Healthcare is in need of fundamental change if we are going to make significant improvements in outcomes while limiting costs. Lean methods, initially developed in the manufacturing industry, have been shown to deliver real improvements in quality, productivity, and safety while using fewer resources in healthcare service settings. These tools, management practices and philosophies have been successfully adapted in operative services at SC. They can transform your system and culture through engagement of front line staff into a continuously improving service with real and sustainable enhancements in clinical outcomes.
El sector de la atención en salud está necesitando un cambio fundamental a fin de mejorar significativamente los resultados y limitar al mismo tiempo los costos. Los métodos Lean desarrollados inicialmente en la industria manufacturera han demostrado mejorar realmente la calidad, la productividad y la seguridad, a la vez que permiten utilizar menos recursos en la situación de la salud. Estas herramientas, prácticas gerenciales y filosofías se han adaptado con éxito en los servicios de cirugía del SC. Pueden transformar el sistema y la cultura mediante el compromiso del personal de asistencia para el desarrollo de un servicio que mejora continuamente y permite obtener mejores resultados clínicos duraderos.
Interest in quality improvement in healthcare has increased over the last decade, principally as a result of calls from the Institute of Medicine.1,2 While some successes have been seen,3 improvements have been slower than expected and highly variable.4,5 A wide spread interest in organizational strategies that enable and incentivize high-quality care to improve clinical outcomes is emerging.6
In response to these growing interests, Lean methodology developed and championed by Toyota7 has begun to be implemented within healthcare. These management practices can be characterized by a set of formal tools and philosophies designed to reduce waste and variation in clinical practices through the collaborative engagement of provider teams. Evidence of the effectiveness of this in healthcare remains limited and consists mostly of single-site studies.8–10
For fifteen years the physician and administrative leaders at Seattle Children's (SC), a 323-bed Pediatric Center affiliated with the University of Washington, have been methodically investigating and developing these practices. These principles have been adapted and utilized by the operative services management team, comprised of the chiefs of surgery, anesthesiology, and surgical services (i.e., nursing). Before describing this healthcare experience, it is important to understand what outcomes Toyota has achieved and why the operative services leadership team made the decision to try Lean within the healthcare setting.
MethodologyA selective review of published English language literature regarding the use of Lean methods in manufacturing and specifically within healthcare service industry was conducted. This review focused on the tools, management practices and philosophy of this methodology. In addition, the quality improvement records within operative services at Seattle Children's were retrospectively reviewed. Representative examples and associated outcomes were identified.
Basic overview and outcomes of Toyota production system improvementToyota is often described as the company that invented Lean production. In fact, Toyota learned from and was inspired by many others, including Henry Ford and practices in American supermarkets.11 Starting in 1945, Toyota set out to improve quality while simultaneously increasing productivity and reducing costs. The post-war setting forced Toyota leaders to be creative if their company was going to survive. By the 1980s, Toyota was successfully competing against their major U.S. competitors. Based on this success, research teams led by James Womack and others began studying the Japanese management practices and coined the term Lean to describe Toyota's management system ability to get by with half of everything (physical space, labor effort, capital investment, and inventory) while seeing fewer than half of the defects and safety incidents. For two decades J.D. Power and other have consistently rated Toyota among the top automotive brands in terms of reliability, initial quality, and long-term durability. Toyota has slowly, iteratively risen to become the largest and most profitable automobile manufacturer in the world.
While early spreading of Lean methods logically occurred first in the U.S. auto industry, it has been successfully introduced into other manufacturing and service industries. SC relied heavily on local Lean expertise gathered in the 1980s and 1990s in the aviation industry at The Boeing Company. Other local healthcare systems, notably Virginia Mason Medical Center12 and Group Health13 have also had success with Lean methods during this same time period.
Taiichi Ohno and Shigeo Shingo were the primary creators of the Toyota Production System (TPS) and defined its goal as “looking at the timeline from the moment a customer gives us an order to the point when we collect the cash”; otherwise known as the value stream. “We are reducing the time spent in the value stream removing the non-value-added wastes”.11 Work is categorized into three groups: (1) value-added, (2) necessary (regulatory), and (3) unnecessary, non-value added work (i.e., waste). Waste is defined as any problem that interferes with people doing their work effectively or any activity that does not provide value for the customer. Removing waste reduces delays and improves quality, safety, efficiency and reliability of the system while decreasing the costs.
Lean adapted to healthcare has been described as a rational and scientific approach to problem solving and learning,14 a frame of reference familiar and aligned with scientifically trained healthcare professionals. Spear outlines four Lean organizational capabilities. (1) Work is designed as a series of experiments that reveal problems. There are three elements in this statement: (a) work is designed, non-random and standardized, (b) work is not static, but rather continuously improving through small kaizen (experimental) events, and (c) work is structured to be visible so that problems can be seen and addressed. (2) Problems are addressed immediately through rapid experimentation. When a problem is discovered, the focus is on solving the problem immediately, at the place the problem occurred and with the input from the people who are struggling with the problem. Problems are seen as opportunities for learning and improvement. (3) Solutions are disseminated adaptively through collaborative experimentation. Local improvements need to be shared with other areas to prevent the waste of unnecessary rework (solving the same problem again and again). (4) People at all levels are taught to become experimentalists. Lean methodology helps employees see their work and processes through new eyes, allowing them to see problems they could not see before and teaching them new ways to the solve these problems.
Continuous performance improvement (CPI): an adaption of TPS in healthcareUsing a ‘learning by doing’ philosophy, SC has tailored many of the management practices inherit in the TPS to fit in the healthcare service industry setting. Our adaption is called Continuous Performance Improvement (CPI). The philosophical approach evolved as practicing Lean spread through our culture. This philosophy includes three major tenets. (1) Focus first on the patient and family. Focus on patients meant involving them as team members working side-by-side with staff making improvements to the system. Only patients and families define value added steps in the process. (2) Support faculty and staff in their work. Staff supports means partnering with them by giving the resources they need to do their best in their jobs. (3) Take a long-term view when making decisions. A long-term view is required when planning for small, incremental improvements in process and people with slow returns on this investment.
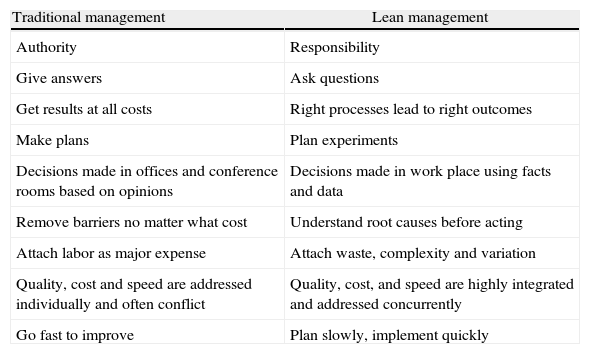
SC has developed a culture of continuous learning and improvement by utilizing the CPI management practices. CPI tools include features found in Lean methods (i.e., 5S, A3, value stream mapping, rapid improvement events) and others common to many process improvement methods (i.e., root cause analysis, failure mode effect analysis). Data rather than opinions are used to make decisions. Data collection starts with clinical observation exercises called three actuals: watching the actual people do the actual work in the actual place. Work sequence maps are generated from these observations and hypotheses are generated to improve the processes and ultimately the outcomes. These hypotheses are tested using the Deming Plan-Do-Check-Act (PDCA) cycle form of the scientific method. All of this work is completed through engagement of front line staff working in multidisciplinary teams. Leaders serve as coaches providing direction, alignment, instruction and encouragement to the teams. This requires a major change in leadership competencies (see Table 1).
Comparison of leadership competencies.
| Traditional management | Lean management |
| Authority | Responsibility |
| Give answers | Ask questions |
| Get results at all costs | Right processes lead to right outcomes |
| Make plans | Plan experiments |
| Decisions made in offices and conference rooms based on opinions | Decisions made in work place using facts and data |
| Remove barriers no matter what cost | Understand root causes before acting |
| Attach labor as major expense | Attach waste, complexity and variation |
| Quality, cost and speed are addressed individually and often conflict | Quality, cost, and speed are highly integrated and addressed concurrently |
| Go fast to improve | Plan slowly, implement quickly |
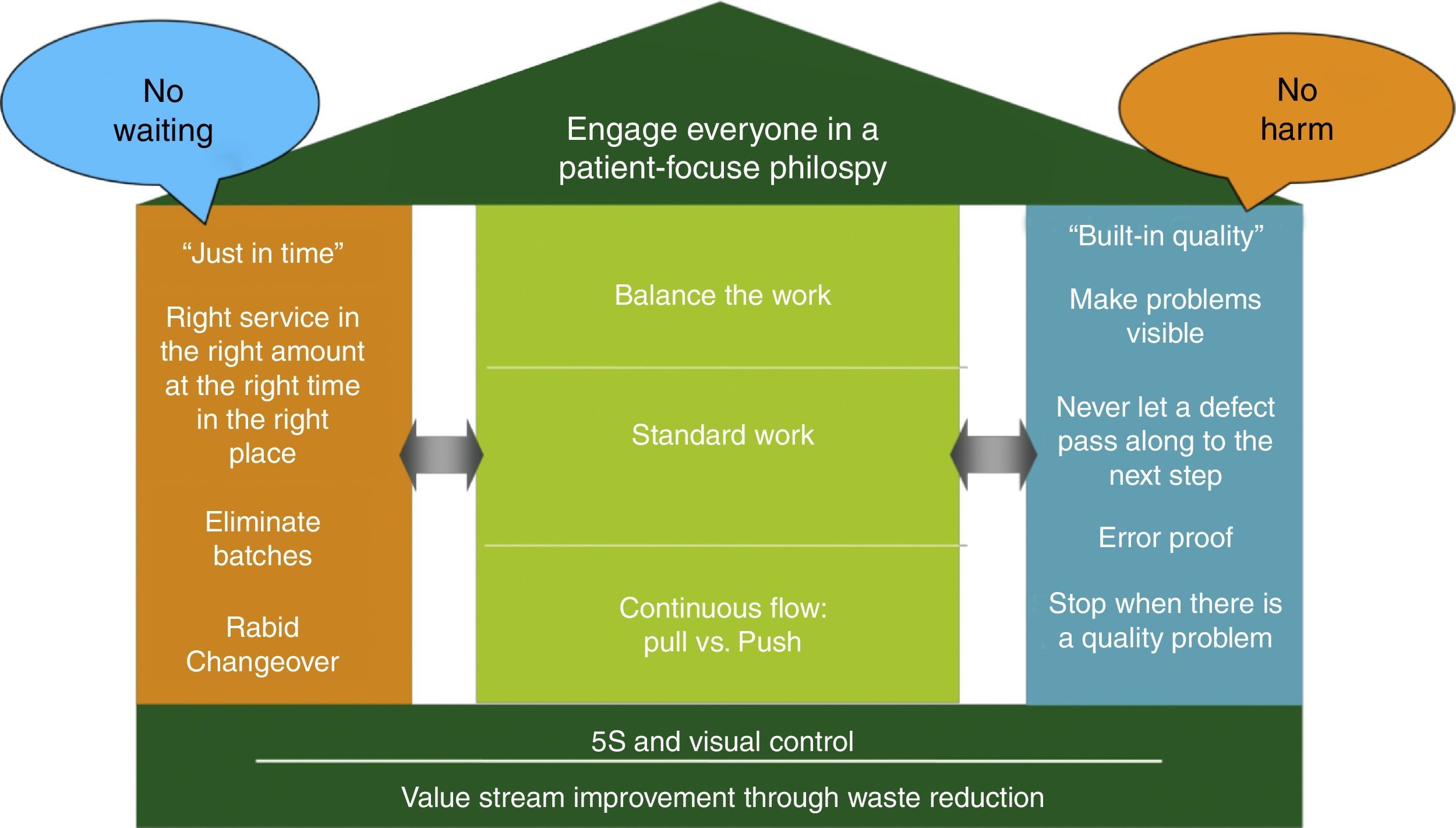
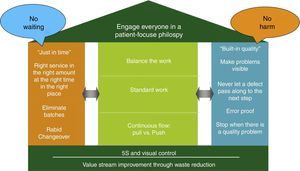
The CPI tools, management practices, and philosophies are summarized by an adaption of the Toyota house (see Fig. 1). The foundation for the house is value stream improvement through waste reduction and creation of a visual work place. The two main pillars of the house are Just-In-Time (the right service and supplies at the right place and time) and Built-In-Quality (problem identification and escalation for rapid resolution) which supports the roof composed of our patient-centric philosophy. This system ultimately improves to the point where there is no waiting for patients, families, or providers and no one (patients, families or providers) is harmed. The measures in the center of the house are necessary key elements to achieve this no wait, no harm outcome.
Using a house metaphor, CPI House depicts a roof as the patient focused philosophy, the foundation built upon value stream management though waste reduction and the two pillars (just in time and built in quality) along with the elements in the house needed to deliver the patient-centric care with no waiting and no harm.
Initially uncertain about the likelihood of success on adapting TPS into the healthcare culture, leaders at SC focused first on point improvements in an escalating risk manner. Small point improvement projects were completed in non-clinical settings (parking garage, loading dock) to test and learn the concepts. Increased staff engagement and sustained improvements in productivity were found in these pilot projects. The second wave of point improvements began touching clinical processes (pharmacy, laboratory), but with no direct physician involvement, again with similar outcomes. The third successful wave of point improvements occurred in the clinical setting led by willing physician leaders eager to trial these new methods.
Operative Services was one of the first clinical sites to begin using these tools, practices and philosophies at SC. This experience demonstrated the challenge of isolated point improvements and led to the formal adoption of value stream mapping and management to improve processes in an integrated manner as experienced by our patients. The next learning was the realization that we could implement simple yet elegant improvements into our clinical processes; however, we lacked the processes to routinely sustain these improvements over time. This lesson led to a focus on daily management, the daily routine monitoring of clinical system performance with rapid identification and resolution of problems. Through these successive waves of learning we have built a system and culture capable of incremental, continuous experimentation in clinical processes that can be sustained over time and resulted in significant improvements in clinical outcomes. Representative examples of this work follow.
Results from CPI outcome improvements in operating servicesDocumentation waste removalIn nearly every process, there are steps that are value-added (steps that our customers, in healthcare our patients/families, would be willing to pay for, steps that bring the desired outcome for customers). However, experts in process improvement note that the majority of the process steps are defined as “waste”, typically more than 90%.15 Some of this “waste” may be necessary, for example a step required by the hospital, state and/or national regulatory organization, but most of the waste can be reduced or eliminated. By focusing on waste, the relative amount of time that is spent on value-added tasks can be increased, yielding the desired outcome more efficiently and reliably.
Waste reduction was first applied to the process of operative documentation.16 Prior to the Rapid Process Improvement Workshop (RPIW), nurses, surgeons and anesthesiologists documented their work on separate paper forms. The patients were asked many questions repeatedly by multiple providers and information was copied from one form to another. In some instances the information gathered by the various providers was not consistent; a significant safety concern and opportunity for adverse outcome.
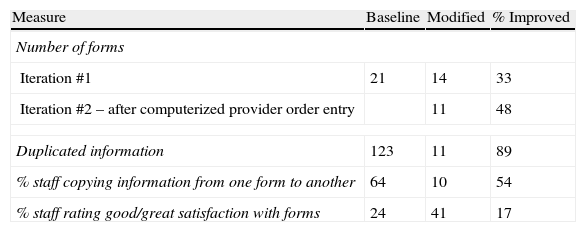
Using those who actually do the work, representatives from the admit nurses, anesthesiologists, and medical assistants (who are the patients’ transporters) were recruited. During the RPIW it was decided that the forms for the registered nurse and anesthesiologist would be merged and that duplicated information would be minimized. The nurse fills out the left side of the consolidated form and the anesthesiologist the right side. This involved not only changing forms, but also designing the new work flow, since both providers could not write on the form at the same time. Results of these efforts are summarized in Table 2.
Documentation improvement outcomes.
| Measure | Baseline | Modified | % Improved |
| Number of forms | |||
| Iteration #1 | 21 | 14 | 33 |
| Iteration #2 – after computerized provider order entry | 11 | 48 | |
| Duplicated information | 123 | 11 | 89 |
| % staff copying information from one form to another | 64 | 10 | 54 |
| % staff rating good/great satisfaction with forms | 24 | 41 | 17 |
One common complaint from parents of children undergoing surgery is the excessively long wait in the pre-operative holding area. Their children are hungry secondary to the pre-operative fasting, anxious in an unfamiliar setting sensing their parent's anxiety regarding the upcoming procedure. Thus, efforts to reduce this wait period are seen as adding value from the customer's perspective.
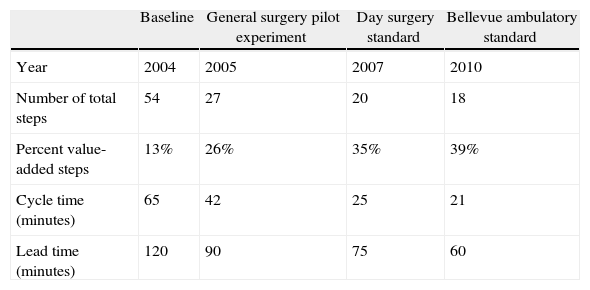
To address this problem, teams first created a detailed work sequence map of the steps completed by all providers from the point of hospital arrival through to anesthesia start. At baseline, they found that 54 steps and 65min of work were necessary. To assure that patients were routinely available when the operating suite was ready, parents were instructed to arrive 120min prior to the scheduled start of their procedure (defined as lead time). Through a series of iterative improvements, waste steps were identified and removed (see Table 3). The teams were ultimately able to reduce the number of steps to 18 and the work time to 21min. These changes allowed a reduction in the scheduled arrival time to 60min without negatively impacting operative suite productivity. Furthermore, parental satisfaction significantly improved.
Incremental improvements in admission process.
| Baseline | General surgery pilot experiment | Day surgery standard | Bellevue ambulatory standard | |
| Year | 2004 | 2005 | 2007 | 2010 |
| Number of total steps | 54 | 27 | 20 | 18 |
| Percent value-added steps | 13% | 26% | 35% | 39% |
| Cycle time (minutes) | 65 | 42 | 25 | 21 |
| Lead time (minutes) | 120 | 90 | 75 | 60 |
Regional anesthesia has many advantages17; however, the administration of a regional anesthetic in children is typically done in children already anesthetized, thus taking up valuable surgical time in the operating suites. In an attempt to address this time constraint, a team was chartered to reduce non-operative time (defined as ‘patient 1 closure’ to ‘patient 2 incision) in children receiving a regional anesthetic.
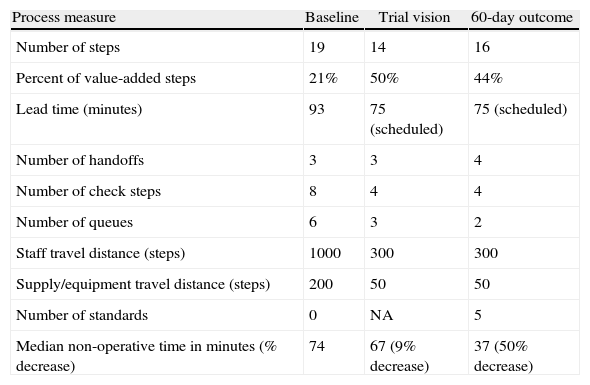
At baseline, regional anesthetic blocks were conducted in various operative suites and required a significant amount of time and distance searching for regional anesthesia supplies and equipment. The team first created a centralized regional anesthesia block room with all necessary supplies and equipment. Next a new work flow that allowed initiation of the anesthesia and regional anesthesia blockade while the preceding case in the operative suite is still underway was tried. Significant improvements were seen and sustained over time (see Table 4). This effort resulted in a significant reduction in non-operative time, improvement in surgeon satisfaction, and enhanced operative suite utilization.
Improvements in regional anesthesia process.
| Process measure | Baseline | Trial vision | 60-day outcome |
| Number of steps | 19 | 14 | 16 |
| Percent of value-added steps | 21% | 50% | 44% |
| Lead time (minutes) | 93 | 75 (scheduled) | 75 (scheduled) |
| Number of handoffs | 3 | 3 | 4 |
| Number of check steps | 8 | 4 | 4 |
| Number of queues | 6 | 3 | 2 |
| Staff travel distance (steps) | 1000 | 300 | 300 |
| Supply/equipment travel distance (steps) | 200 | 50 | 50 |
| Number of standards | 0 | NA | 5 |
| Median non-operative time in minutes (% decrease) | 74 | 67 (9% decrease) | 37 (50% decrease) |
Integrated Facility Design (IFD) is an adaptation of the Toyota 3P (Production Preparation Process). The goals of Toyota 3P are (1) shorter development time and (2) lower start up costs. These principles can easily be utilized in starting a new healthcare service and/or site of practice. Architects, engineers, and clinicians collaborate to form a Design Core Team. Utilizing customer requirements, this team develops a vision and a set of guiding principles that forms the basis for a sequential design process. The design process begins with a Concept Design, with macro-scale determination of size and key adjacencies. This process is facilitated by the use of full-scale mock-ups. Once the adjacencies and size elements have been determined, a Functional Design is created again utilizing mock-ups. The functional level design determines room level elements. The final step is Detail Design which is a detailed design of individual work spaces. Lean principles are utilized in every phase of the design process.
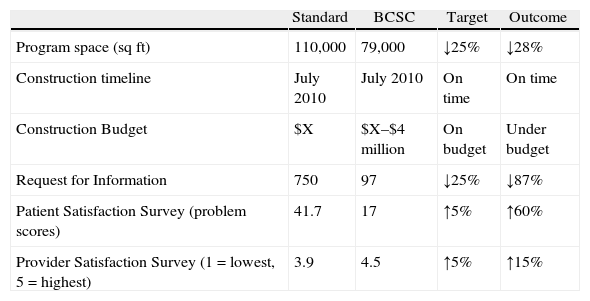
Utilizing IFD, SC designed and built the Bellevue Clinics and Surgery Center (BCSC), an ambulatory center located 10 miles from the hospital. The goal was to produce a facility with excellent patient and provider flow, improved safety, and high patient and employee satisfaction, and to do so at significantly lower construction costs. Specific goals set for the team included: (1) reduction of the space requirements by 25% over industry standards while still achieving capacity of expected patient volumes and services; (2) completion of the project at or under the estimated construction timeline; (3) completion of the project at or under target cost; (4) reduction of construction Request for Information (RFI) by 25%; (5) improvement of patient satisfaction scores by 5% on surveys; and (6) an increase in staff satisfaction as measured by pre-and post-work survey results. All goals were met or exceeded (see Table 5), including a cost avoidance of approximately $10 million in contingency costs.18
Integrated facility design (IFD) outcomes.
| Standard | BCSC | Target | Outcome | |
| Program space (sqft) | 110,000 | 79,000 | ↓25% | ↓28% |
| Construction timeline | July 2010 | July 2010 | On time | On time |
| Construction Budget | $X | $X–$4 million | On budget | Under budget |
| Request for Information | 750 | 97 | ↓25% | ↓87% |
| Patient Satisfaction Survey (problem scores) | 41.7 | 17 | ↑5% | ↑60% |
| Provider Satisfaction Survey (1=lowest, 5=highest) | 3.9 | 4.5 | ↑5% | ↑15% |
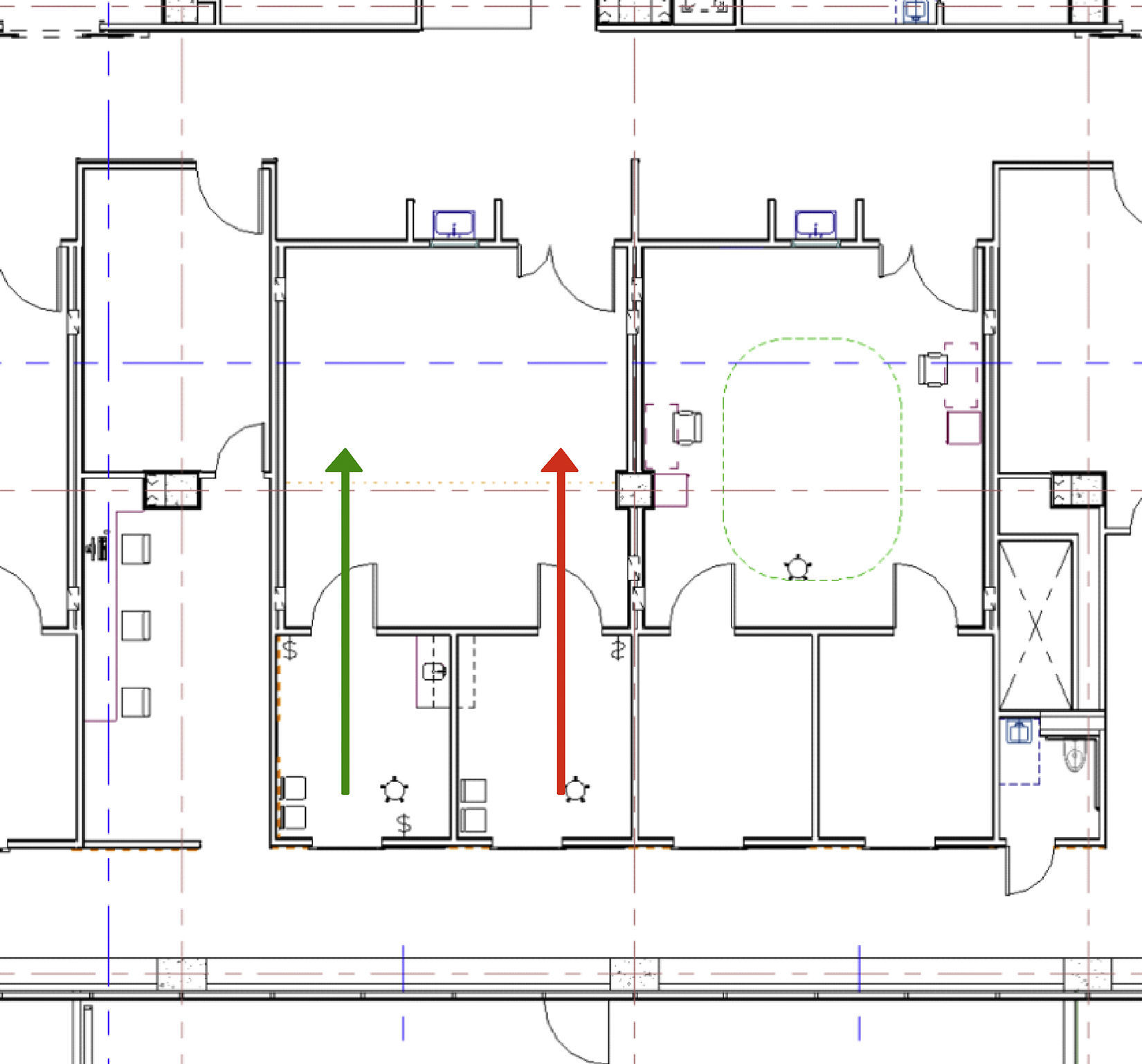
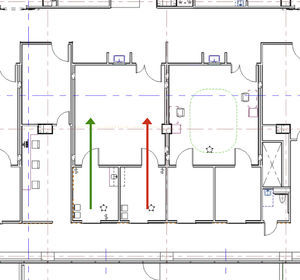
To optimize surgeon productivity, many surgery centers provide surgeons with two complete ORs and teams; however, this creates underutilization of space and personnel. In the BCSC, an innovative design using two induction rooms connected to each operating room was developed to provide highly efficient provider flows (see Fig. 2). Flow is simplified when a patient enters one induction room, receives anesthesia, and then advances through the surgical cycle; moving from the operating room, to the recovery area, and then through discharge while never crossing the path of another patient. Efficiency is enhanced by having the next patient receive anesthesia in the second induction room while the first patient is undergoing surgery. Once surgery is complete and the first patient advances to the recovery area, a brief room turnover occurs after which the second patient is pulled into the operating room for his or her procedure. As the second patient's surgical procedure commences, a third patient receives anesthesia in the initial induction room. This pattern continues until the surgical schedule is completed. Cycle times of various steps in the ambulatory surgery flow were computer modeled with a goal of having an anesthetized patient always ready to move into the operating room when the operating room was cleaned and ready. The two induction rooms per surgical suite model resulted in >50% decrease in non-operative time and allowed for optimal OR and surgeon utilization and operational efficiency.
Standard work – catheter-associated blood stream infections (CABSI)Over several years standardized practices concerning the insertion and maintenance of central intravenous lines, based upon national guidelines, were introduced in our intensive care units (ICU's) to reduce CABSI's. After an initial decrease in the incidence of CABSI's, the infection rates leveled. Further study revealed an excessive number of infections in patients who traveled out of the ICU for an invasive or radiologic procedure under the care of anesthesiologists. Therefore, anesthesia practice was examined to identify potential changes that could reduce the number of CABSI's.
Publications show that anesthesiologists do not consistently practice good hand hygiene.19–21 Travel to the OR has also been shown to be an independent risk factor for developing infection.22 Anesthesia providers contaminate their hands23 and workspace,24 and these episodes of contamination become sources of transmission of bacteria to patients. Contamination of surfaces, peripheral intravenous lines (PIV) and stopcocks can be reduced if frequent hand hygiene is practiced.25 Initial observations of our colleagues confirmed many episodes of potential contamination of IV lines and the surrounding workspace.
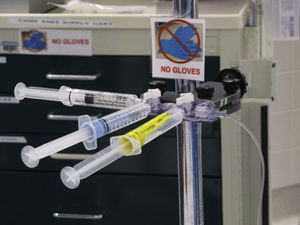
A team of anesthesia providers, technicians, operating room nurses, infectious disease specialists and an improvement consultant was formed to address this problem. They first collected data via direct observation of clinical practice and developed a list of potential countermeasures. Most medication administrations were not considered “clean” because providers failed to follow the recommended but time-consuming procedure for obtaining clean access to an iv line. To change behavior, the team decided to treat both peripheral and central intravenous lines in a new “clean” manner. They developed a clean medication administration process using a “medication manifold” (see Fig. 3). By attaching a med-line with a series of stopcocks that would be secured in a “clean” area and handled only with “clean” hands, all medications could be administered in a clean manner.
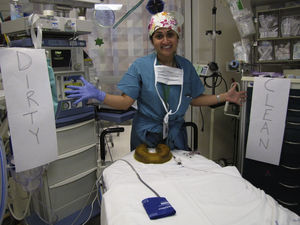
The team found that anesthesia providers tend to put on gloves and then considered their hands to be clean at all times. However, direct observation revealed that hands were not clean after certain tasks until the gloves have been removed and hands either washed or cleaned with gel. They organized the work space and designated a “clean zone” for medications’ preparation and administration using ungloved, clean hands and at a patient table and the anesthesia machine designated as “dirty zone”, tasks are completed with gloved hands (see Fig. 4).
The set of countermeasures were first trialed in simulated cases, then by team members in a single, rotating anesthesia site until all anesthesia sites were tested and finally by non-team members in all locations with a team member available for real-time coaching and feedback. Results of these efforts recently published26 showed the rates of CABSI's in patients traveling from the ICU for procedures fell from 14 to 9 infections per 1000 trips (↓36%). The overall hospital CABSI rate also dropped from 3.5 to 2.2 infections per 1000 catheter days (↓37%). This work demonstrates that standard practices designed to improve anesthesia provider hand hygiene can lessen infectious complications in surgical patients.
Patient Flow Checklists for Safety: The links between aviation, the operating room, and checklists, their use, evolution and adoption are growing. Not so clearly described is how checklists fit into the culture of aviation. Understanding how that culture looks and feels is essential to understanding why the culture in medicine is starkly different.
In the 1970s, aviation diagnosed problems with their culture relating to teamwork by learning from their accidents. Systematic training in human factors was implemented by the early 1980s. Aviation called this “crew resource management” (CRM), a system which teaches teams how to effectively use all available resources (people, equipment and processes) to maximize the efficiency and safety of flight operations, both routine and emergent. The domains of CRM include leadership, decision-making, situational awareness, communication and teamwork. The training does not end when a pilot is trained; these ‘non-technical skills’ are part of their ongoing professional assessments. Aviation recognizes specific behaviors which can be desirable or destructive and these are objectively and repeatedly assessed.
The latest review by the U.S. Joint Commission still demonstrates the leading causes of sentinel (harmful) events are failures in human factors, leadership, and communication.27 However, training in these skills still does not commonly occur in graduate or postgraduate medical programs. After the Institute of Medicine publication “To Err is Human” in 1999,1 there fortunately has been a massive increase in the number of grants, publication, editorials, letters and reviews on human factors in medicine.
Medicine is at a tipping point, like aviation in the 1970s when they began to understand what was causing their planes to crash. There are national programs (i.e., Team STEPPS™) which attempt to standardize the training of human factor skills. Recent data prove that team training saves lives,28 yet transformational culture change in healthcare has yet to occur. This change would (1) allow teams to openly communicate without fear of reprimand; (2) facilitate communication across disciplines; and (3) flatten power hierarchies (barriers to communication). We need change from “captain knows best” and toward the “captain listens to team members concern”. Pronovost29 demonstrated the link between teamwork and clinical outcomes with his work with CABSI's in ICUs.
For checklists to be successful, the whole team needs to be engaged in their development, evolution and have a shared mental model of how they work (i.e., when should it be triggered, who says what and when, what to do if there is an interruption, what to do if it is not been completed properly). Teams designing checklists need to understand the different types (e.g. challenge-response vs. read-do) since different work flows will be supported by different styles of checklist.30 There's been success in SC and other hospitals in re-formatting the World Health Organization safer surgery checklists into aviation style ‘challenge-response’ checklists.31 Team training and coaching increased team engagement by adopting a standard style with standard roles. They also have adopted patient-flow checklists which are used at critical points in the patient pathway to ensure the team, patient and equipment are correctly configured for each stage of the surgical journey.32 This work resulted in enhancing staff satisfaction with the checklists (74%) and teamwork (100%).
Witnessing a team expertly executing a checklist, it's not about the checklist. You are seeing expert teamwork; a team which understands that humans will make errors, but recognizes that these can be anticipated and neutralized before they do harm if team members effectively cross-monitor each other. It is a team in which the quietest member has a voice to speak up if they perceive a problem with safety and a team where the leader listens when concerns are raised. These practices will need to be replicated throughout healthcare before significant, sustained improvements in patient safety can be realized.
FundingNone.
Conflict of interestsNone.
Please cite this article as: Martin LD, Rampersad SE, Low DKW, Reed MA. Mejoramiento de los procesos en el quirófano mediante la aplicación de la metodología Lean de Toyota. Rev Colomb Anestesiol. 2014;42:220–228.