, III, or IV) on the basis of their clinical characteristics, family history and fracture antecedents. Classification of the type of OI in each case was based upon the presence of blue sclera, dentinogenesis imperfecta, short stature, kyphoscoliosis or other bone deformities, and hearing loss confirmed by audiometry.
Inclusion criteria- -
Age≥17 years.
- -
Densitometric study with a lumbar spine and/or total femoral T-score of <−2.
- -
Hormone tests ruling out other causes of osteoporosis (primary hyperparathyroidism, hyperthyroidism, growth hormone [GH] deficiency, hypogonadism and hypercortisolism).
- -
The obtaining of written informed consent before a witness, and an application for the use of the drug (zoledronic acid) on a compassionate basis (accepted in each case by the competent authorities).
- -
A desire for pregnancy in women (the advisability of contraceptive measures was stressed in premenopausal women).
- -
Fractures in the consolidation phase.
- -
Renal failure with creatinine clearance <60ml/min.
- -
Serious concomitant illness.
Zoledronic acid (4mg diluted in 0.9% saline as a continuous i.v. infusion over a period of at least 15min) was administered every 6 months for the first three years, followed by annual doses, with reinforced water intake during the hours before and after the administration of the drug. All patients received daily supplements of calcium (500–1000mg) and vitamin D (400–800IU).
Previous antiresorptive treatments were documented.
DensitometryDual energy X-ray densitometry was performed before the start of treatment and then annually with a HOLOGIC densitometer in the femur (coefficient of variation 1.72%) and/or lumbar spine (L2–L4) (coefficient of variation 1.37%). The BMD measurements were expressed in g/cm2. In patients with bilateral femoral prostheses, BMD could only be measured at the lumbar level. In patients that had been operated upon for spinal deformities, with the implantation of metallic material, BMD was only measured at hip level.
Biochemical parametersThe following parameters were determined at baseline and every 12 months thereafter: total calcium, phosphorus, iPTH (chemiluminescence immunoassay, SIEMENS®), 25-OH-vitamin D (chemiluminescence immunoassay, SIEMENS®), bone alkaline phosphatase (immunoelectrophoresis, SIEMENS®), βcross-laps (electrochemoluminescence, SIEMENS®) and fasting blood count before drug infusion, as well as deoxypyridoline (chemiluminescence, SIEMENS®) in the second morning urine sample. Likewise, control laboratory tests were made corresponding to total calcium, creatinine, phosphorus and fasting blood count 24 and 48h after the first drug infusion.
SafetyIn addition to the laboratory parameters, we recorded the clinical side effects in the first 48h after the first drug infusions: the presence of fever or febricula, headache, asthenia and muscle or osteoarticular pain (flu-like syndrome), nausea or other gastrointestinal symptoms or ocular discomfort.
We also documented the new fractures during follow-up.
Statistical analysisThe results are reported as the mean± standard deviation (SD). The comparison of values was based on the Student t-test for paired samples after the confirmation of normal data distribution with the Shapiro–Wilk test. The Fisher–Pitman statistic for paired samples was used in the case of non-normal data distribution. The association between previous treatment with bisphosphonates and the presence of flu-like syndrome was explored by means of the Fisher exact test. The association between previous treatment with bisphosphonates and the BMD values during follow-up was analyzed using a linear regression model in which the BMD value before the start of treatment constituted the independent variable, BMD during follow-up represented the dependent variable, and previous bisphosphonate treatment was the adjustment variable. Statistical significance was considered for p≤0.05.
The data corresponding to the extension of a previous study involving 10 patients with a maximum follow-up period of 36 months are presented.9
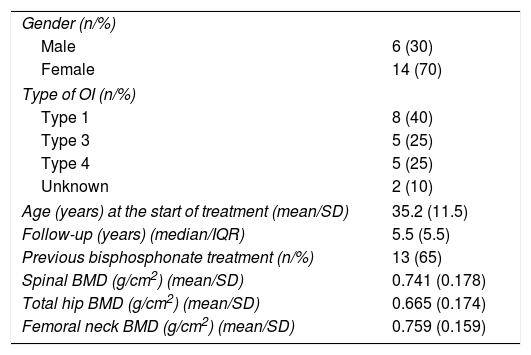
ResultsTwenty patients (6 men and 14 women) aged 17–60 years received treatment. Table 1 shows the baseline clinical characteristics of the patients. All but one of the women was pre-menopausal. Nine patients had received previous treatment with oral bisphosphonates. One-half of the patients (n=10) were followed-up on for over 5 years.
Patient characteristics at the start of treatment.
| Gender (n/%) | |
| Male | 6 (30) |
| Female | 14 (70) |
| Type of OI (n/%) | |
| Type 1 | 8 (40) |
| Type 3 | 5 (25) |
| Type 4 | 5 (25) |
| Unknown | 2 (10) |
| Age (years) at the start of treatment (mean/SD) | 35.2 (11.5) |
| Follow-up (years) (median/IQR) | 5.5 (5.5) |
| Previous bisphosphonate treatment (n/%) | 13 (65) |
| Spinal BMD (g/cm2) (mean/SD) | 0.741 (0.178) |
| Total hip BMD (g/cm2) (mean/SD) | 0.665 (0.174) |
| Femoral neck BMD (g/cm2) (mean/SD) | 0.759 (0.159) |
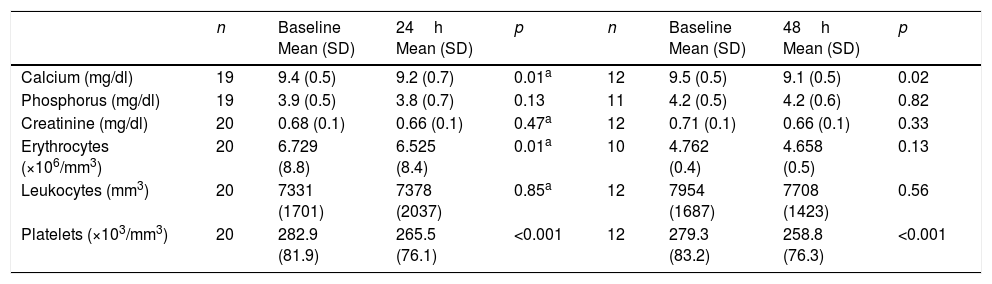
The total calcium levels decreased significantly both 24 and 48h after the first infusion with respect to baseline. Serum calcium levels of under 8.5mg/dl were recorded in three patients (after 24h in two patients and after 48h in one), followed by spontaneous normalization over the next 24h. No patient developed clinical manifestations of hypocalcemia. The red cell count after 24h and the platelet count after 24 and 48h decreased significantly, though no patient reached the lower limit of normal (Table 2). Seven patients developed symptoms consistent with flu-like syndrome, three patients experienced gastrointestinal symptoms, and no patients suffered eye discomfort after the infusion of the drug. The patients that had been previously treated with bisphosphonates presented a significantly lower risk of flu-like syndrome (odds ratio [OR]: 13.7, 95% confidence interval [95%CI]: 1.7–113; p=0.02). After the second dose, only three patients presented symptoms consistent with flu-like syndrome. After a third dose, only one patient presented such symptoms.
Acute biochemical effects of intravenous zoledronic acid.
| n | Baseline Mean (SD) | 24h Mean (SD) | p | n | Baseline Mean (SD) | 48h Mean (SD) | p | |
|---|---|---|---|---|---|---|---|---|
| Calcium (mg/dl) | 19 | 9.4 (0.5) | 9.2 (0.7) | 0.01a | 12 | 9.5 (0.5) | 9.1 (0.5) | 0.02 |
| Phosphorus (mg/dl) | 19 | 3.9 (0.5) | 3.8 (0.7) | 0.13 | 11 | 4.2 (0.5) | 4.2 (0.6) | 0.82 |
| Creatinine (mg/dl) | 20 | 0.68 (0.1) | 0.66 (0.1) | 0.47a | 12 | 0.71 (0.1) | 0.66 (0.1) | 0.33 |
| Erythrocytes (×106/mm3) | 20 | 6.729 (8.8) | 6.525 (8.4) | 0.01a | 10 | 4.762 (0.4) | 4.658 (0.5) | 0.13 |
| Leukocytes (mm3) | 20 | 7331 (1701) | 7378 (2037) | 0.85a | 12 | 7954 (1687) | 7708 (1423) | 0.56 |
| Platelets (×103/mm3) | 20 | 282.9 (81.9) | 265.5 (76.1) | <0.001 | 12 | 279.3 (83.2) | 258.8 (76.3) | <0.001 |
n: patients entered in the analysis; p: Student t-test for paired samples.
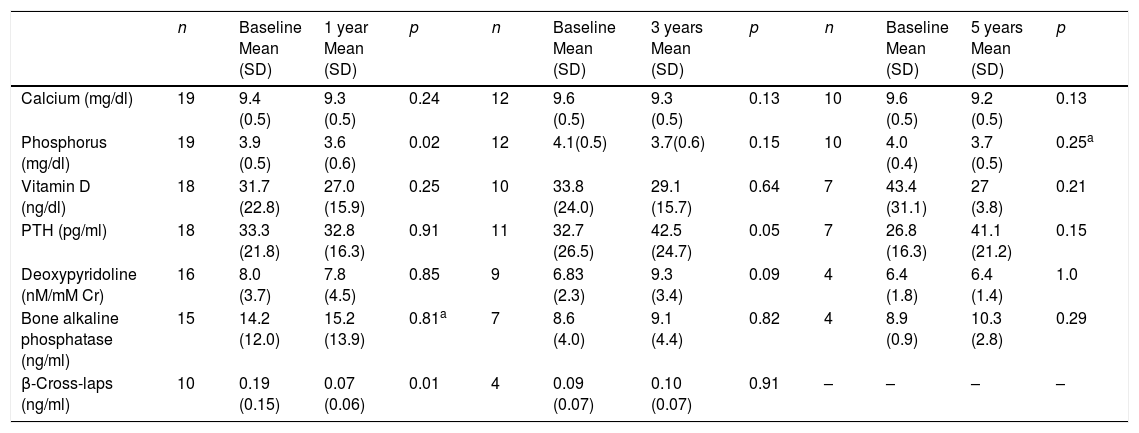
No significant differences were observed in the calcium or 25-OH-vitamin D levels during follow-up. The phosphorus values decreased significantly after one year of follow-up, though without reaching the lower limit of normal (2.5mg/dl) in any of the cases. The iPTH levels increased significantly at three years (42.5±7.4 vs 32.7±8pg/ml; p=0.05). The β-cross-laps decreased significantly after one year of follow-up. There were no significant changes in the deoxypyridoline or bone alkaline phosphatase values (Table 3).
Long-term biochemical effects of intravenous zoledronic acid.
| n | Baseline Mean (SD) | 1 year Mean (SD) | p | n | Baseline Mean (SD) | 3 years Mean (SD) | p | n | Baseline Mean (SD) | 5 years Mean (SD) | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcium (mg/dl) | 19 | 9.4 (0.5) | 9.3 (0.5) | 0.24 | 12 | 9.6 (0.5) | 9.3 (0.5) | 0.13 | 10 | 9.6 (0.5) | 9.2 (0.5) | 0.13 |
| Phosphorus (mg/dl) | 19 | 3.9 (0.5) | 3.6 (0.6) | 0.02 | 12 | 4.1(0.5) | 3.7(0.6) | 0.15 | 10 | 4.0 (0.4) | 3.7 (0.5) | 0.25a |
| Vitamin D (ng/dl) | 18 | 31.7 (22.8) | 27.0 (15.9) | 0.25 | 10 | 33.8 (24.0) | 29.1 (15.7) | 0.64 | 7 | 43.4 (31.1) | 27 (3.8) | 0.21 |
| PTH (pg/ml) | 18 | 33.3 (21.8) | 32.8 (16.3) | 0.91 | 11 | 32.7 (26.5) | 42.5 (24.7) | 0.05 | 7 | 26.8 (16.3) | 41.1 (21.2) | 0.15 |
| Deoxypyridoline (nM/mM Cr) | 16 | 8.0 (3.7) | 7.8 (4.5) | 0.85 | 9 | 6.83 (2.3) | 9.3 (3.4) | 0.09 | 4 | 6.4 (1.8) | 6.4 (1.4) | 1.0 |
| Bone alkaline phosphatase (ng/ml) | 15 | 14.2 (12.0) | 15.2 (13.9) | 0.81a | 7 | 8.6 (4.0) | 9.1 (4.4) | 0.82 | 4 | 8.9 (0.9) | 10.3 (2.8) | 0.29 |
| β-Cross-laps (ng/ml) | 10 | 0.19 (0.15) | 0.07 (0.06) | 0.01 | 4 | 0.09 (0.07) | 0.10 (0.07) | 0.91 | – | – | – | – |
n: patients involved in the analysis; p: Student's t test for paired samples.
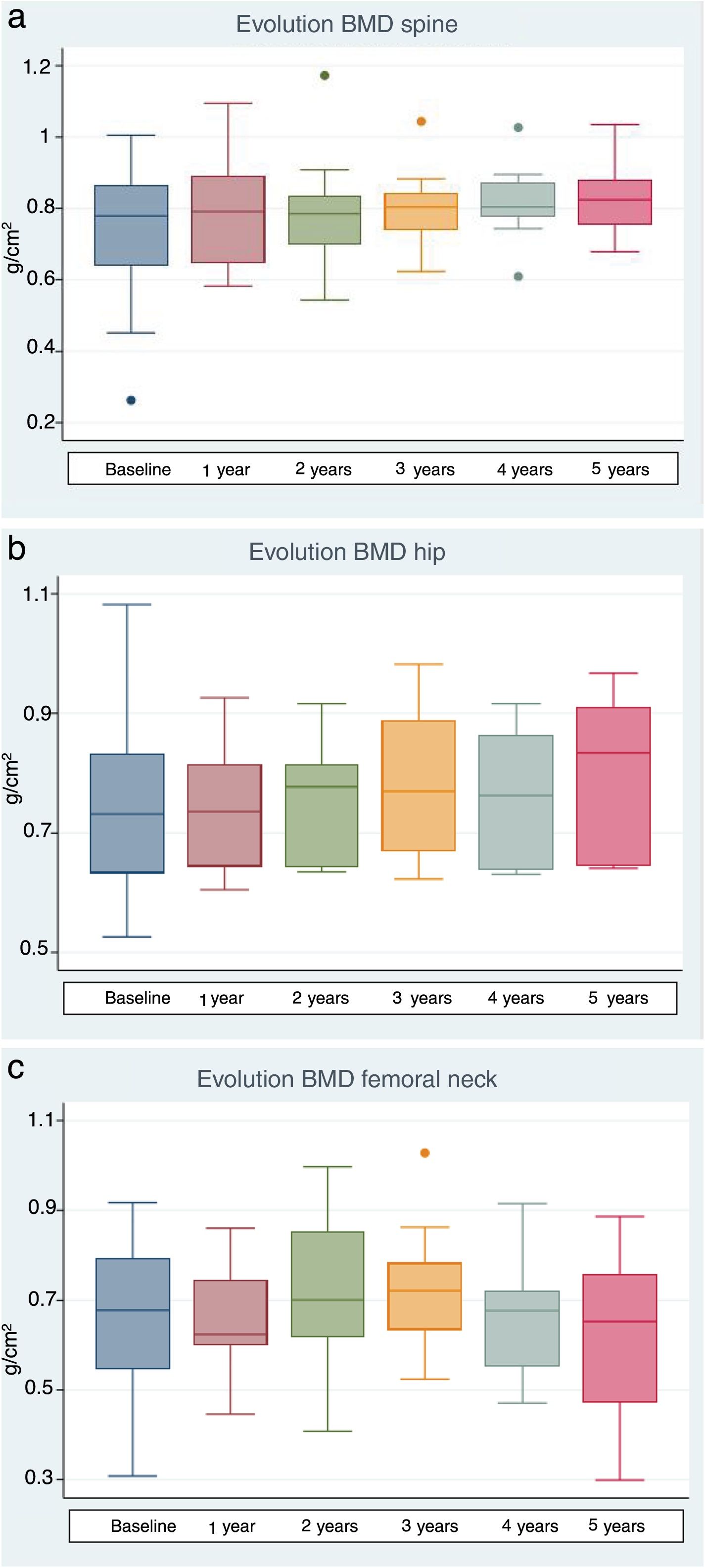
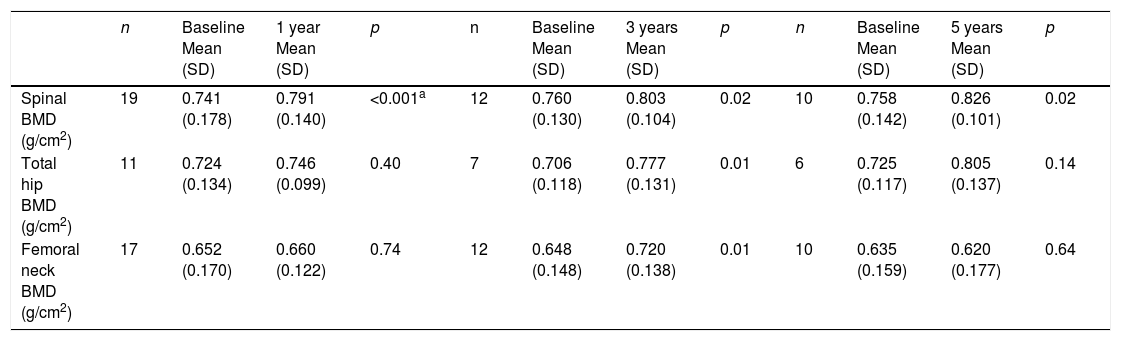
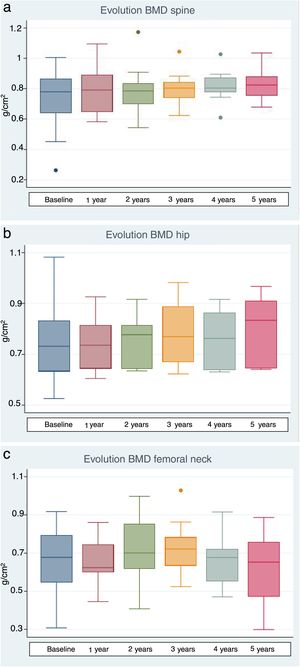
The lumbar spine BMD values increased by an average of 6.7%, 5.7% and 9% after 1, 3 and 5 years of treatment, respectively, the results being statistically significant. On examining the total hip values, an increase in BMD of 3%, 10.1% and 11% was observed after 1, 3 and 5 years of treatment, though statistical significance was only reached after 3 years of treatment. With regard to the BMD values at femoral neck level, a 1.2% and 11.1% increase was recorded after one and three years of treatment, respectively, with statistically significant results after three years of treatment. After 5 years of therapy, the BMD values tended to decrease, though statistical significance was not reached (Table 4 and Fig. 1a–c). Previous treatment with bisphosphonates did not influence the results. Only one patient suffered new fractures during follow-up as a consequence of severe trauma.
Long-term bone mineral density effects of intravenous zoledronic acid.
| n | Baseline Mean (SD) | 1 year Mean (SD) | p | n | Baseline Mean (SD) | 3 years Mean (SD) | p | n | Baseline Mean (SD) | 5 years Mean (SD) | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spinal BMD (g/cm2) | 19 | 0.741 (0.178) | 0.791 (0.140) | <0.001a | 12 | 0.760 (0.130) | 0.803 (0.104) | 0.02 | 10 | 0.758 (0.142) | 0.826 (0.101) | 0.02 |
| Total hip BMD (g/cm2) | 11 | 0.724 (0.134) | 0.746 (0.099) | 0.40 | 7 | 0.706 (0.118) | 0.777 (0.131) | 0.01 | 6 | 0.725 (0.117) | 0.805 (0.137) | 0.14 |
| Femoral neck BMD (g/cm2) | 17 | 0.652 (0.170) | 0.660 (0.122) | 0.74 | 12 | 0.648 (0.148) | 0.720 (0.138) | 0.01 | 10 | 0.635 (0.159) | 0.620 (0.177) | 0.64 |
n: patients involved in the analysis; p: Student's t test for paired samples.
Most adult patients with OI have osteoporosis for a number of reasons: a failure to achieve adequate bone mass during childhood; periods of immobilization after repeated fractures; and, in many cases, very limited physical activity (whether present or past). The bone histomorphometric study of these patients shows increased bone remodeling, which may also cause bone mass loss. It is estimated that over 25% of all fractures in OI patients occur during adult life.10
The management of OI requires a multidisciplinary approach in order to improve patient quality of life, muscle strength and mobility and independence, and to reduce pain and the number of fractures. General recommendations include smoking cessation, the avoidance of excess alcohol intake, moderate physical exercise (at least 30min a day, depending on the circumstances of each patient) and/or physiotherapy, the maintenance of adequate body weight, and a sufficient intake of both calcium and vitamin D.11–13
The ideal pharmacological approach to these patients has not been clearly established. However, due to the increased bone remodeling involved in OI, antireabsorptive therapy is preferred.
To date, the drugs most commonly used in patients with OI are bisphosphonates, administered both orally and intravenously. In children, these drugs have been shown to be effective in increasing BMD, and some studies have also demonstrated beneficial effects in terms of growth, final height, mobility, quality of life and possibly fracture incidence (though this latter aspect remains subject to controversy).14–19
Zoledronic acid is a third generation bisphosphonate of great potency (100–1000 times more than pamidronate), with a high affinity for bone and a long duration of action (6–12 months in adult series).20 Zoledronic acid has been shown to be effective in postmenopausal osteoporosis and osteoporosis secondary to hematological diseases, in treatment with glucocorticoids, in hypogonadism and in transplant patients, among other conditions.21–25
The drug has recently been shown to offer an efficacy and safety profile similar to that of pamidronate in a series of children with OI.18,19 Its side effects are similar to those reported for other intravenous bisphosphonates.20 The most common effects are acute phase reactions in the form of a flu-like syndrome with febricula, asthenia, headache or muscle pain occurring in the first 24–72h after infusion, and disappearing in less than three days. Thirty-five percent of the patients in our study experienced some of these symptoms to a mild degree, and 15% also experienced mild gastrointestinal symptoms. The frequency of these effects decreases with subsequent doses. Transient hypocalcemia has been reported as a possible side effect. We observed a decrease in calcium levels 24 and 48h after the first infusion, followed by spontaneous recovery. Three patients presented mild biochemical test hypocalcemia with no clinical impact. It should be noted that all patients received calcium and vitamin D supplements, which may have minimized the hypocalcemic effect. There have also been reports of transient creatinine elevation in 1.2% of the patients treated with zoledronate.21 Water intake was reinforced before and after infusion in all of our patients, with no evidence of renal functional impairment in any of the cases. Likewise, no relevant changes in blood count were noted.
Studies of the long-term effects of bisphosphonate treatment in adults with OI are scarce. In this respect, the present study involves the longest period of follow-up to date. The limitations of our study include its small sample size, which is explained by the low prevalence of the disease, and the lack of a control group. We observed a significant increase in BMD at lumbar spine level after one year of treatment, and this increase was maintained after three and 5 years, with increments of 6.7%, 5.7% and 9% respectively. Total hip BMD also showed improvement, with increments of 3%, 10.1% and 11% after one, three and 5 years, respectively. The results obtained at femoral neck level after 5 years of treatment could be explained by the small sample size involved.
In a study published by Adami et al., 23 men and 23 premenopausal women (OI types I, III and IV) were randomized to either neridronate 100mg i.v. every three months or placebo (2:1 ratio) for one year. Both groups received treatment during the second year. The mean age of the study population was 35 years, and the mean lumbar spine T-score was −3.4. Bone mineral density increased significantly in the treatment group as compared to the control group. After two years, the BMD values were seen to increase by 6.4% in men and by 7.5% in women in the treatment group, with a significant decrease in fracture incidence.26 In a recent three-year extension study published by Viapiana et al., involving 114 patients, neridronate was found to exert positive effects upon BMD and bone remodeling markers, with an adequate safety profile. The authors were unable to demonstrate a significant effect upon fracture risk. The percentage of patients with fractures did not change, though the number of fractures per patient did decrease significantly.27 Our study was not designed to assess the influence of treatment on fractures, though it should be noted that only one patient experienced a new fracture after the start of treatment, and this incident was attributed to severe trauma.
In another observational study, 90 adults with OI (types I, III, and IV) were treated with different bisphosphonates: pamidronate i.v. (n=28), oral alendronate (n=10) or oral risedronate (n=17), or received no treatment (n=35), for an average period of 52 months. A significant increase in BMD was observed per year of treatment in all OI patients treated with pamidronate, but only in the OI type I patients treated with oral bisphosphonates. The fracture rate only decreased in OI types III and IV (the most severe forms) treated with pamidronate.27
More recently, 60 patients were randomized to alendronate 70mg weekly via the oral route or zoledronic acid 5mg as an annual intravenous infusion (2:1) for a period of two years. A total of 52 patients completed the study. The BMD values in the lumbar spine, femoral neck and hip increased similarly in both treatment groups: 10.5%, 13.2% and 14.7% in the alendronate group versus 11.3%, 13.7% and 11.7% in the zoledronic acid group. The incidence of clinical fractures was significantly reduced in both groups compared with the previous fracture rates.28
Experience with other antiresorptive agents such as denosumab is limited to small case series in children with OI type VI, where promising results have been obtained.29,30 There are no publications regarding such treatment in adult patients with OI.
In another study involving osteoanabolic treatment with teriparatide, 79 adult patients with OI were randomized to 20μg of recombinant PTH or placebo for an 18 month period in the context of a double-blind trial. The patients, particularly those with milder OI (type I), experienced a comparative increase in BMD (6.1±1.0% vs 2.8±1.0% in the lumbar spine; p<0.05 and 2.6±1.0% vs −2.4±1.0% in the hip; p<0.001), but no differences were observed in more severely ill patients with OI types III and IV.31
ConclusionsZoledronic acid is a convenient and effective drug for the treatment of osteoporosis in adult patients with OI, since it induces a significant increase in BMD at both lumbar and hip level. Its safety profile is acceptable. The optimum dose, the dosing interval, the duration of treatment and its long-term safety remain to be established.
Further longer-term studies involving a larger number of patients are needed to confirm and contrast our findings.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Pavón de Paz I, Rosado Sierra JA, Pérez Blanco C, Modroño Móstoles N, Guijarro de Armas G, Navea Aguilera C. Efectos agudos y a largo plazo del tratamiento con zolendronato en pacientes adultos con osteogénesis imperfecta. Estudio español observacional con 5 años de seguimiento. Endocrinol Diabetes Nutr. 2019;66:108–116.