Spinal anesthesia has been part of the pediatric anesthesia practice for more than 100 years. Its use has been increasing in recent years because of its effectiveness, efficiency and safety. We report a successful case in a patient with a difficult airway.
ObjectiveTo report a case of spinal anesthesia and sedation with remifentanil, together with a review of the literature including alpha 2 agonists for locoregional procedures in pediatrics.
MethodsSearch of relevant references in PubMed, MD consult and BIREME. The search resulted in 306 articles, and 23 considered relevant by the authors were finally selected.
ResultsWe present a case of a 1-year-old boy with an expected difficult airway because of the presence of a cavernous hemangioma of the lower lip, scheduled for surgical correction of bilateral club foot. Spinal anesthesia consisted of 0.5% hyperbaric carbonated bupivacaine plus 30μg of clonidine (1.3ml total), maintaining sedation-analgesia at 3–4/6 on the Ramsay scale with remifentanil 0.05–0.075μg/kg/min, 50% oxygen with facial mask, and spontaneous ventilation, with no hemodynamic or respiratory adverse effects.
ConclusionsSpinal anesthesia is an option in cases of predicted difficult airway. Clonidine (alpha 2 agonist) prolongs blockade with no hemodynamic or respiratory complications. Remifentanil used for sedation in pediatric locoregional procedures is easy to titrate with predictable results.
la anestesia espinal lleva más de 100 años en la práctica anestésica pediátrica. Actualmente viene aumentando su uso por ser eficaz, eficiente y segura. Se expone un caso exitoso en paciente con vía aérea difícil.
ObjetivoReportar un caso de anestesia espinal y sedación con remifentanilo, haciendo la revisión de la literatura incluyendo fármacos alfa 2 agonistas, para procedimientos locorregionales pediátricos.
Métodosbúsqueda bibliográfica relevante en las bases bibliográficas PubMed, MD consult y BIREME. Inicialmente se obtienen 306 artículos, seleccionando 25 considerados relevantes por los autores.
Resultadosse presenta el caso de un niño de un año de edad, con vía aérea difícil predicha por un hemangioma cavernoso en labio inferior, programado para corrección quirúrgica de pie chapín bilateral. Se administra anestesia espinal con bupivacaína hiperbárica 0,5% carbonatada 5 mg y clonidina 30 g(1,3 ml total), manteniendo sedoanalgesia 3-4/6 de Ramsay con remifentanilo 0,05-0,075 g/kg/min, con oxígeno 50% por máscara facial y ventilación espontánea sin efectos adversos hemodinámicos o respiratorios.
Conclusionesla anestesia espinal es una alternativa ante una vía aérea difícil predicha. La clonidina (alfa 2 agonista) prolonga la duración del bloqueo sin complicaciones hemodinámicas o respiratorias. El remifentanilo para sedación en los procedimientos locorregionales pediátricos es de fácil titulación, con resultados predecibles.
Case review and topic review based on a search that included medical practice guidelines, meta-analysis, systematic reviews, clinical trials, case reports and review of the literature of the past 5 years, both in English and Spanish in the PubMed, MD consultation and BIREME databases. MESH terms used: spinal anesthesia, clonidine, remifentanil, sedation, pediatrics.
Of the initial 306 articles, 8 met the selection criteria. Results were obtained through reading, interpreting and analyzing each of the articles, some of which led to additional references, for a total of 23.
Case presentationThe case was a twelve-month-old male child 75cm tall, weighing 10kg and coming from the rural area of Huila with a congenital equinovarus foot (club foot) and lower-lip cavernous hemangioma, who was scheduled for surgical correction of the bilateral club foot. He had no significant personal or family history.
The physical examination revealed a difficult airway because of the presence of a tumor mass 2cm high×3cm wide in the lower lip. Both feet were completely inverted, with the forefoot in adduction.
At the pre-anesthesia assessment, the anesthesiologist found an ASA 2/5 patient, with low surgical risk and a difficult airway because of a prominent mass in the lower lip. The anesthesia plan was designed to include spinal anesthesia plus sedation and analgesia with remifentanil and spontaneous ventilation through a facemask.
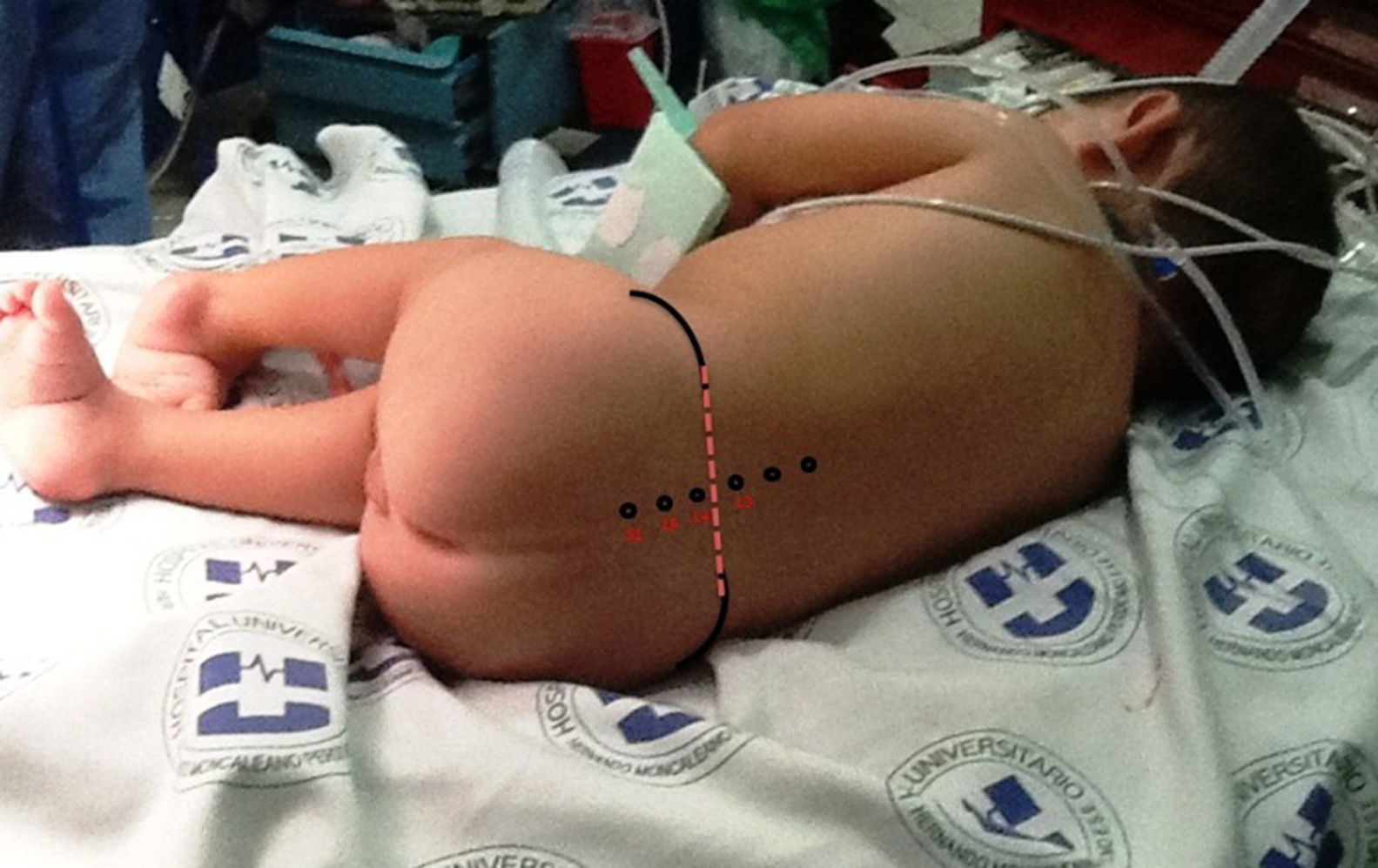
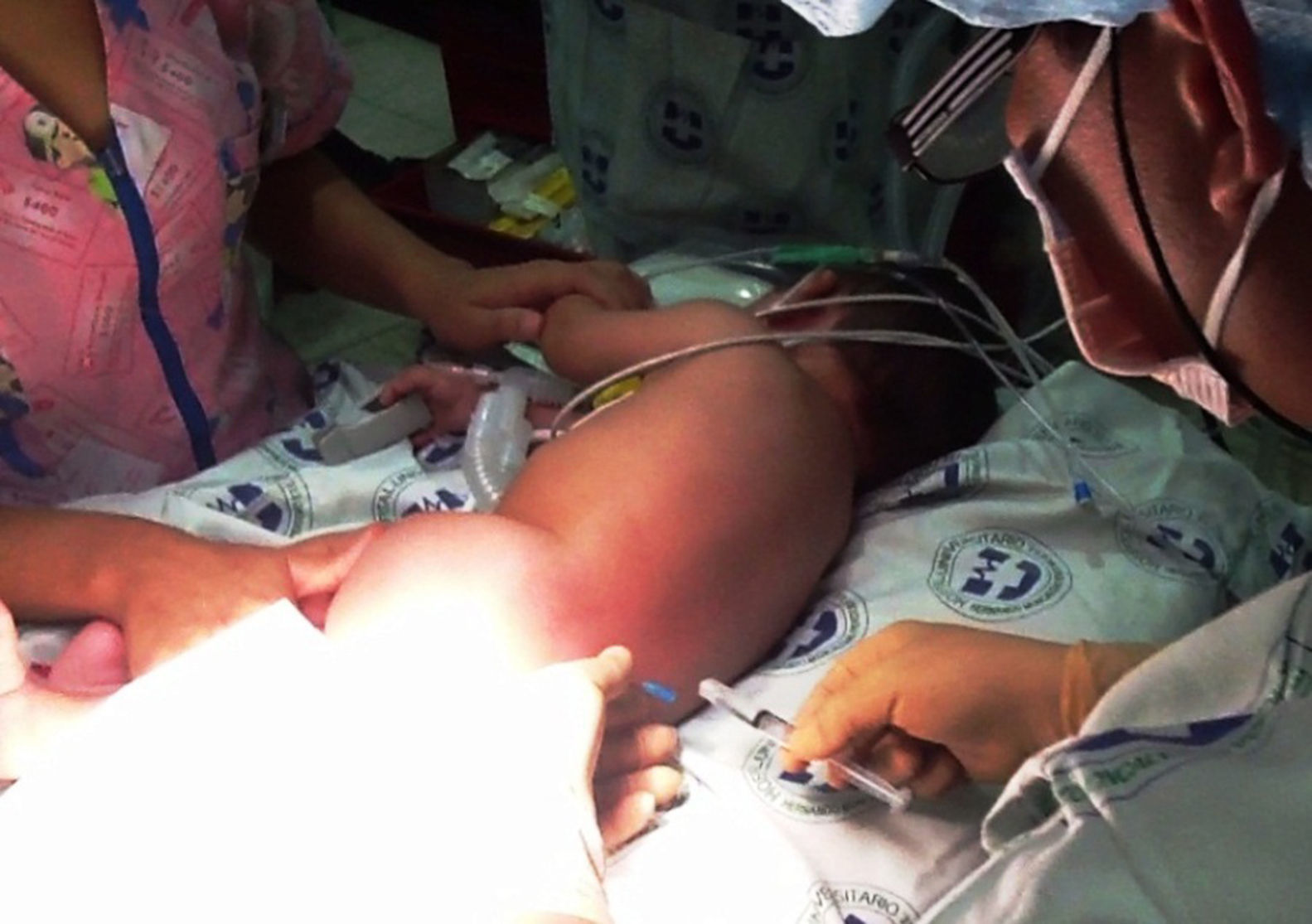
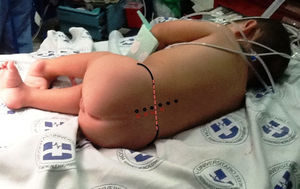
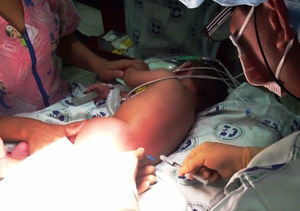
Upon admission the patient was given ketamine 10mg i.v. plus midazolam 0.5mg i.v., achieving a sedation of 5/6 on the Ramsay scale (Table 1). Intraoperative basic monitoring was set up (cardioscope in DII, pulse oximetry, non-invasive blood pressure every 5min, and capnography). The remifentanil infusion was started at a rate of 0.1μg/kg/min following which the patient was placed on a right lateral decubitus fetal position. After asepsis and antisepsis, the L3–L4 intervertebral space was localized (Fig. 1), a lumbar puncture was performed using a number 22 hypodermic needle, the return of clear CSF is observed, and 1.3ml of 0.5% hyperbaric carbonated bupivacaine 5mg plus clonidine 30μg were administered (Fig. 2). The dose of remifentanil was titrated between 0.05 and 0.075μg/kg/min, with the goal of maintaining a state of sedation and analgesia of 4/6 on the Ramsay scale, and spontaneous ventilation. Dexamethasone 1mg, Ketorolac 2.5mg, Ondansetron 1mg and Cefazoline 500mg were given intravenously before the surgical incision.
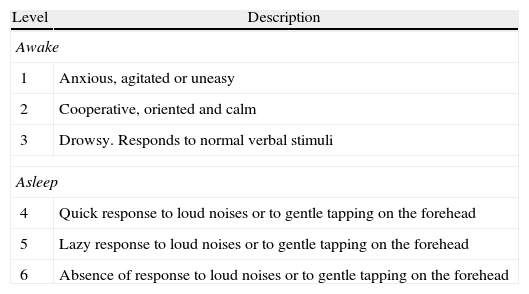
Ramsay scale.
| Level | Description |
| Awake | |
| 1 | Anxious, agitated or uneasy |
| 2 | Cooperative, oriented and calm |
| 3 | Drowsy. Responds to normal verbal stimuli |
| Asleep | |
| 4 | Quick response to loud noises or to gentle tapping on the forehead |
| 5 | Lazy response to loud noises or to gentle tapping on the forehead |
| 6 | Absence of response to loud noises or to gentle tapping on the forehead |
Modified from Ramsay et al. BMJ, 1974; 2: 656-659.
The surgical procedure lasted 4h and a good anesthetic block was maintained throughout. The patient remained hemodynamically stable, with no bradycardia, hypotension, apnea or arterial desaturation. The patient received, overall, 180cm3 of crystalloids and had an estimated blood loss of 50cm3. There were no surgical or anesthetic complications. Remifentanil was interrupted at the end of the surgery.
The patient was transferred to the post-anesthetic care unit with a level of sedation of 2/6 on the Ramsay scale and no lower limb motor blockade. He remained in the PACU for 1h with no evidence of requiring additional analgesia or management of nausea or vomiting. The patient was admitted and then discharged 24h later, with recommendations and instructions for a follow-up visit to the orthopedics outpatient clinic in eight days.
Topic reviewSpinal anesthesia in pediatricsAugust Bier was the first to report in 1899 a case series of successful spinal blockade using subarachnoid cocaine in pediatric patients.1 Later, locoregional anesthesia was used massively during the first decades of the 20th century in the pediatric population, driven by inaccuracy and lack of safety with the use of chloroform or ether-based general anesthesia. With new advances in the pharmacological knowledge of muscle relaxants, inhaled and intravenous anesthesia, and the development of new equipment and greater experience in the management of the airway and the use of mechanical ventilation, locoregional techniques in pediatrics were left by the sideway gradually.2 Starting in the 1980s there was a reemergence of regional techniques in pediatrics because of the undeniable advantages demonstrated in the literature for the intra- and post-operative periods and for the management of chronic pain.3
Subarachnoid anesthesia is indicated in a large number of infraumbilical surgical procedures. There is a clear indication in neonates under 60 weeks of postconceptional age because of the high risk of respiratory complications and apnea during the post-operative period after general anesthesia, a risk which is greater when there have been episodes of apnea or a hematocrit of less than 30% during the pre-operative period. Experts recommend spinal anesthesia as an alternative in the following clinical situations:
- 1.
Potentially difficult airway.4–6
- 2.
Chronic respiratory diseases.
- 3.
Malignant hyperthermia.
- 4.
Congenital heart diseases, helping to minimize hemodynamic fluctuations.7
- 5.
Epidermolysis bullosa, where airway manipulation must be minimized.8,9
- 6.
Sleep disorders.
In order to perform the technique, the anesthesiologist must be very familiar with the spinal anatomy and its differences with the adult spine. At birth, the spinal cord ends at the level of L4–L5, with cephalad advancement during the first year of life, until if finally comes to rest between lumbar segments L1 and L2. Meninges are localized at the level of the sacral vertebrae S2–S3, remaining at this level throughout growth. The distance between the skin and the dura mater in the neonatal lumbar spine is often 1–1.5cm; an approximate calculation may be done using the formula of (weight+10)×0.8 or also 1mm per kg.10
CSF volume is a determining factor for local anesthetic diffusion in the neural axis. The volume of CSF in neonates is 4ml/kg (twice the adult volume), of which 50% is found in the spinal canal (25% in adults), resulting in greater dilution of intrathecal drugs. On the other hand, neonates have large neuronal structures in proportion to their skeletal mass, and the low concentration of Ranvier nodules means that high concentrations of local anesthetics are required.11,12
Spinal anesthesia in the pediatric population does not alter hemodynamic stability, and this is a relevant consideration. It has been observed that blood pressure and heart rate are maintained even with blocks at T4 without previous hydration. This is explained by the fact that infants have less venous capacitance in their lower limbs, depend less on the vasomotor tone, and have parasympathetic predominance.13
Ventilation will not be affected as long as the block does not go beyond T1, because neonates maintain their breathing primarily with the support of the diaphragm.14
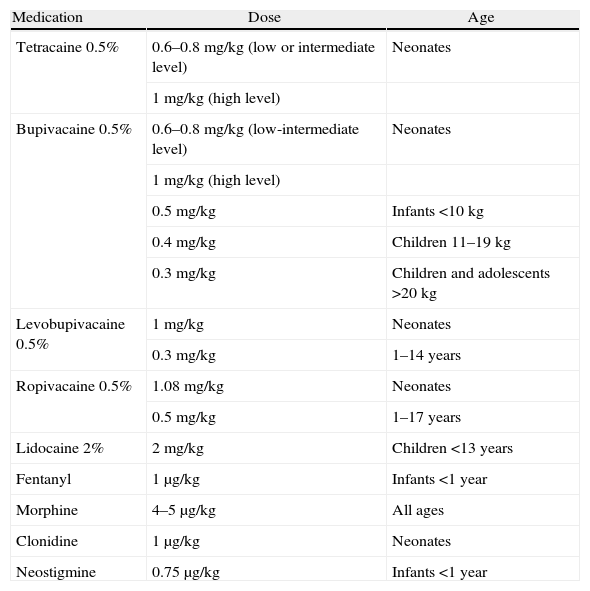
For infraumbilical procedures, a T8–T9 level is sufficient. The most commonly used drugs are 0.5% bupivacaine and 0.5% tetracaine. The recommended dose to obtain mean spinal blocks is 0.6–0.8mg/kg and the duration of the block with the two drugs ranges between 90 and 120min. Pediatric patients between six months and 14 years of age require a lower dose, and hyperbaric bupivacaine is indicated at 0.3mg/kg with a 98% success rate.15 Recently, 0.5% ropivacaine at 0.5mg/kg16 and 0.5% levobupivacaine at 0.3mg/kg have been used in children 1–14 years of age with a lower incidence of toxicity and motor blockade, and this is recommended in cases of liver dysfunction (see Table 2).
Drugs used for spinal anesthesia in pediatrics.
| Medication | Dose | Age |
| Tetracaine 0.5% | 0.6–0.8mg/kg (low or intermediate level) | Neonates |
| 1mg/kg (high level) | ||
| Bupivacaine 0.5% | 0.6–0.8mg/kg (low-intermediate level) | Neonates |
| 1mg/kg (high level) | ||
| 0.5mg/kg | Infants <10kg | |
| 0.4mg/kg | Children 11–19kg | |
| 0.3mg/kg | Children and adolescents >20kg | |
| Levobupivacaine 0.5% | 1mg/kg | Neonates |
| 0.3mg/kg | 1–14 years | |
| Ropivacaine 0.5% | 1.08mg/kg | Neonates |
| 0.5mg/kg | 1–17 years | |
| Lidocaine 2% | 2mg/kg | Children <13 years |
| Fentanyl | 1μg/kg | Infants <1 year |
| Morphine | 4–5μg/kg | All ages |
| Clonidine | 1μg/kg | Neonates |
| Neostigmine | 0.75μg/kg | Infants <1 year |
Taken from Lopez T et al. Spinal anesthesia in pediatric patients. Minerva Anestesiol, 2012; 78(1): 78-87. Printed with con permission.
Hypoxemia, intraoperative apnea and bradycardia are frequent complications in premature babies and neonates, possibly as a result of high spinal blockade, excess sedation during the procedure, or maximum neck flexion during the lumbar puncture. Other complications have been described, including post-puncture headache – ranging from 0.4% to 15% depending on the type of procedure and the size of the needle17,18 – transient neurologic syndrome (1.5%), and menigitis.19
Neuroaxial alpha-2 agonist in pediatricsClonidine, prototype of alpha-2 agonists, acts on the pre-synaptic and post-synaptic alpha-2 adrenergic receptors, and has also a weak action on the alpha-1 receptors. It is indicated as a centrally acting antihypertensive drug, and in regional anesthesia for sedation and analgesia as an adjunct for opioids in the treatment of pain.
In the pediatric population, clonidine is used at 0.5–5μg/kg given to the subarachnoid space without producing ventilation or hemodynamic compromise.20 Different authors suggest caution in children under 1 year of age or weighing less than 10kg until studies with a higher level of evidence support its use.
Neuroaxial clonidine at higher doses may lead to hypotension, bradycardia and sedation.21 Hypotension is considered as an interaction in the spinal cord vasomotor center, localized in the lateral reticular nucleus of the brain stem, leading to a reduced norepinephrine turnover from the sympathetic endings to the peripheral tissues. Bradycardia is secondary to parasympathetic hyperstimulation and concurrent reduction of the sympathetic tone.22 The sedative effect has a central action.
Remifentanil for sedation in regional anesthesiaRemifentanil is an ultra short acting opioid used primarily in adults for anesthesia and conscious sedation in painful procedures. There are few references about its use in the pediatric literature.23–27
Its main advantages relate to the rapid onset and cessation of its clinical effects (maximum effect lasting 90–120s). Analgesic plasma concentration is 0.5–1.5μg/ml. The optimal balance between patient comfort and safety requires careful dosing of the drug under adequate monitoring of the central nervous, cardiovascular and respiratory systems, and good communication with the patient and the surgeon or interventional specialist.
In deep sedation, airway protection reflexes are altered, increasing the risk. Multi-center trials propose remifentanil for sedation analgesia combined with locoregional anesthesia, starting the infusion at a rate of 0.1μg/kg/min until the blockade is completed, and continuing at a rate of 0.05μg/kg/min until the end of the procedure;28 this permits autoprotection of the airway without the need for manipulation.
ConclusionsThere is evidence in the literature of the advantages and low risk of spinal anesthesia in pediatrics when compared with general anesthesia.29 It has been shown to be highly effective and efficient in the hands of experienced anesthesiologists. However, it continues to be underutilized by most institutions and anesthesiologists.
Intrathecal clonidine, an alpha-2 agonist, is used as an adjunct to local anesthetics allowing a dose reduction and diminishing their deleterious side-effects.
Remifentanil is a good option for conscious sedation in locoregional anesthetic procedures in pediatric patients, maintaining good hemodynamic stability and spontaneous ventilation. The anesthesiologist is required to perform adequate and close monitoring due to the risk of respiratory depression which, in most cases, only requires reducing or interrupting the infusion or stimulating the patient.
FundingThe authors’ own resources.
Conflict of interestsNone declared.
Please cite this article as: Diaz Herrera D, et al. Reporte de caso: anestesia espinal multimodal en paciente pediátrico con vía aérea difícil. Rev Colomb Anestesiol. 2013. http://dx.doi.org/10.1016/j.rca.2013.05.001.