Reduction of cardiovascular events in patients with hypertriglyceridaemia
More infoIn addition to low-density lipoproteins (LDL), those containing apolipoprotein (Apo) B and with a diameter less than 70 nm, including the smaller triglyceride-rich lipoproteins, remnant particles, and lipoprotein(a), may independently contribute to atherosclerosis because they also cross the endothelium and penetrate the arterial intima. Although mild/moderate hypertriglyceridemia is a recognized vascular risk factor, only two studies, the Japan EPA Lipid Intervention (JELIS) and the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT), using pure eicosapentaenoic acid (EPA) or icosapent ethyl (IPE), the stable ethyl ester of EPA, respectively, rather than a combination of docosahexaenoic acid (DHA) and EPA, have demonstrated a reduction in the rate of cardiovascular events. For this reason, it was deemed appropriate to examine the implications and applicability of the REDUCE-IT study in real-life settings. This analysis suggests a transversal therapeutic approach, based on both LDL cholesterol and triglycerides, for patients at very high cardiovascular risk to achieve an effective prevention. Furthermore, among patients in secondary prevention, treatment with IPE should focus on those with the highest vascular risk (recent acute coronary syndrome, post-infarction, angioplasty, and coronary bypass grafting).
Además de las lipoproteínas de baja densidad (LDL), aquellas que contienen la apolipoproteína (Apo) B y un diámetro inferior a 70 nm, incluidas las lipoproteínas ricas en triglicéridos más pequeñas, las partículas remanentes y la lipoproteína(a), pueden contribuir de forma independiente a la aterosclerosis, ya que también atraviesan el endotelio y penetran en la íntima arterial. Si bien la hipertrigliceridemia leve/moderada es un reconocido factor de riesgo vascular, sólo dos estudios, el Japan EPA Lipid Intervention (JELIS) y el Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT), utilizando ácido eicosapentanoico (EPA) puro, o icosapento de etilo (IPE), éster etílico estable del EPA, respectivamente, en lugar de una combinación de ácido docosahexanoico (DHA) y EPA, han demostrado descensos en la tasa de episodios cardiovasculares. Por dicho motivo se ha considerado oportuno examinar las implicaciones y la aplicabilidad del estudio REDUCE-IT en la vida real. El presente análisis da pie a proponer una planificación terapéutica transversal, basada tanto en colesterol LDL como en triglicéridos, en los pacientes de muy alto riesgo cardiovascular para conseguir una prevención eficaz. Es más, de los pacientes en prevención secundaria, el tratamiento con IPE debe focalizarse en aquellos con mayor riesgo vascular (síndrome coronario agudo reciente, post-infarto, angioplastia y baipás coronario).
Three aspects of great clinical significance must be highlighted from what has been discussed in the previous chapters of this monograph in order to address the issue at hand. Firstly, in addition to low-density lipoproteins (LDL), other lipoproteins containing apolipoprotein (Apo) B with a diameter of less than 70 nm, including smaller triglyceride-rich lipoproteins, remnant particles and lipoprotein(a), can contribute independently to atherosclerosis because they also cross the endothelium and penetrate the arterial intima. Triglyceride-rich lipoproteins may have difficulty leaving the intima due to their size or they may become trapped by components of the intima. While LDL requires modification to be absorbed by macrophages, remnant particles are taken up by members of the LDL receptor family in their native state. Furthermore, the hydrolysis of triglycerides by lipoprotein lipase from remnant particles increases the inflammatory response of macrophages. Secondly, both mild-moderate and severe hypertriglyceridaemia are associated with an increased cardiovascular risk (CVR) and mortality in the general population. Similarly, an increased CVR has been documented in patients with cardiovascular disease (CVD) when triglycerides are elevated. Conversely, not all omega-3 fatty acids have cardiovascular benefits. Early studies such as the Diet and Reinfarction Trial (DART)1 and the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione2 aimed to reduce all-cause mortality by 29% and 14%, respectively, in survivors of myocardial infarction. Since then, numerous intervention studies with omega-3 fatty acids have been conducted in both primary and secondary prevention, but most have yielded null results.3 However, two studies - the Japan EPA Lipid Intervention (JELIS)4 and the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT),5 used pure eicosapentaenoic acid (EPA) and or icosapent ethyl (IPE), a stable ethyl ester of EPA, respectively, instead of a combination of docosahexaenoic acid (DHA) and EPA, and showed reductions in the rate of cardiovascular events.
While the mechanisms by which IPE exerts its cardioprotective effects are complex, it is agreed that it reduces concentrations of triglycerides, remnant particles, oxidised LDL, and Apo CIII at the lipid level. It may also favourably modify the functions of high-density lipoproteins (HDL) and the endothelium. Anti-inflammatory and antioxidant properties, increased mediators such as resolvins, protectins, and maresins, antithrombotic effects, and membrane stabilisation have also been proposed as possible mechanisms to explain the cardiovascular results.6 The Effect of Vascepa on Improving Coronary Atherosclerosis in People with High Triglycerides Taking Statin Therapy (EVAPORATE)7 study validated the beneficial effects of IPE on the composition and regression of coronary atheromatous plaque in patients receiving active treatment compared to those receiving a mineral oil placebo. Necropsy and imaging studies have shown that EPA enrichment in plaque reduces necrotic core volume, lipid accumulation and cholesterol crystal formation. Therefore, it can be concluded that IPE has a significant atheroprotective impact.
Implications and applicability of the REDUCE-IT studyIn the REDUCE-IT study,5 IPE (4 μg/day) has been shown to effectively reduce cardiovascular morbidity and mortality in patients with CVD and/or type 2 diabetes mellitus (T2DM) with at least one CV risk factor and LDL cholesterol levels <100 mg/dl and triglycerides <500 mg/dl. These results were consistent across all baseline triglyceride tertiles, regardless of LDL cholesterol concentrations.8 At this point, it should be noted that the 8,179 patients in the REDUCE-IT study had a mean baseline LDL cholesterol concentration of 74 mg/dl, given that all were on statin therapy based on the LDL cholesterol inclusion criterion. Furthermore, the efficacy of IPE was greater than expected, given the 18.3% reduction in triglycerides. This finding reinforces the concept that IPE has effects beyond triglyceride lowering. With a median follow-up period of 4.9 years, the risk of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, unstable angina, or coronary revascularisation was reduced by 25% in the IPE group, as was the secondary endpoint of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke.5 This meant that the number needed to treat (NNT) to prevent one cardiovascular event at 5 years was 21; moreover, in the population with acute coronary syndrome alone, the NNT at 5 years was 11.9
In the recent the Randomized Trial for Evaluation in Secondary Prevention Efficacy of Combined Therapy–Statin and Eicosapentaenoic Acid (RESPECT-EPA),10 treatment with IPE (1.8 g/d) significantly reduced the rate of cardiovascular death, nonfatal myocardial infarction, non-fatal stroke, unstable angina, or revascularization in patients with chronic coronary artery disease and an EPA/arachidonic acid ratio <.4 who were receiving statin therapy. However, IPE showed a significant 27% reduction in coronary events (secondary endpoint). As the authors indicate, the study was not sufficiently powered, despite two protocol modifications to increase the sample size and follow-up period. It should be noted that the dropout rates were much higher than in the JELIS4 and REDUCE-IT studies.5
Despite the accessibility and availability of a new cardiovascular drug, a frequent barrier to translating evidence from randomised controlled trials into clinical practice is establishing the applicability of the results obtained. There are numerous determinants of the external validity of such clinical trials. Among these, both patient selection and characteristics are fundamental. Therefore, it is important to assess the applicability of the REDUCE-IT population in cohorts of patients with CVD or equivalent risk. Table 1 shows the main eligibility studies of IPE in different cohorts and registries. On average, between 15% and 20% of patients with persistent CVR could benefit from this treatment.11–22 The variability in eligibility for IPE observed in these studies depended on various factors, including whether the studies included highly selected population groups versus broader populations, which and how many of the REDUCE-IT criteria, regulatory frameworks, or guidelines were used, and the type of cardiovascular prevention (primary, secondary, or both). It would be unfair not to mention here that one of the most common reasons for ineligibility for IPE was an LDL cholesterol concentration >100 mg/dl, suggesting that high-intensity lipid-lowering strategies remain underutilised in patients with high/very high CVR.
Main eligibility studies of icosapent ethyl in different cohorts and registries.
| Cohort/registry | Population | Sample size | Eligibility criteria REDUCE-IT (5) |
|---|---|---|---|
| CLARIFY (11) | Stable IHD | 24,146 | CVD: 15.5% |
| REACH (12) | Age ≥45 years with CVD or ≥3 CVRF | 62,464 | CVD: 11.2% T2DM: 12.3% |
| US Veterans Affairs (13) | CVD and age ≥45 years. T2DM without CVD and age >50 years | 1,695,750 | CVD: 14.5% T2DM: 17.1% |
| NHANES 1996-2016 (14) | Not hospitalised and age >20 years | 21,548 | 2.8% |
| CANHEART (15) | Established CVD | 196,717 | CVD: 25.4% |
| Québec Heart Database (16) | Coronary artery bypass | 12,641 | CVD: 21.9% |
| MESA, CARDIA, Dallas Heart, and Heinz Nixdorf Recall (17) | Hypertriglyceridaemia without CVD | 2,345 | 17%a |
| VERTIS-CV (18) | T2DM and CVD | 8,246 | CVD + T2DM: 29.6% |
| EMPA-REG (19) | T2DM and CVD | 7,020 | CVD + T2DM: 25.8% |
| FAST-MI (20) | AMI | 9,459 | CVD: 12.5% |
| Western Denmark Heart (21) | Non-urgent symptoms of IHD in patients undergoing angio-CT | 23,759 | CVD: 9% |
| Tertiary Hospital Australia (22) | Coronary artery bypass | 484 | CVD: 25.6% |
AMI: acute myocardial infarction; CT: Computerised tomography scan; CVD: cardiovascular disease; CVRF: cardiovascular risk factor; IHD: ischaemic heart disease; T2DM: type 2 diabetes mellitus.
On the other hand, cost-effectiveness studies have shown that IPE is more advantageous in secondary prevention, although several found it to be cost-effective in both clinical settings. Differences in the cost-effectiveness of IPE between studies are largely attributed to medication costs and the models used.23,24
Given the budgetary constraints of healthcare, identifying which populations may be priorities for treatment with IPE is a critical consideration. Table 2 shows subgroups of patients with CVD who were treated with IPE and who, due to their higher CVR, showed a greater reduction in the absolute risk of cardiovascular events and, therefore, lower NNT.5,9,25–27
Subgroups of patients with cardiovascular disease treated with icosapent ethyl according to absolute risk reduction in cardiovascular events.
| Primary objective | |||||
|---|---|---|---|---|---|
| Substudy | Icosapent ethyl | Placebo | RR | ARR | NNT4,9 years |
| Global (5) | 705/4,098 (17.2) | 901/4,090 (22.0) | .75 (.68−.83) | 4.8 | 21 |
| Recent ACS (9) | 81/433 (18.7) | 114/407 (28.0) | .63 (.48−084) | 9.3 | 11 |
| Post-angioplasty (25) | 362/1,737 (20.8) | 491/1,671 (29.4) | .66 (.58−.76) | 8.5 | 12 |
| Post-bypass (26) | 179/897 (22.0) | 265/940 (28.2) | .76 (.63−.92) | 6.2 | 16 |
| Post-MI (27) | 378/1,870 (20.2) | 475/1,823 (26.1) | .74 (.65−.85) | 5.9 | 17 |
ACS: acute coronary syndrome; ARR: absolute risk reduction; MI: myocardial infarction; NNT: number needed to treat; RR: risk ratio.
Around thirty scientific societies have included IPE in their clinical practice guidelines, recommending it for patients with high/very high cardiovascular risk (CVR) and residual hypertriglyceridaemia who are being treated with statins. These recommendations largely reflect the inclusion criteria of the REDUCE-IT study5; however, some organisations restrict its use to individuals with cardiovascular disease (CVD).
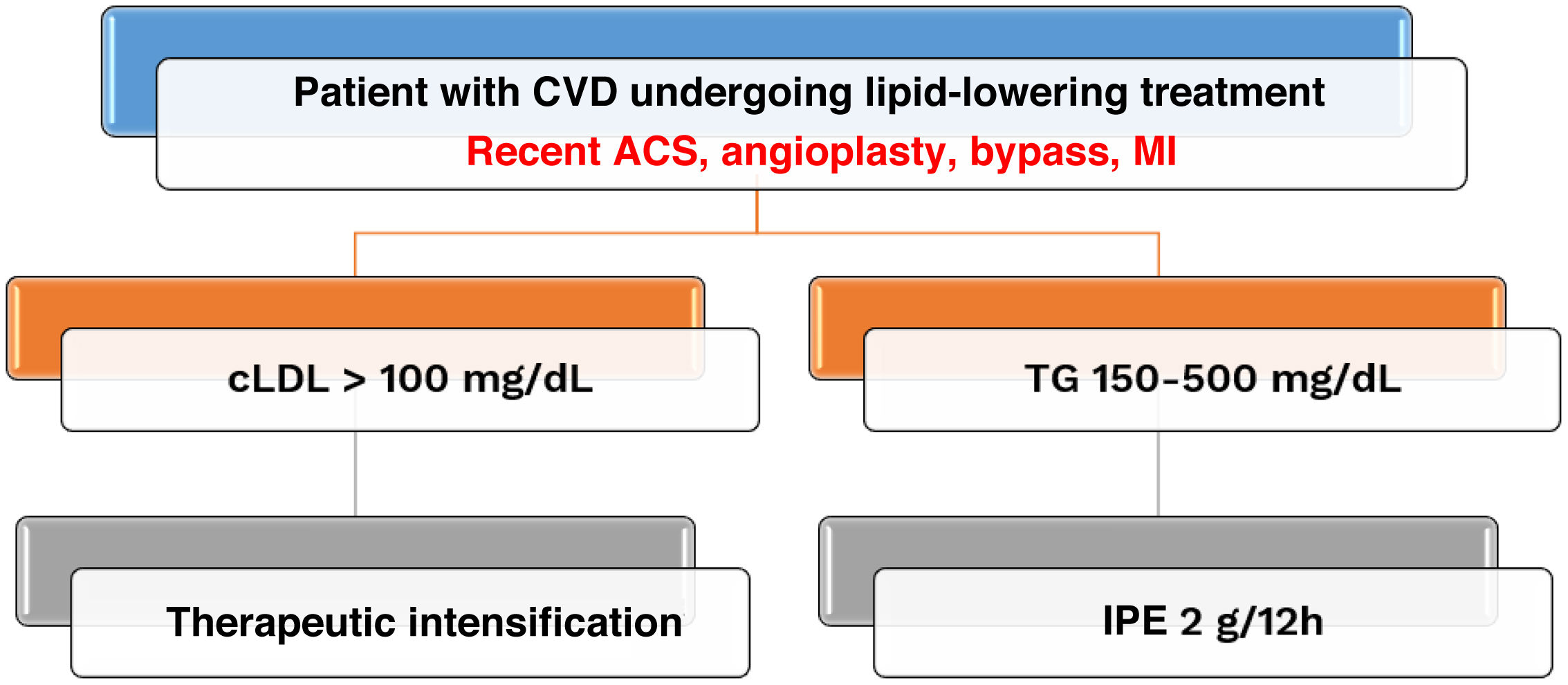
To optimise the indication for IPE, two key aspects of secondary prevention must be considered. When treating patients at very high CVR, time is of the essence and, therefore, we must move from sequential to cross-sectional therapeutic planning. Following this line of reasoning, it is not advisable to wait until LDL cholesterol levels reach <100 mg/dl before starting treatment with IPE when triglyceride levels are between 150 and 500 mg/dl. Furthermore, IPE reduced the rate of cardiovascular events in patients treated with statins with high CVR and elevated triglycerides, regardless of baseline LDL cholesterol.5 The large reduction in cardiovascular targets recently achieved in patients with LDL cholesterol <55 mg/dl,28 suggests that IPE should be considered for cardiovascular prevention in patients with very high CVR even when LDL cholesterol is optimally controlled. Therefore, a cross-sectional approach should be adopted, and therapeutic targets for LDL cholesterol and triglycerides should be pursued simultaneously. Secondly, according to the therapeutic positioning report issued by the Spanish Association of Medicines and Health Products,29 IPE is only funded for patients with CVD. This therapeutic strategy should focus on patients with recent acute coronary syndrome, or who have undergone angioplasty, coronary artery bypass surgery, or post-myocardial infarction, as these subgroups have a higher CVR and have experienced reductions in absolute risk greater than 5. These considerations are reflected in the therapeutic algorithm in Fig. 1.
Therapeutic algorithm in patients with cardiovascular disease: towards cross-sectional planning in high-risk subgroups. ACS: acute coronary syndrome; CVD: cardiovascular disease; IPE: icosapent ethyl; LDL-C: Low-density lipoprotein cholesterol; MI: myocardial infarction; TG: triglycerides.
In addition to LDL, other Apo B-containing particles such as triglyceride-rich lipoproteins and remnant particles may contribute independently to the atherogenic process. Even when LDL cholesterol concentrations are within the target range, incorporating IPE into lipid-lowering strategies can further reduce cardiovascular events. For this reason, a cross-sectional therapeutic plan (targeting LDL and TG) is proposed in patients at very high CVR in order to achieve effective prevention. Furthermore, IPE treatment among patients in secondary prevention should focus on those with a higher CVR (recent acute coronary syndrome, post-infarction, angioplasty, and coronary bypass). It is important to note in this clinical setting that the benefits of IPE cannot be generalised to other omega-3 fatty acid formulations.
FundingThis study was sponsored by the Spanish Society of Arteriosclerosis with funding from Amarin, which did not participate in the design or preparation of this manuscript.
Information about the supplementThis article is part of the supplement entitled “Reduction of cardiovascular events in patients with hypertriglyceridaemia,” which was sponsored by the Spanish Society of Atherosclerosis, with funding from Amarin.
The author has no conflict of interest to declare.