OSAHS is a respiratory disorder with perioperative complications, which increase morbidity and mortality. The characteristics of the pediatric population require a careful perioperative assessment.
ObjectiveThis article addresses relevant physiopathology, clinical and paraclinical topics, perioperative treatment and care of pediatric patients with OSAHS.
Materials and methodsA literature search was conducted in PubMed and Embase databases using the MeSH terms: sleep apnea syndromes, obstructive sleep apnea, physiopathology, perioperative period, and perioperative care. The titles and/or the abstracts of each of the obtained results were reviewed and 60 articles were finally selected.
Results and conclusionThe knowledge of the physiopathology, the adequate preanesthetic assessment and the pertinent request of paraclinical care, allows for the adjustment of the perioperative care and to improve the results of the patient.
El SAHOS, es un trastorno respiratorio con complicaciones perioperatorias, que aumentan la morbimortalidad; las características de la población pediátrica hacen necesaria una cuidadosa valoración perioperatoria.
ObjetivoEste artículo aborda tópicos relevantes de la fisiopatología, la clínica, paraclínicos, tratamientos y cuidados perioperatorios de los pacientes pediátricos con SAHOS.
Materiales y métodosSe realizó una búsqueda de literatura, en base de datos de Pubmed y Embase, con los términos MESH: Syndromes sleep apnea, obstructive sleep apnea, Physiopathology, Perioperative Period,Perioperative Care. Se revisaron los títulos y/o resúmenes de cada uno de los resultados obtenidos y se escogieron finalmente 60 artículos.
Resultados y conclusiónEl conocimiento de la fisiopatología, la adecuada valoración preanestésica, la pertinente solicitud de paraclínicos, permite establecer el ajuste de los cuidados perioperatorios y mejorar los resultados del paciente.
Obstructive sleep apnea/hypopnea syndrome (OSAHS) is part of a group of related diseases, known as sleep-disordered breathing (SDB), which includes: primary snoring (PS), upper airway resistance syndrome (UARS) and OSAHS. The latter is the most severe form and it has a higher risk of perioperative complications and systemic repercussions that increase morbidity.1–4
The American Thoracic Society and the International Classification of Sleep Disorders1 defines OSAHS as “a sleep-disordered breathing characterized by partial or complete, prolonged and intermittent obstruction of the upper airway that alters normal ventilation during sleep and normal sleep patterns”.1
The episodes are characterized by snoring, desaturation, hypercapnia, respiratory effort, changes in intrathoracic pressure followed by cortical and subcortical activation with intermittent return to normal sleep patterns. Its prevalence ranges from 1% to 5% and occurs mainly between 2 and 8 years of age.5–8
The aim of the present review is to show the approach toward the pediatric patient with OSAHS from the physiopathological perspective, considering relevant epidemiological data, addressing the preanesthetic assessment (clinical and paraclinical) and the recommendations about preoperative treatment.
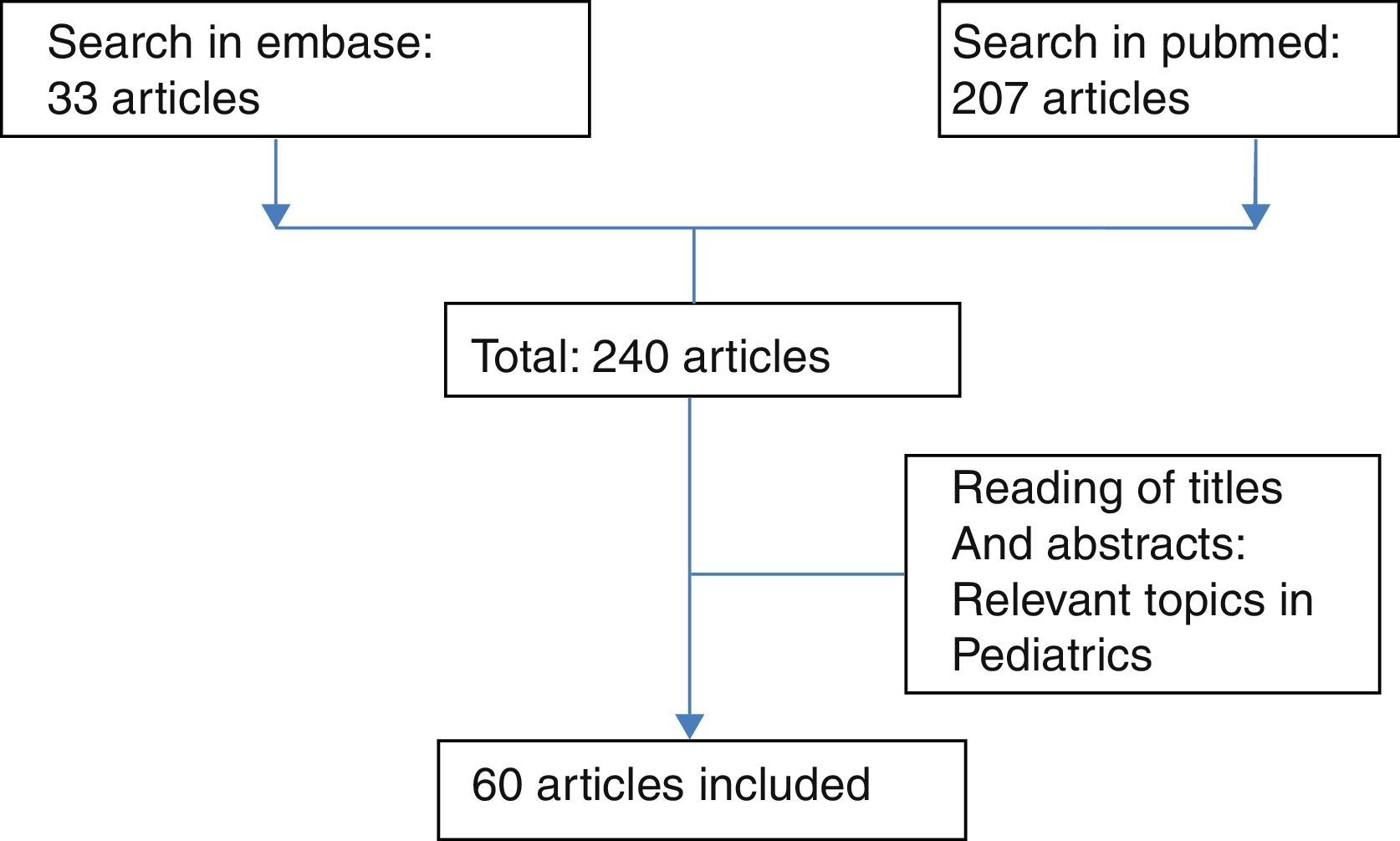
MethodologyA search of the literature was performed with the MeSH terms: sleep apnea syndromes, obstructive sleep apnea, physiopathology, perioperative period and perioperative care in the Pubmed (207 articles) and Embase (33 articles) databases, finding a total of 240 articles of which titles and abstracts were reviewed. Finally, 60 articles were selected according to the inclusion of relevant topics in the pediatric population (see Fig. 1).
The results of the reading of the selected articles are presented as a summary in subtopics such as: physiopathology, morbidity, epidemiology, preanesthetic assessment and preoperative treatment. Also, conclusions were drawn from the authors’ interpretation of the articles.
PhysiopatologyPermeability of pharyngeal airwayThere are two described models for understanding the phenomena that determine the airway permeability: The neural model and the anatomical model.
Neural modelThe size of the pharyngeal airway is determined by the balance between two opposing forces. The ones that collapse the pharynx are produced by the contraction of the inspiratory muscles (diaphragm and external intercostals) and generate a negative pressure gradient within the pharynx, which promotes the collapse of the extrathoracic airway.9–11 This collapse is prevented by forces of the pharynx produced by the contraction of the dilator muscles. The activity of these two forces is regulated by neural mechanisms (chemical stimuli, state of consciousness, airway reflexes) that interact to maintain the airway permeable. Neural mechanisms work best during waking states. When the level of consciousness is reduced, the activity of the pharyngeal dilator muscles decreases rather than the activity of the inspiratory muscles, resulting in a loss of balance of the neural balance favoring the narrowing of the pharyngeal airway.10,11
Anatomical modelProposed by Isono,12 it describes that the upper airway passes through a compartment within which is the pharyngeal soft tissue. This, at the same time, is limited by several bone structures such as the base of skull, cervical spine and mandible.10,11,13 During sleep, airway caliber is determined by soft tissue, bone structures, and by the tone of the pharyngeal dilator muscles (genioglossus, geniohyoid, and velopalatine muscles).10,11,13 During the contraction of the inspiratory muscles forces are generated that collapse the airway by the intraluminal negative pressure. Usually, the pharyngeal airway does not collapse until subatmospheric pressures are reached. In patients with OSAHS, collapse of the pharyngeal airway occurs at a positive pressure.12
The balance between the two forces (closure tendency, opening tendency) determines the anatomical balance of the pharyngeal airway. Its size is conditioned by the interaction between neural and anatomical mechanisms.10,14–16
Increased upper airway resistance during sleep is an essential feature of OSAHS. In children, it is usually a multifactorial disorder in which various factors converge, including: stenosis of the upper respiratory tract (e.g., tonsillar hypertrophy), abnormal airway muscle tone, and genetic predisposition.17
Physiopathological factors involved can be divided into:
Craniofacial anatomical factors: Small mandible, large or retroposed tongue, enlarged pharyngeal fat deposit, hypertrophic lymphoid tissues in the upper respiratory tract (particularly adenoids and tonsils), inferior nasal turbinate hypertrophy, deviated nasal septum.
Factors that promote greater collapsibility: Inflammation of the upper airway and alteration of the neurological reflexes that control upper respiratory tract muscles.10,18
Obstructive sleep events in children with OSAHS have an inverse pattern in relation to the sleep phase, compared with that observed in adults with OSAHS. 80% of obstructive events occur during REM sleep, while in adults 80% of obstructive events occur during non-REM sleep. Non-REM obstructive events occur less frequently in children, but they tend to increase in older children, African-American children, those in a lateral position, and those with low levels of oxyhemoglobin saturation. Non-REM obstructive events leading to wakefulness occur at a higher oxygen saturation than events during REM sleep.16,19
OSAHS can be divided into two types: Type I associated with marked lymphadenoid hypertrophy in the absence of obesity and Type II associated with obesity and with mild hyperplasia of lymphadenoid tissue.6
The cross-sectional area of the pharynx in children with OSAHS is 5%–10% smaller than in healthy children. Breathing in an awake child with this pathology is normal. During general anesthesia and neuromuscular block, the upper airway decreases in caliber. The relative increase in the soft pharyngeal tissue is caused by inhibition of the dilator muscles of the airway and decrease in lung volume, which results in a cephalic displacement of the mediastinum and a decrease in longitudinal tension on the upper airway, increasing collapsibility of the pharyngeal airway.12,20
MorbidityOSAHS has systemic repercussions that increase the morbidity of patients who suffer from it.3,6 Alterations in behavior have been observed in these patients (hyperactivity, attention deficit, cognitive deficit and poor school performance), along with daytime sleepiness, cardiovascular alterations, metabolic alterations, alterations in somatic growth, metabolic syndrome.21
OSAHS and obesity are considered inflammatory diseases. An increase in C-reactive protein has been observed that could constitute an important marker in the diagnosis of OSAHS. The current evidence suggests the existence of a genetic predisposition demonstrated by apolipoprotein epsilon 4.22,23 Children with OSAHS, particularly the obese ones, tend to develop metabolic syndrome and a fatty liver.6,24,25
Cardiovascular morbidity may be attributable to a complex interaction between intermittent hypoxia, episodic hypercapnia, recurrent fluctuations of intrathoracic pressure and sleep interruption. OSAHS can affect endothelial function causing hypertension due to increased release of endothelin and decreased nitric oxide production; In addition, sympathetic activation may exert direct action on the endothelium causing vasoconstriction. Inflammation may contribute to endothelial dysfunction by increasing the adhesion of inflammatory mediators and generating hypercoagulability. Some studies have shown that these alterations can be reversed 4 to 6 weeks after tonsillectomy was performed.22,26,27
In some patients with OSAHS, delayed growth and development have been observed, which may be secondary to an alteration in the functioning and/or release of insulin-like growth factor 1 and growth hormone.6
EpidemiologyIn 2008, Lumeng and Chervin carried out a systematic review of studies on the epidemiology of sleep-disordered breathing (SDB), finding the following prevalences: SDBs reported by parents 4%–11%; habitual snoring 1.5%–12%; sleep apnea events 0.2%–4%. OSAHS diagnosed by different criteria in diagnostic studies varies widely between 0.1 and 13%, but most studies report a figure between 1% and 4%.2,13,25 In general, SBD are more common in males, overweight children and African-American children. There are no data that support clear differences in age-based prevalence. However, some data shows a peak prevalence between 2 and 8 years of age.11,18,28
In children younger than 2 years, OSAHS is frequently associated with facial/skull malformations, neurological abnormalities and obesity. In these cases, OSAHS is usually more severe and with a greater recurrence or incomplete recovery after surgery.29
The incidence of postoperative respiratory complications in patients with OSAHS reaches 27% (6.4–27%), which varies according to age, severity of OSAHS, diagnostic criteria used and comorbidities. Children <3 years and an AHI (Apnea/Hypopnea Index) >10 have twice as much risk as children between 3 and 6 years of age. These complications include oxygen desaturation <90%, increased respiratory effort, radiographic changes (edema, atelectasis, infiltrates, pneumotorax, pneumomediastinum, pleural effusion), laryngospasm, apnea, crisis of pulmonary hypertension, pneumonia and perioperative death, and negative pressure pulmonary edema.17 Respiratory complications may be related to an alteration in the response to hypoxemia and hypercapnia and overexpression of opioid receptors.22,30–32
Preanesthetic assessmentIn all patients, a complete medical history including a review of systems, emphasizing clinical risk factors, and a specific physical examination should be performed. The child with OSAHS is at risk for potentially fatal perioperative respiratory complications, so the perioperative approach must be multidisciplinary (pediatrics, surgery, anesthesia).14,33,34
Most of the complications are respiratory. Children with OSAHS have a 20% greater chance of complications compared to healthy children undergoing adenotonsillectomy. In the general population, 21% of complications occur in the intraoperative period, 33% in the postanesthetic care unit and 46% in the patient's room. These complications can lead to severe neurological damage or death.35–37 Other less frequent complications such as negative pressure pulmonary edema and bleeding have been observed.22
The majority of the literature focuses on the perioperative management of the child with OSAHS who will be taken to adenotonsillectomy, from which the management principles for other surgical interventions have been extrapolated.14 General principles of management include:
Preoperative evaluation of snoring.
Strict observation and respiratory monitoring.
Analgesic and anesthetic opioid sparing techniques.30
Regional anesthesia techniques when possible.11
The clinical presentation varies depending on the age. In childhood, there should be high suspicion of craniofacial dysmorphia, growth retardation, susceptibility to infections or delayed speech development. At school age it can manifest as agitation, attention deficit, poor school performance or secondary enuresis. In adolescence the clinical presentation becomes similar to the adult.14,28,33,38
In the absence of characteristic symptoms of OSAHS in the pediatric age, the situation can be clarified with the systematic application of screening questions, emphasizing the presentation of nocturnal symptoms (snoring, excessive sweating, restless sleep, mouth breathing, apneas, wheezing, paradoxical or labored breathing, hyperextension of the neck during sleep). Also with the presence of diurnal symptoms such as difficulty concentrating, behavioral and mood disorders, morning headaches, excessive daytime sleepiness, and delayed growth.39 It should be questioned directly by the presence of snoring (most children with OSAHS snore, and the absence of snoring makes a diagnosis of OSAHS much less likely, with a sensitivity of 91% and a specificity of 75%).17,27,33
On physical examination, the findings suggestive of OSAHS include: mouth breathing, elongated facies, triangular and/or small chin, retroposition of the mandible, high palate, chest retractions, extreme body habits (developmental delay in infants, and obesity in children and adolescents), developmental delay. The magnitude of the tonsillar hypertrophy is not correlated with the severity of OSAHS.17,33
Questionnaires and clinical history are useful but not adequate in clinical practice to differentiate primary snoring in children from pediatric OSAHS (normal polysomnographic studies); therefore, additional diagnostic testing is required. The accuracy of the clinical evaluation of pediatric OSAHS in predicting the positivity of sleep studies varies between 30% and 85%.14
Polysomnography performed in a sleep laboratory at night is considered the gold standard for the diagnosis and evaluation of OSAHS in children. Ideally, this test should include cardiorespiratory, electroencephalographic, electrooculographic and electromyographic monitoring.1,17,40 Polysomnography is indicated in patients at high risk and in the evaluation of the following clinical conditions: growth failure, unexplained polycythemia (particularly if it is associated with snoring), sickle cell disease associated with a medical history suggestive of OSAHS or frequent veno-occlusive crisis, morbid obesity, craniofacial anomalies, neuromuscular disorders, cor pulmonale, systemic arterial hypertension.14,41
In polysomnography the following measurements are defined.17,18,33
Apnea–Hypopnea Index (AHI)Mean number of obstructive events (apnea–hypopnea) per hour of sleep. Diagnostic criteria for OSAHS in adults are a product of expert consensus and include an AHI of 5 or more and evidence of restless or disturbed sleep, daytime sleepiness, or other diurnal symptoms. IAH cut-off points of 5, 15 and 30events/hour have been suggested to indicate mild, moderate and severe levels.14,42 There are few data available in the literature to establish diagnostic criteria for specific OSAHS in children from AHI. Currently, an AHI of 1–5 events per hour is used in research to identify children with OSAHS; however, widely varying definitions have been used1,33 (see Table 1).
Oxygen saturation nadir (see Table 1)Respiratory Disturbance Index (RDI): Number of apnea/hypopnea events per hour of sleep. It is not specific for OSAHS, since it includes episodes of central and obstructive apnea.18
Due to the cost and the limited availability in many communities of polysomnography performed in the sleep laboratory, significant efforts have been made to explore other forms of diagnosis. Recording of audio at home at night, video recording, pulse oximetry, cardiorespiratory studies, and home polysomnography have been used. However, the validity of each of these methods has not been compared with the gold standard in the pediatric population.43 It cannot be ruled out that any of these modalities may have some diagnostic value in specific populations. Brouillette et al.44 concluded that in children with suspected OSAHS, severe isolated oxygen desaturation (SaO2 <80%) or desaturation clusters (more than three episodes of SaO2 <90%) are considered abnormal and have a positive predictive value of 97%, but a negative result does not rule out the diagnosis of OSAHS.
During the evaluation of the child with OSAHS, the airway obstruction site should be identified. The study of dynamic airway collapse patterns during sleep identifies the anatomical causes of the obstruction and facilitates the planning of interventions to alleviate such obstruction. To study these, differential pharyngeal pressures through catheters placed at different levels in the upper airway have been used; cine-fluoroscopy; video endoscopy; computed tomography; fiberoptic bronchoscopy.14,33
Although most children scheduled for adenotonsillectomy do not require cardiac evaluation, patients with evidence of systemic hypertension and/or chronic hypercapnia (multiple episodes of severe hypoxemia <70%, compensatory metabolic alkalosis), which usually have increased pulmonary pressures and therefore are at risk of cor pulmonale, should have at least one evaluation with echocardiogram before proceeding with elective surgery. These cardiopulmonary abnormalities are seen less frequently in children than in adults.24,26,46
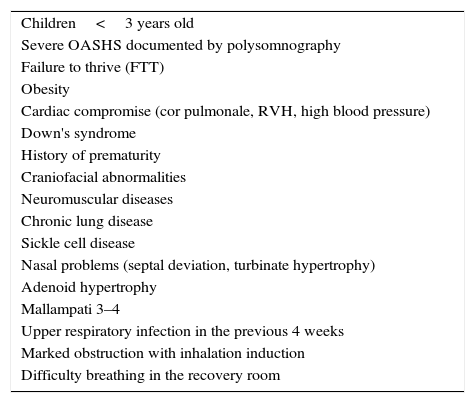
Children at high risk for postoperative respiratory complications should be identified from preanesthetic assessment to plan their postoperative stay. Although there is no clear evidence of relevant observation time, there are some recommendations drawn from the literature that may contribute to the adequate planning of the perioperative treatment of the patient and to reduce the incidence of complications through appropriate postoperative monitoring (see Table 2).14,17,26,47
Risk factors for pulmonary postoperative complications after adenotonsillectomy.
| Children<3 years old |
| Severe OASHS documented by polysomnography |
| Failure to thrive (FTT) |
| Obesity |
| Cardiac compromise (cor pulmonale, RVH, high blood pressure) |
| Down's syndrome |
| History of prematurity |
| Craniofacial abnormalities |
| Neuromuscular diseases |
| Chronic lung disease |
| Sickle cell disease |
| Nasal problems (septal deviation, turbinate hypertrophy) |
| Adenoid hypertrophy |
| Mallampati 3–4 |
| Upper respiratory infection in the previous 4 weeks |
| Marked obstruction with inhalation induction |
| Difficulty breathing in the recovery room |
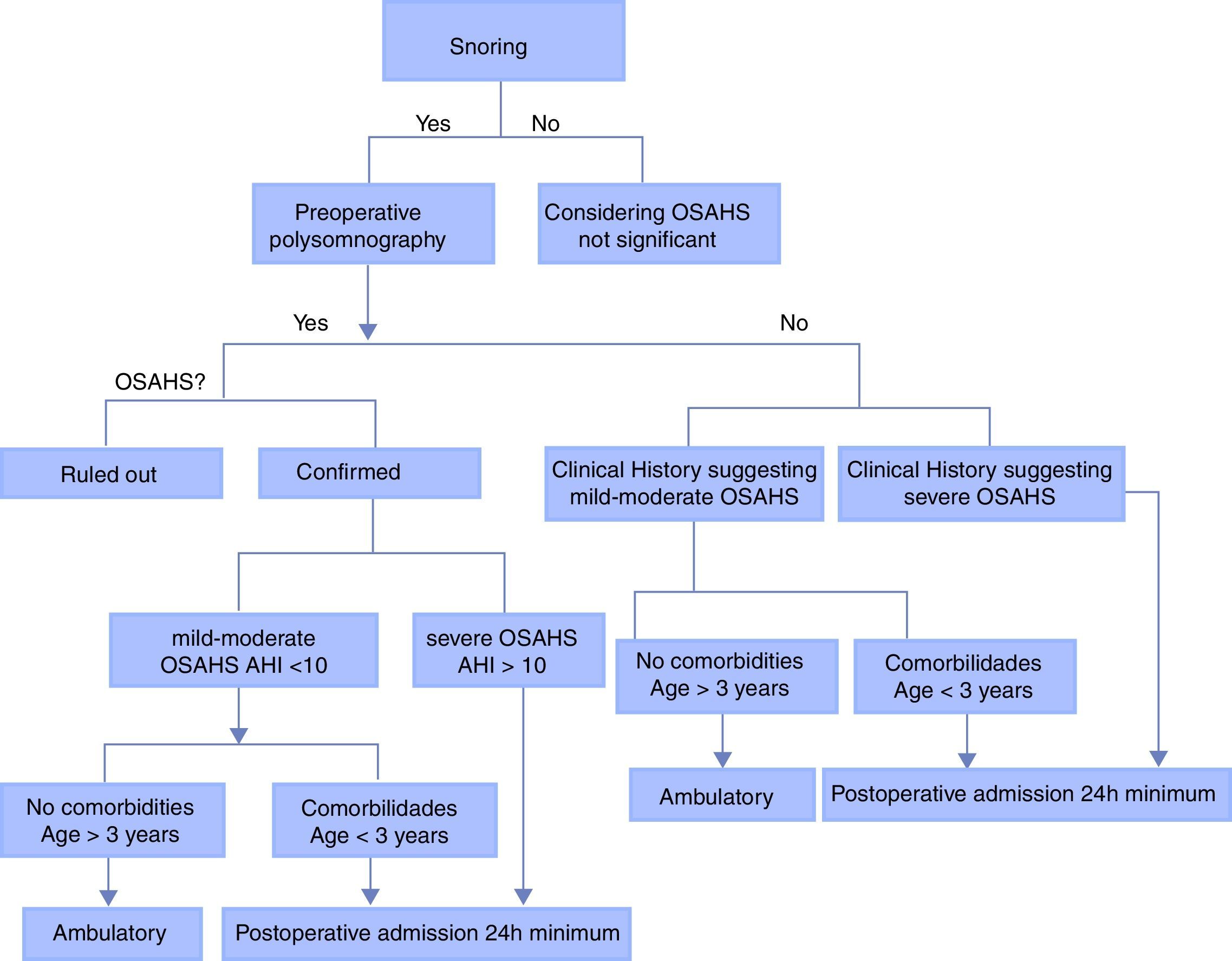
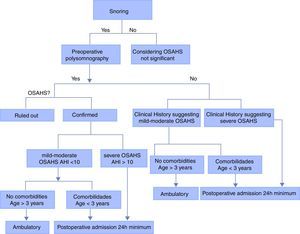
Mild or moderate OSAHS without any risk factors: 3–7h observation
Moderate/mild OSAHS without risk factors but with desaturation, oxygen requirement, difficult intraoperative airway management or postoperative obstruction: intermediate care for 24h.
Mild/moderate OSAHS without risk factors but with severe pain requiring opioid management: intermediate care for 24h.
Mild or moderate OSAHS with risk factors: intermediate care for 24h.
Severe OSAHS: intensive care for 24h (see Fig. 2).
Perioperative treatmentAdenotonsillectomy is the recommended initial treatment for pediatric OSAHS.34,48 Positive-pressure ventilation (BiPAP/CPAP) in the preoperative period has been used to decrease the rate of postoperative complications and to increase airway permeability in adults. It may be useful in pediatric patients with severe OSAHS and secondary cardiovascular complications,9,17,26,49 since improvement in pulmonary hypertension and reduction of surgical risk have been observed. The pressures adjusted by the pediatric pulmonologist in the sleep laboratory are those used in the postoperative period.17,50,51
Several studies compare early adenotonsillectomy with expectant management in different pediatric ages of patients with OSAHS and other lung diseases, finding that it does not significantly improve care or executive function measured by neuropsychological tests, but reduces symptoms and improves secondary outcomes of behavior, quality of life and polysomnography.51–56
The following are evidence-based practice recommendations for the diagnosis and treatment of OSAHS57,58:
Clinical history and physical examination are not sufficient to differentiate between primary snoring and OSAHS. Polysomnography is the gold standard for the diagnosis of OSAHS.
Children with Down's syndrome, craniofacial anomalies, obesity, or Prader–Willi syndrome (PWS) are at increased risk for OSAHS and often require polysomnography and specific therapies.
The pillars of therapy for OSAHS in children are adenotonsillectomy and the use of positive airway pressure devices.9 The use of continuous positive airway pressure therapy (CPAP) is an effective treatment in children. However, like what occurs in adults, adherence is a major problem and there may be side effects in children with long-term use.50
Non-surgical therapies, including anti-inflammatory drugs, dental appliances and weight loss may be helpful in certain circumstances, such as mild OSAHS, either primary or persistent after adenotonsillectomy, although more data is needed.9
Airway surgery has been shown to be effective in children with craniofacial abnormalities, but not in the general pediatric population. Bariatric surgery is an option for extremely obese adolescents.
As for premedication, caution should be exercised with sedatives in children with severe OSAHS. If they have been received, they should be continuously monitored by clinical observation and, at least, continuous pulse oximetry until the transfer to surgical rooms. The residual effect of these drugs can be observed even in the recovery stage, especially in short-term procedures.14 Some data suggest that children with OSAHS may receive sedatives, but require monitoring until full recovery can be demonstrated.17
The strategies to keep the pharyngeal airway permeable consist of: Expansion of the bone limits, reduction of pharyngeal soft tissue volume and activation of the pharyngeal dilator muscles.12 The expansion of the bone limit can be obtained with the triple airway maneuver. The reduction of the volume of the pharyngeal soft tissue can be obtained with the use of continuous positive airway pressure (CPAP) that acts like a pneumatic splint preventing the soft tissue from invading the upper airway. It also increases lung volume, resulting in a caudal displacement of the mediastinum that applies longitudinal tension over the pharyngeal airway, decreasing its collapse.12,50 Recruitment of the upper airway dilator muscles is not easy due to the selective inhibition of many anesthetics. Propofol apparently preserves genioglossal muscle activity.12
The position of the body influences the volume of the soft pharyngeal tissue. In the supine position, airway occlusion in children with OSAHS occurs in the upper 2/3 of the pharyngeal airway in the area between the adenoid tissue and the tonsils. The lateral position displaces the soft tissue outside the airway.12,26
ConclusionsOSAHS is a disease whose prevalence in the pediatric population requires an in-depth knowledge of its physiopathology and the comorbidities that it involves at the pulmonary, cardiovascular, neurological, metabolic and hematological levels. Similarly, the approach in preanesthetic assessment should focus on a broad clinical evaluation, considering polysomnography and derived information as a fundamental diagnostic tool, that allows to determine the pertinence of a broader paraclinical evaluation to define the parameters of preoperative treatment and postoperative care in order to reduce complications.
FundingThe authors did not receive sponsorship to carry out this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Amézquita-Trujillo Á, Garzón JF. Consideraciones en el paciente pediátrico con síndrome de apnea/hipopnea obstructiva del sueño (SAHOS): desde la fisiopatología al perioperatorio. Rev Colomb Anestesiol. 2017;45:173–181.