Polycystic ovary syndrome (PCOS) is the most common endocrinopathy affecting approximately 5–10% of women of reproductive age and it is also a major cause of anovulatory infertility. PCOS is associated with obesity and conditions like hirsutism, acne, diabetes, and irregular periods.
AimThe present study aimed to evaluate the serum-free testosterone (FT) levels of women afflicted with hirsutism, one of the main physical manifestations of PCOS versus healthy women and determine whether their serum testosterone levels correlate with polycystic ovaries, glucose levels, menstrual abnormalities, and obesity.
MethodsThis study assessed 180 women; this included 140 females who suffered from excessive and unwanted hair growth on the chin and 40 healthy women as a control group. Free testosterone levels and fasting blood glucose levels were taken. Prior to the study, ultrasonographic (US) tests were performed for all patients to diagnose polycystic ovaries.
ResultsPatients with hirsutism exhibited a significant elevation in free testosterone (FT) compared to the control group. Approximately half of these women were confirmed to have a polycystic ovary, and 42.8% of them were overweight and obese. Additionally, women between 18 and 20 years old (G1) present with the highest level of FT.
ConclusionSerum FT levels were significantly increased in hirsute women, and this positively correlated with BMI and glucose levels in women with PCOS. Glucose levels may serve as a potentially effective biomarker in evaluating the severity of hirsutism in women suspected of having PCOS.
El síndrome de ovario poliquístico (SOP) es la endocrinopatía más común que afecta aproximadamente al 5-10% de las mujeres en edad reproductiva y también es una de las principales causas de infertilidad anovulatoria. El SOP está asociado con la obesidad y condiciones como hirsutismo, acné, diabetes y períodos irregulares.
ObjetivoEl presente estudio tuvo como objetivo evaluar los niveles séricos de testosterona libre (FT, del inglés) de mujeres con hirsutismo, una de las principales manifestaciones físicas del síndrome de ovario poliquístico, en comparación con mujeres sanas y determinar si los niveles séricos de testosterona se correlacionan con ovarios poliquísticos, niveles de glucosa, anomalías menstruales y obesidad.
MétodosEste estudio evaluó a 180 mujeres; esto incluyó a 140 mujeres que sufrían de un crecimiento de vello excesivo e indeseado en la barbilla y 40 mujeres sanas como grupo de control. Se midieron el nivel de FT y el nivel de azúcar en sangre en ayunas. Antes del estudio, se realizaron pruebas ultrasonográficas a todas las pacientes para diagnosticar ovarios poliquísticos.
ResultadosLos pacientes con hirsutismo exhibieron una elevación significativa en la FT en comparación con el grupo de control. Se confirmó que aproximadamente la mitad de estas mujeres tenían un ovario poliquístico y el 42,8% de ellas tenían sobrepeso y obesidad. Adicionalmente, las mujeres entre 18 y 20 años (G1) presentan el mayor nivel de FT.
ConclusiónLos niveles séricos de FT aumentaron significativamente en mujeres hirsutas, y esto se correlacionó positivamente con el IMC y los niveles de glucosa en mujeres con SOP. Los niveles de glucosa pueden servir como un biomarcador potencialmente eficaz para evaluar la gravedad del hirsutismo en mujeres con sospecha de SOP.
Polycystic ovarian syndrome (PCOS) is a multifactorial endocrine disease, and its hallmark features include anovulation, hyperandrogenism, and increased ovarian volume. The most upsetting aspect of this common disease for any patient woman may change with time. Clinically, this can be presented with hirsutism, acne and androgenic alopecia.1 Other symptoms include weight gain, menstrual irregularities and sub- or in-fertility.2
The prevalence of hirsutism in women with PCOS can reach up to 70–80%, in contrast to 4–11% of women in the general population. Hirsutism is the chief characteristic of hyperandrogenism in PCOS.3 The degree of hirsutism also differs depending on the ethnicity of the population. Women suffering from PCOS have an increased risk of developing metabolic abnormalities than women without PCOS.4 Some prospective studies have confirmed that insulin resistance (their bodies can make insulin but can’t use it effectively) is a key feature in the pathogenesis of PCOS.5
In women suffering from PCOS, a higher level of androgens, namely testosterone, is observed. Testosterone is a steroid hormone secreted mainly by the testicles in men and, in a small amount, by the placental tissues and ovaries in women.6 Free testosterone is the fraction of total testosterone not bound to any transport protein and is readily available to cells.7 Biochemically, hyperandrogenism is an increase in the level of testosterone and other related parameters of androgen excess, such as free testosterone and free androgen index. Hence, increased total testosterone or free testosterone level is a critical diagnostic biochemical marker of hyperandrogenism.
However, the majority of the circulating testosterone in humans is bound to its binding proteins – sex hormone – globulin, serum albumin, and cortisol-binding globulin. Binding proteins are essential in testosterone transport, tissue delivery, bioactivity, and metabolism.8 The bioactive level of testosterone and free testosterone might be suitable for revealing the relationships between testosterone and other metabolic abnormalities. Free testosterone has a closer association with metabolic parameters than total testosterone.
Furthermore, Lerchbaum et al. mentioned that higher levels of free testosterone and androstenedione in women who have PCOS illustrate that they have abnormal metabolic status.9 Approximately 20% of females with PCOS frequently experienced sleep apnoea, depression, and anxiety.10
Furthermore, the increasing worldwide prevalence of obesity and depression is a series of health issues nowadays, specifically for women.11 In addition to PCOS, several bacterial, parasitic, and fungal diseases infect women, particularly women of childbearing age, which can lead to inflammation, infertility or miscarriage.12,13
This work aimed to determine the relationship between excessive hair growth on the chin and serum-free testosterone level, blood glucose level, presence, or absence of PCO, BMI, and menstrual regularity among hirsute females in Soran city/Kurdistan-Iraq.
The main objective of this paper is to find out the relationship between hirsutism as an emerging phenomenon among women in Erbil-Kurdistan region, Iraq with free testosterone, polycystic ovaries, obesity, and glucose levels, as there are only very limited investigations have been conducted on this topic in this region.
Materials and methodsStudy subjectsRandomly selected females suffering from hirsutism who attended the private gynecological clinics in Soran city/Erbil-Iraq from October 2021 to February 2022 were eligible for the current study. In comparison, age-matched healthy women were considered as a control group. Physician permission was obtained, and all patients signed a consent form. A questionnaire was filled out regarding their medical status to confirm the diagnosis. The questionnaire included age, where they lived, educational level, occupation, marital status, family history, and menstrual regularity.
Sample selection and collectionA total of one hundred forty females who complained of abnormal hair growth on their faces and were admitted by physicians were enrolled in this study. In addition, blood samples from forty healthy age-matched women were collected from Soran city as a control group. Initially, 3ml of peripheral blood samples were taken from the study subjects during the first five days of their menstrual cycle.14 Blood samples were taken while the patient was at least 6–8h after meals. None of the participants smoked tobacco, took any medications, or were pregnant. All women in the control group were healthy, had regular menstrual periods, had no clinical or biochemical hyperandrogenemia, no significant medical history, and none were on any treatments, including oral contraceptive pills. All the participants gave written informed consent.
Clinical examinationPolycystic ovary syndrome was diagnosed depending on Rotterdam criteria, such as irregular menstruation, hyperandrogenism, and the occurrence of cysts in the ovaries.15 Ultrasound tests were performed by a sonographer using Medison SonAce R3, Korea, to determine the presence of cysts on the ovaries. These women were identified by a gynecologist as having polycystic ovarian syndrome, ruling out any other possible endocrine disorders as the source of their hyperandrogenism. For instance, other causes of anovulation as well as androgen-secreting tumors, hyperprolactinemia, thyroid disorders, and medication-induced androgen were disregarded.16
The body mass index (BMI) of all study subjects was calculated according to the standard BMI formula.17
Participants were inquired about the symptoms associated with abnormal hair growth, androgen levels, menstrual regularity and whether they had a diagnosis of PCOS. As well as that, the selected participants were asked if they had been on any hormonal therapy for hirsutism in the last two months prior to the blood sample collection. The participants who had received any treatment were excluded from the study.
Serum preparationA volume of 3ml of peripheral blood samples was taken from female patients and healthy control women. The blood samples were kept in gel tubes for sera separation. Then the blood was centrifuged for 15min at 3000rpm, and sera samples were collected and stored at −20°C until further analysis.
Hormonal and biochemical examinationsThe sera of patients and control subjects were used to estimate testosterone-free hormones. In addition to that, blood glucose tests were also done for all the samples to monitor the glucose level.
Estimation of serum-free testosterone levelThe serum level of free testosterone was assessed by enzyme-linked immunosorbent assay (ELISA). Human-free testosterone ELISA kit as free testosterone AccuBind ELISA based on enzyme immunoassay (USA Catalog Number. RX 5325-300) was used. The investigation was performed according to the manufacturer's instructions for the kit. This involved taking 20μl of the samples and placing them, into each well on the ELISA plate. This was incubated for 60min at room temperature. Then, the contents were discarded and washed three times. After that, 100μl of the working substrate was added to each well and incubated in a dark room for 15min. Finally, each well was added to an aliquot of 50μl stop solution. They were mixed gently, and the color change was immediately observed. Finally, each well's optical density (OD) was measured within 5min using the BIOBASE Elisa microplate reader (Model BK-EL 10 C, China) at 540nm. An ELISA computer reader automatically drew the ELISA standard curve, plotting the OD values on the Y-axis and calibration concentrations (pg/ml) on the X-axis. A standard curve was prepared to evaluate the concentration of tested samples.
Estimation of blood glucose levelA blood glucose test was performed by the fully automated Cobas C111 system using Gluc2 CAN: 767, Glucose HK kit manufactured by (Roche Diagnostics GmbH). The tests were carried out immediately after collecting the sample.
Statistical analysisThe present data were analyzed statistically by Graph Pad Prism 7.04 software. The variables were investigated using visual (histograms) and analytical methods (Kolmogorow–Simirnov test/Shapiro–Wilk test) to determine whether or not they were normally distributed. A p value of <0.05 was considered to be statistically significant. Pearson correlation was used to assess the correlations between free testosterone serum level and other study subjects’ parameters included in this investigation, such as age, blood glucose, PCOS, and body mass index.
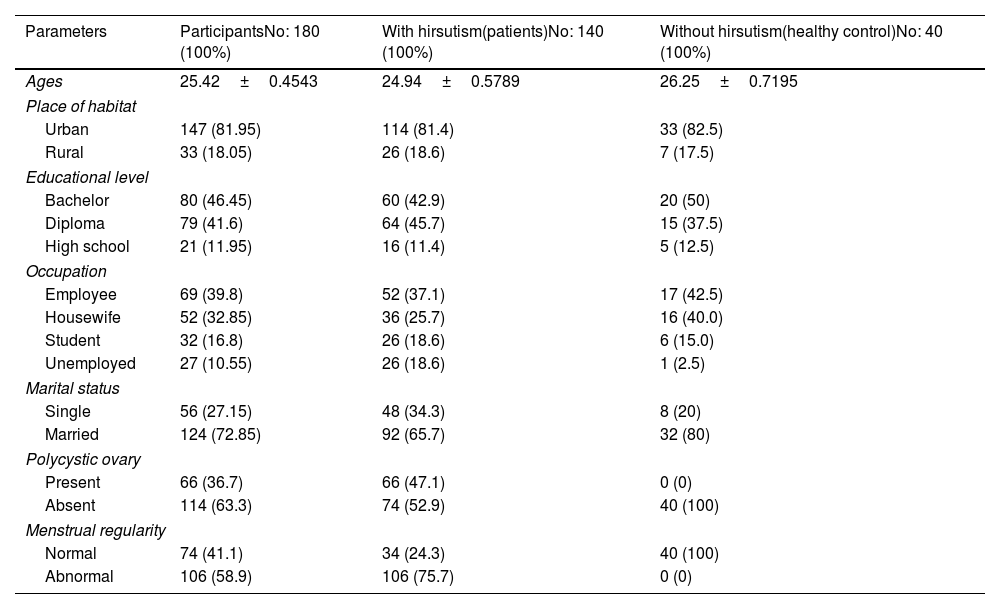
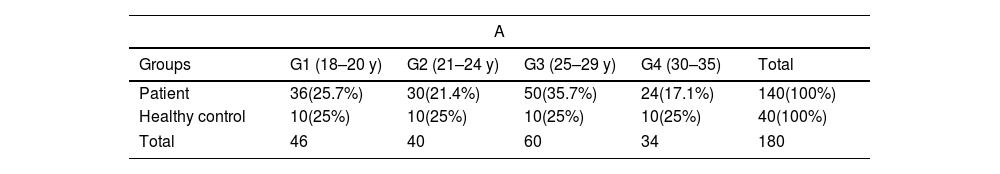
ResultsStudy subjectIn this study, one hundred forty blood samples were taken from women who suffered from hirsutism and who had attended gynecologist-led clinics in Soran city/Erbil-Iraq. They were compared with 40 age-matched healthy control women. The mean age of patient participants was (24.94±4.85), with an age range of 18–35. The prevalence of participants in urban areas was 114 (81.4%); this was more than those who lived in rural areas 26 (18.6%). The baseline demographics and clinical characteristics of hirsute women are given in Tables 1 and 2A. Moreover, when we compared the polycystic ovary cases in the BMI subgroups of patients (obese and overweight versus normal body weight), we found that all the obese women had PCOS, and the majority of the patients in the overweight sub-group had PCOS (100% vs 21.4%), and (82.6% vs 21.4%), respectively. In the present study, the association between PCOS and BMI was found to be statistically significant at p<0.0001.
Distribution of study subjects according to demographic and clinical characteristics.
| Parameters | ParticipantsNo: 180 (100%) | With hirsutism(patients)No: 140 (100%) | Without hirsutism(healthy control)No: 40 (100%) |
|---|---|---|---|
| Ages | 25.42±0.4543 | 24.94±0.5789 | 26.25±0.7195 |
| Place of habitat | |||
| Urban | 147 (81.95) | 114 (81.4) | 33 (82.5) |
| Rural | 33 (18.05) | 26 (18.6) | 7 (17.5) |
| Educational level | |||
| Bachelor | 80 (46.45) | 60 (42.9) | 20 (50) |
| Diploma | 79 (41.6) | 64 (45.7) | 15 (37.5) |
| High school | 21 (11.95) | 16 (11.4) | 5 (12.5) |
| Occupation | |||
| Employee | 69 (39.8) | 52 (37.1) | 17 (42.5) |
| Housewife | 52 (32.85) | 36 (25.7) | 16 (40.0) |
| Student | 32 (16.8) | 26 (18.6) | 6 (15.0) |
| Unemployed | 27 (10.55) | 26 (18.6) | 1 (2.5) |
| Marital status | |||
| Single | 56 (27.15) | 48 (34.3) | 8 (20) |
| Married | 124 (72.85) | 92 (65.7) | 32 (80) |
| Polycystic ovary | |||
| Present | 66 (36.7) | 66 (47.1) | 0 (0) |
| Absent | 114 (63.3) | 74 (52.9) | 40 (100) |
| Menstrual regularity | |||
| Normal | 74 (41.1) | 34 (24.3) | 40 (100) |
| Abnormal | 106 (58.9) | 106 (75.7) | 0 (0) |
(A) Distribution of study subjects according to age groups. (B) Pearson's correlation values for the relationships among FT, BMI, age, and glucose level in female patients.
| A | |||||
|---|---|---|---|---|---|
| Groups | G1 (18–20 y) | G2 (21–24 y) | G3 (25–29 y) | G4 (30–35) | Total |
| Patient | 36(25.7%) | 30(21.4%) | 50(35.7%) | 24(17.1%) | 140(100%) |
| Healthy control | 10(25%) | 10(25%) | 10(25%) | 10(25%) | 40(100%) |
| Total | 46 | 40 | 60 | 34 | 180 |
| B | ||||||
|---|---|---|---|---|---|---|
| Free testosterone | Female patients with PCOS | Female patients without PCOS | ||||
| BMI | Age | Glucose | BMI | Age | Glucose | |
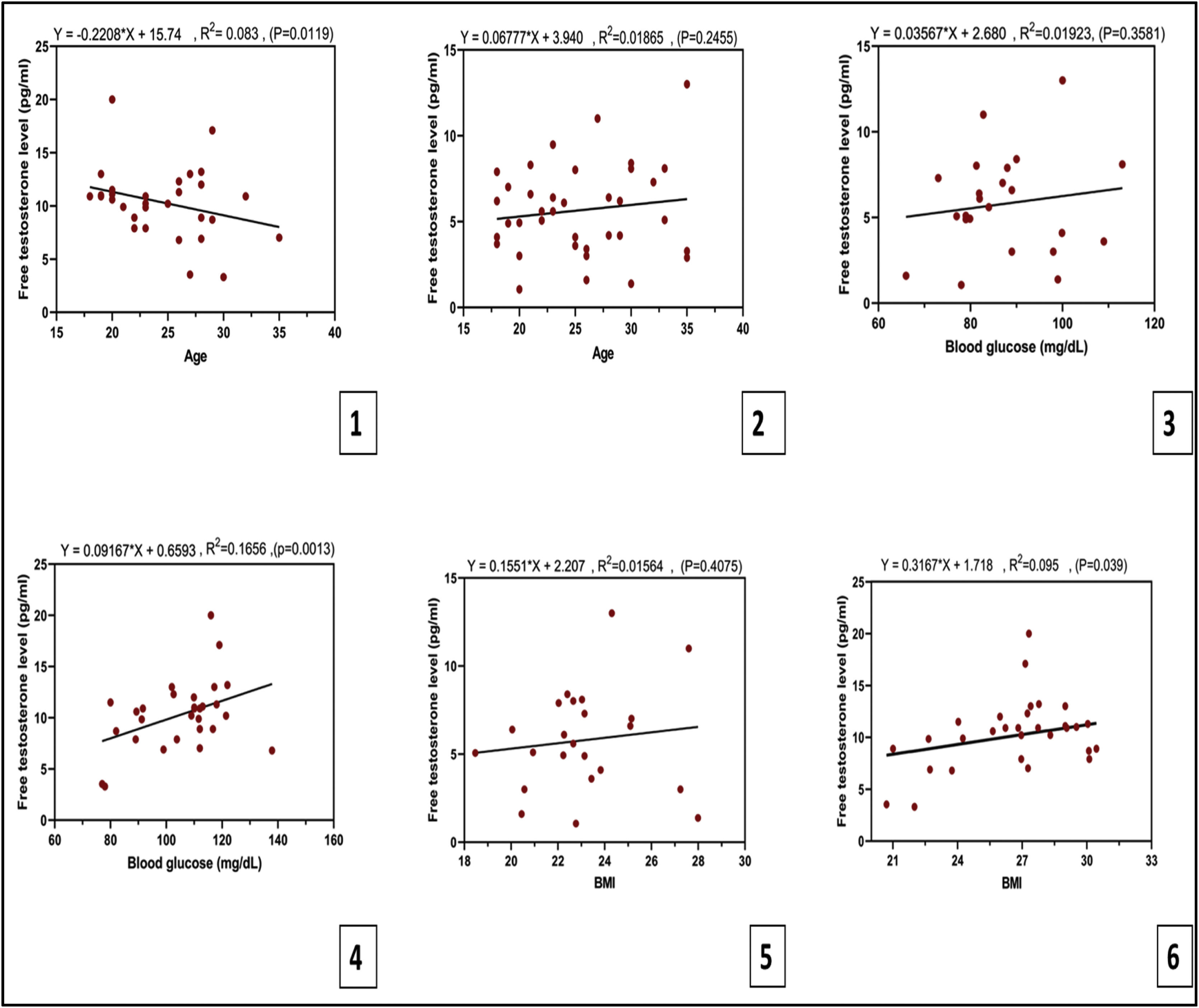
| r square | 0.3076 | −0.308 | 0.4069 | 0.1251 | 0.1367 | 0.1387 |
| p value | 0.0398* | 0.0119* | 0.0013* | 0.4075 | 0.2455 | 0.3581 |
The results showed a significant increase in free testosterone level with a value of 7.8±0.4pg/mL in the patient group compared to the healthy control, which was recorded as 3.8±0.2pg/mL (p<0.05), as illustrated in Fig. 1A. Further analysis showed that in the hirsute patient, there were significant differences between the mean of PCOS (10.3±0.6) and the mean of healthy women without PCOS (5.7±0.4). This study showed a significant increase in serum FT levels in the patient group with abnormal menstruation compared to patients with regular menstruation (p<0.05), as shown in Fig. 1A.
Serum-free testosterone levels and blood glucose in the control group versus patients women. (A) There are differences in serum-free testosterone levels between healthy control women and hirsute women with and without PCOS and normal and abnormal menstruation. (B) Free testosterone levels according to age groups of patients and control groups. (C) Free testosterone levels according to BMI of patient's women. (D) Fasting blood glucose of healthy control women compared to patients with and without polycystic ovaries (PCOS).
The present findings reported a significant elevation in FT levels in the patient groups compared to HC (p<0.05). Furthermore, a high level of FT was detected in the sera of the G1 group, followed by G2, G3, and G4, Fig. 1B.
Serum level of free testosterone according to body mass indexThe BMI values for each patient were calculated and categorized into three categories, normal weight (BMI=18.5–24.9), overweight (BMI=25–29.9), and obese (BMI>30). The results of this study showed a significant increase in serum FT levels in those patients in the overweight and obese categories (p<0.05), as shown in Fig. 1C.
Blood glucose levels in patients and control groupsThe patient's fasting blood glucose concentration was 96.1±1.8mg/dL, while that of the healthy control group was 80.2±0.7mg/dL. Additionally, the mean blood glucose value of the PCOS patients was 104.8±2.6mg/dL, whereas those patients with no PCOS had a value of 88.3±1.8mg/dL. Thus, there was a highly significant difference between the mean fasting blood glucose in healthy women and patient groups with PCOS. In contrast, there was no significant difference between the means of fasting blood glucose in the healthy control group and the patients without PCOS, Fig. 1D.
Pearson's correlation between age, BMI and blood glucose with free testosteroneThe correlation between the free testosterone values and the age of the hirsute patients’ group is shown in Table 2B and Fig. 2A and B. A significant negative (inverse) Pearson's correlation was observed between the serum level of FT and the age of the patients with PCOS. Contrastingly, a non-significant weak positive correlation was detected between the mean of FT and the ages of patients without PCOS.
(A) A significant negative Pearson's correlation between the serum level of FT and the age of the patients with PCOS. (B) A non-significant positive Pearson's correlation between the level of FT and the ages of patients without PCOS. (C) Significant positive Pearson's correlation between the level of FT and the BMI of the patients with PCOS. The Pearson's correlation=0.3.07. (D) There is a non-significant weak positive Pearson correlation between FT levels and the BMI of the patient without PCOS. Pearson's correlation=0.125.
Regarding the correlation between FT levels and BMI, a significant positive Pearson's correlation (0.307) was recorded between the level of FT and the BMI of the patients with PCOS. In addition to that, a non-significant weak positive Pearson correlation was observed between FT levels and the BMI of the patients without PCOS with a Pearson's correlation score of 0.125, as shown in Table 2B and Fig. 2C and D.
Furthermore, a significant moderate positive Pearson's correlation of 0.4821 was noticed between serum FT levels and blood glucose in the patient group having PCOS, and a non-significant weak positive Pearson's correlation of 0.138 was found between serum FT levels and blood glucose of the patient group without PCOS. Table 2B and Fig. 3A and B summarize the correlations.
Linear regressionIn linear regression, the present study showed that age was significantly and negatively associated with FT levels in women with PCOS, while it correlated-non significantly with FT in patients without PCOS. Also, a non-significant positive association was found between blood glucose and FT in women without PCOS. Whereas, a significant positive association was observed between blood glucose and FT in patients with PCOS. Moreover, non-significant association between BMI and FT in women without PCOS. In contrast, a significant positive association between BMI and FT was found in women with PCOS (Fig. 4).
A linear regression models. 1. Age was significantly and negatively associated with FT levels in women with PCOS. 2. Age correlated-non significantly with FT in patients without PCOS. 3. Non-significant positive association between blood glucose and FT in women without PCOS. 4. Significant positive association between blood glucose and FT in patients with PCOS. 5. Non-significant association between BMI and FT in women without PCOS. 6. Significant positive association between BMI and FT in women with PCOS. A p value <0.05 is statistically significant.
Hirsutism is extreme terminal and coarse hair that appears in a male-like pattern: this is different to hypertrichosis which is a diffuse growth in hair follicles. Hirsutism results from the high amounts of circulating androgen and the conversion of testosterone to dihydrotestosterone under the action of 5α-reductase in the pilosebaceous unit. This process transforms the thin, less visible vellus hair into terminal hair via androgen receptor activation.18
Dismally, hirsutism is the major gynecologic problem that Iraqi women who were of childbearing age facing this phenomenon. It is suggested that this issue, in addition to psychological factors are also might be associated with low diet quality and environmental disorders.19
This study revealed a significantly increased free testosterone level of 7.8±0.4pg/mL in the hirsute women compared to the healthy control. A similar result was observed among Filipino women. They concluded that Hirsute women are more likely to have elevated free testosterone than non-hirsute patients.20 Moreover, high serum-free testosterone is considered a possible indicator of hyperandrogenic syndrome.17 The increased level of total testosterone or free testosterone is a critical diagnostic biochemical marker of hyperandrogenism. Genes like CYP11, CYP17, and CYP19 are overexpressed in the ovarian theca cells, leading to the amplified production of 17-hydroxyprogesterone, testosterone, and androstenedione, decreasing the activity of aromatase and further upregulating the androgen production.2
The current study revealed that 47.1% of hirsute women have PCOS, and 53% do not. This finding is inconsistent with the results that hirsutism is more frequent in women with PCOS than in other patients.20
The present work demonstrated that overweight and obese patients expressed high levels of FT compared to normal-weight patients. Our results showed a significant increase in serum FT levels in the patient groups who had a high BMI (overweight or obese) (p<0.05). Moreover, this study observed a significant moderate positive Pearson's correlation between the level of FT and BMI in the patient group having menstrual abnormalities with PCOS. This finding agrees with Gilbert et al., they found out that, PCOS is closely related to metabolic disorders such as obesity and insulin resistance. Many women with PCOS are obese or overweight.21
Women with PCOS reveal increased circulating levels of luteinizing hormone, which stimulates the ovarian theca cells to secrete androgens. Elevated levels of this hormone will also stimulate androgen synthesis in ovarian theca cells resulting in hyperandrogenemia and as aforementioned, this manifests as hirsutism, acne and alopecia: all of which are common symptoms in women afflicted with PCOS.22
Khomami et al. found that 68% of women with PCOS exhibited hirsutism. Other studies have documented that hirsutism had the most substantial impact on the health and the QoL of Iranian women suffering from PCOS.23
Previous results have shown that calculated values of free testosterone help evaluate hirsutism scores in all hirsute women, despite ovarian or adrenal hyperandrogenemia.24
In humans, four structurally different binding proteins link to the circulating testosterone. Sex hormone-binding globulin (SHBG) has attracted light due to its high binding affinity for testosterone. These binding proteins provide tissue bioavailability and metabolic clearance percentage of testosterone by regulating the quantity of free testosterone available for biological activity in human tissue. The circulating testosterone not bound to any plasma protein is called free testosterone.9
It is important to mention that hirsutism is just one indication of hyperandrogenism while assessing it. Menstrual abnormalities, infertility, obesity, and other symptoms linked with androgen excess are commonly reported in patients, and diagnosis is usually a clinical one rather than through laboratory investigations. The results of high serum levels of FT were also linked with mood problems; Stanikova et al., reported that premenopausal women with depressive symptoms showed higher free testosterone levels than non-depressed women.25 Additionally, data from a prospective survey documented that serum testosterone is pivotal in initiating and developing PCOS and PCOS-associated kidney fibrotic injury in PCOS women.26
In terms of hirsute women and their blood glucose level, our findings discriminate between patients with polycystic ovaries and those without this finding. The first group showed a high glucose level of 104.8mg/dL compared to the second group who had a mean level of 88.3mg/dL. As well as that, the Pearson correlation results proved a significant positive correlation was observed (0.406) between the free testosterone levels and glucose levels in women with polycystic ovaries and hirsute women compared to the women patients without PCOS. In a previous study, Zhu et al., pointed out that women with the PCOS and hyperandrogenemia had a higher and more considerable mean glucose reading, which may place them at an increased risk of developing type 2 diabetes mellitus T2DM).27 In a recent review, Livadas et al., revised the data on the epidemiology of dysglycemia in PCOS and its pathophysiology.28 They concluded that the association of PCOS with increased T2DM risk is relatively strong; therefore, it should not be ignored in any women with these complications. They advised that patients should be told to maintain an optimal weight through a healthy diet coupled with exercise and that menopausal females with a history of PCOS should be repeatedly checked for T2DM since they are, perhaps, at a higher risk, particularly if they are overweight or obese.28
PCOS is closely related to metabolic disorders. A recent study emphasizes that circulating galactose levels positively correlate with PCOS and PCOS-associated insulin resistance and can be proposed as a potential biomarker for this disease diagnosis.29 The linear regression analysis of the present data revealed that age was significantly and negatively associated with FT levels in women with PCOS, Also, a significant positive association was observed between both blood glucose and BMI with FT in patients women with PCOS. These findings were in line with the results of the Pearson correlation. A recent study concluded that BMI was one of the influencing factors of testosterone levels.30
The limitation of this work is the use of enzyme-linked immunosorbent assay in the hormonal assessment due to the instrument's accessibility and the absence of liquid chromatography–tandem mass spectrometry which possesses superior specificity. Moreover, insulin assessment considers another conflicting point. Therefore, we will take it into account in future studies to validate the present results. Generally, the lack of funding to support our study was also a conflict.
ConclusionThis study was conducted on hirsute females who suffered from the appearance of excessive hair on their chin compared to healthy control ones. The present findings revealed a significant elevation in serum FT levels in hirsute women and this positively correlated with the BMI and glucose levels in those patients. There was a significant moderate positive Pearson's correlation between the level of FT and the BMI in the patient group having menstrual abnormalities and PCOS. Moreover, the glucose values of patients with PCOS correlated significantly with their testosterone levels. Therefore, it could be assumed that glucose level may serve as a potentially effective biomarker in evaluating the severity of hirsutism disease in these women. Further investigations need to be conducted to validate these findings.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Patient's consentThe authors declare that this article has been conducted according to international and local guidelines ensuring an ethically conducted research. The patient's privacy has been respected; the patient's identity is completely removed in this article research.
FundingThe authors received no specific funding for this manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank the gynecological clinic staffs in Soran city for their assistant to perform this study.