A moderate level of physical activity (PA), such as a daily 30-min walk, reduces cardiovascular risk. There is a lack of evidence about the cardiovascular benefits of PA below this recommendation of minimum PA level.
ObjectiveWe aimed to study the impact of a lower level of PA on cardiovascular health.
DesignSixty-four overweight/obese men and women were enrolled in a community programme consisting of 4 months of 1h, low-intensity PA two days per week. Before and after the intervention, PA level (METs/h/wk), endogenous antioxidant status (SOD and GPX concentration and activity and oxidised LDL), ADMA concentrations, endothelial function by small artery reactive hyperaemia index (saRHI), and resting heart rate (RHR) were assessed.
ResultsAfter the intervention, significant increases in saRHI (P=0.031), SOD and GPX activities, and a decrease in ADMA plasma concentrations, and RHR (P<0.001 for all) were observed. Increases in PA were positively associated with increases in saRHI (r=0.341, P=0.022), GPx (r=0.303, P=0.047) and decreases in RHR (r=−0.302, P=0.047). Multivariate analyses showed that independent predictors of saRHI improvement were an increase in PA (2.65, 95%CI: 1.21–4.01), decrease in RHR (1.91, 95%CI: 1.01–4.98), and an increase in GPx (2.61, 95%CI: 1.16–5.01).
ConclusionIn obese and overweight men and women, an increase in PA, even below the minimal international recommendations, improves antioxidant capacity, RHR and peripheral small artery reactivity.
Los niveles moderados de actividad física (AF), 30min al día de caminar, reducen el riesgo cardiovascular. No existe evidencia si los niveles bajos de actividad física, por debajo de las recomendaciones internacionales, afectan la salud cardiovascular.
ObjetivoEstudiar el efecto de los niveles bajos de actividad física sobre la salud cardiovascular
DiseñoSe seleccionaron 64 hombres y mujeres con sobrepeso u obesidad para completar un programa comunitario de actividad física consistente en 1h, 2días a la semana, durante 4meses, de AF de intensidad baja. Antes y después del programa se evaluó la AF (MET/h/semana), el estado antioxidante endógeno (SOD y GPX concentración y actividad), las concentraciones de ADMA, la función endotelial de pequeña arteria mediante el índice de hiperemia reactiva (saRHI) y la frecuencia cardíaca (FC) en reposo.
ResultadosDespués de la intervención se observó un aumento significativo en el saRHI (p=0,031), en la actividad de la SOD y la GPX, y una disminución de las concentraciones plasmáticas de ADMA y de la FC (p<0,001 para todos). El aumento en la AF se asoció directamente con el aumento del saRHI (r=0,341, p=0,022), GPx (r=0,303, p=0,047) y disminución en FC (r=–0.297, p=0,047). Los predictores independientes de la mejora del saRHI fueron un aumento en la AF (2,65; IC95%: 1,21-4,01), la disminución de la FC (1,91; IC95%:1,01-4,98) y el aumento de la GPx (2,61; IC95%:1,16-5,01).
ConclusionesUn aumento de la AF, incluso por debajo de las recomendaciones internacionales de AF, mejoró la capacidad antioxidante, la FC y la función endotelial de las pequeñas arterias en hombres y mujeres con sobrepeso u obesidad.
Regular physical activity (PA) is associated with a reduced risk of cardiovascular events and is therefore a central component in primary and secondary prevention of cardiovascular disease.1,2 Several major scientific bodies currently recommend and encourage a minimum of 2.5–5h/week of physical activity or aerobic exercise training of at least moderate intensity or 1–2.5h/week of vigorous intensity exercise.3,4
Among the benefits on classical cardiovascular risk factors, increasing physical activity modulates oxidative stress and nitric oxide (NO) bioavailability, both of which contribute to improved endothelial function and reduced cardiovascular risk.3–6 It is known that sedentary men and women have impaired endothelium-dependent dilatation. Endothelial function is carefully controlled, mainly by the bioavailability of NO and sympathetic and parasympathetic drive reflexes.7 NO bioavailability is in part the result of the balance between the production and the neutralisation of reactive oxygen species (ROS). In this context, increased concentrations of asymmetrical dimethylarginine (ADMA), an endogenous l-arginine analogue, may uncouple endothelial NO synthase (eNOS), which impairs NO production but also increases superoxide production in the vascular endothelium.8 Human and animal data show a clear association between ROS production and the activation of the sympathetic drive, which may cause impairment of NO release and function.9,10 Although physical activity has a clear positive impact on cardiovascular risk, some types and levels of PA have been associated with increased oxidation in the short term and also the impact on endothelial function is controversial.11,12 Moreover, reported low adherence to physical activity interventions in large community-based studies reflects the difficulty of implementing a physical activity plan.13,14 This low adherence also contributes to the differences in the above mentioned studies. Engaging chronically sedentary groups who have physical boundaries such as ageing and obesity into a PA programme is rather difficult. A low intensity PA programme could increase adherence. However, the real impact of this level of PA on cardiovascular performance and intermediate factors for cardiovascular risk (CVR), such as oxidative stress and endothelial function, have not been assessed. In this work, we aim to determine whether implementing a low-intensity physical activity programme in a group of sedentary overweight/obese men and women has any impact on oxidative stress and small artery vascular function.
Materials and methodsDesign, population, clinical assessment and interventionThis is a nested study from a primary care community programme “Step by Step” involving all of the primary care centres in the city of Reus (Spain). Participants in this programme are men and women at increased cardiovascular risk or with established cardiovascular disease. The aim of this community programme is to boost a healthy lifestyle in people at increased cardiovascular risk. It consists of 1h low-intensity walking sessions twice a week. These sessions are directed by health care and physical education professionals. Before every session, participants signed an attendance list.
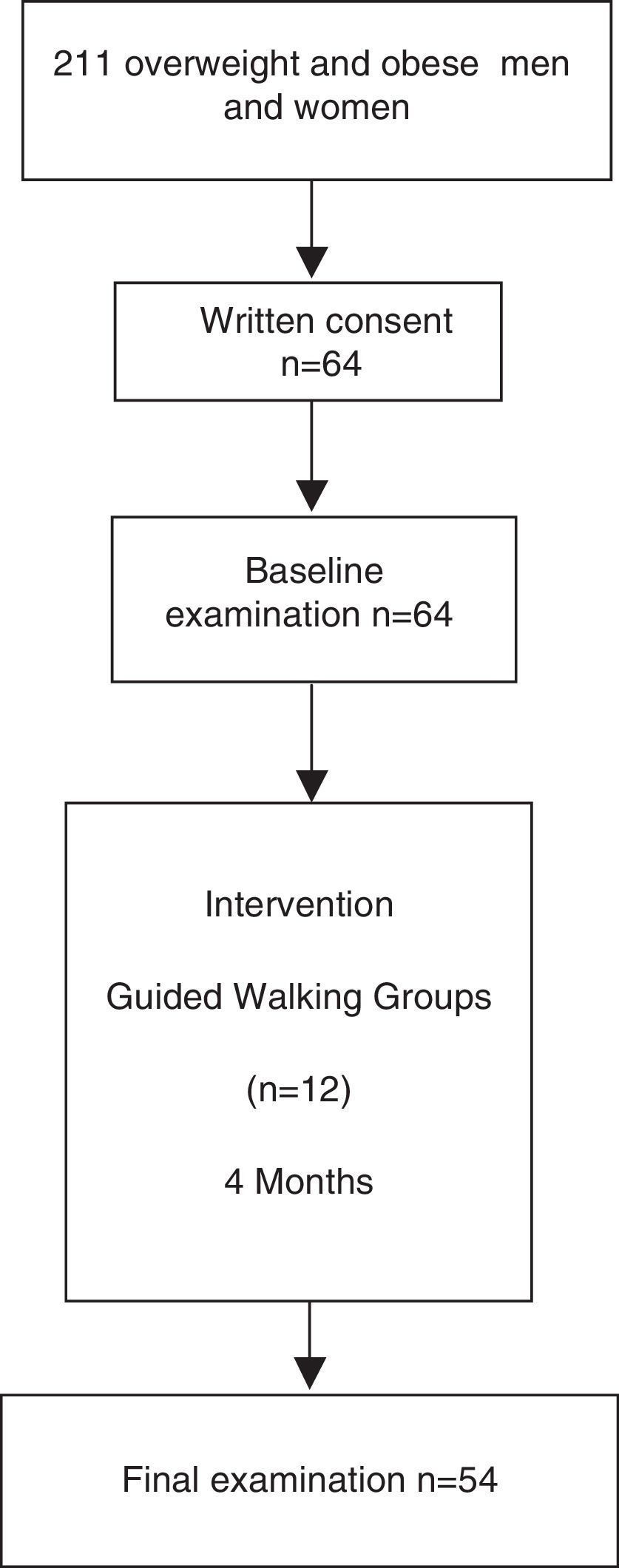
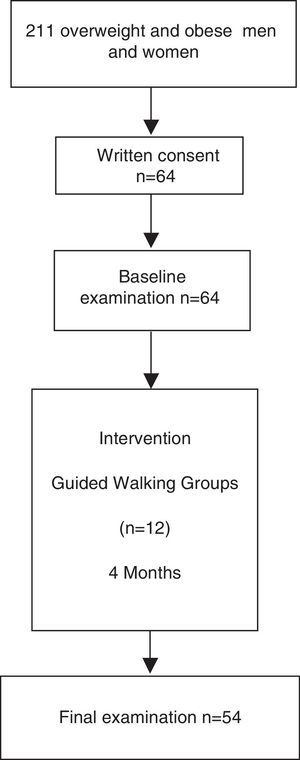
The inclusion criteria for the present study were men and women aged between 30 and 70 years with increased cardiovascular risk who were overweight, defined as body mass index between 25 and 30kg/m2, or obese, defined as a body mass index higher than 30kg/m2 or waist circumference higher than 102cm in men and 88cm in women as specified by the ATPIII clinical criteria for abdominal obesity. Exclusion criteria were as follows: unwillingness to cooperate in the study; severe disease that may interfere with the ability to comply with study protocol; shorten life expectancy; and patients diagnosed with neoplasia or cardiovascular disease. Out of 211 eligible volunteers of the Step by Step programme, sixty-four fulfilled the inclusion criteria and were included in this study.
A complete physical examination including lifestyle components (diet, physical activity and smoking), anthropometry assessment, and biochemical, abdominal fat and vascular studies (small artery reactive hyperaemia index, saRHI) were performed before and after the 4-month follow-up period. Leisure-time physical activity was quantified as Metabolic Equivalents (METs/week) using the Minnesota questionnaire adapted for the Spanish population.15 A validated semi-quantitative food frequency questionnaire was used to evaluate diet.16 Tobacco status was assessed using standardised questionnaires administered by heath care providers.17 The patients’ usual pharmacological treatment was maintained. The sample size considering an 80% power to detect a difference between means of 0.04 in saRHI, 0.02 in ADMA and 0.01 in SODe and Gpxe, with a level of significance (alpha) of 0.05 (two-tailed) was calculated regarding these conditions. The sample size required was 45 participants. This study was conducted according to the guidelines of the Declaration of Helsinki, and all procedures involving human patients were approved by the Ethical and Clinical Investigation Committee of the hospital. Written informed consent was obtained from all patients.
Resting heart rate and small artery function measurementResting heart rate (RHR) and saRHI were measured using peripheral artery tonometry (PAT) technology (EndoPAT-2000 device, Itamar Medical Ltd., Israel). Both measurements were performed in a quiet room with a controlled temperature (22–24°C) after patients had fasted for 12h and refrained from smoking or strenuous exercise for 24h. The patients lay in a relaxed, quiet and evenly illuminated environment while the device recorded changes in pulse waves in the digital arteries. The PAT technique has been described elsewhere.18 There is a 5min baseline measurement, after which the cuff is inflated enough above baseline systolic pressure to produce ischaemia. After 5min of occlusion, the cuff is rapidly deflated and the PAT tracing is recorded for an additional 5min. Blood flow measurements from two fingertips on different hands—one a test, the other a control—were compared after a stabilisation period. A second pair of measurements for comparison was taken before and after five minutes of brachial ischaemia in the test arm. The results were processed with specific software to calculate the post-ischaemia reflex vasodilatation observed when measurements from the test arm (before and after ischaemia) were compared to those from the control arm. The value generated was termed saRHI. Arterial stiffness, measured as augmentation index (AIx), was also determined during the same exploration and processed by specific software by analysing the differences in pulse wave amplitude before and after ischaemia in comparison to the control arm. We then calculated the AIx adjusted to 75 beats per minute variable (AIx@75).
Subcutaneous and visceral fat determinationA MyLab 50 X-Vision ultrasonograph (Esaote, Italy) equipped with a linear array ultrasound probe (7.5–12MHz) was used to assess body fat according to the ultrasound image review consensus.19 Briefly, the thicknesses of subcutaneous and preperitoneal fat were measured by placing the probe perpendicular to the skin on the epigastrium. The thickness of the subcutaneous fat is defined as the distance between the anterior surface of linea alba and the fat–skin barrier. The preperitoneal fat extends from the anterior surface of the liver (left lobe) to the posterior surface of linea alba.
Biochemical determinationsStandard biochemical parameters were determined via the usual methods. Glucose, cholesterol, triglyceride, apolipoprotein B100, apolipoprotein A1, direct LDL-cholesterol, HDL-cholesterol, and high-sensitivity C-reactive protein (hs-CRP) were measured using enzymatic and immunoturbidimetric assays (Spinreact, SA, Spain and Wako Chemicals GmbH, Germany) adapted to the Cobas Mira Plus autoanalyser (Roche Diagnostics, Spain). SOD and GPX plasma and erythrocyte lysate levels were determined by commercially available assays according to the manufacturer's instructions (Cayman Chemical Company, USA). ADMA and LDLox plasma levels were determined by commercially available immunoassays according to manufacturer's instructions (ADMA–ELISA, Immunodiagnostik AG, Germany; oxLDL-ELISA, Mercodia, Sweden). FABP4 levels were determined with commercial ELISA kits (Bio Vendor Laboratory Medicine Inc., Brno, Czech Republic).
Statistical analysesData are presented as the mean±SD or as the median and interquartile range for continuous variables and as frequencies for categorical variables. Normality of the distribution of variables was assessed with the Kolmogorov–Smirnov test. Differences between physical activity, anthropometric, biochemical or vascular data were analysed using the ANOVA or Kruskall–Wallis test for continuous variables or with the chi-squared test for categorical variables. Univariate association was tested by Spearman correlation analysis. A multiple stepwise logistic regression test was performed to assess the determinants of saRHI in our study group patients. The dependent variable of interest was the saRHI. Independent variables were selected based on univariate analysis and from the variables known to be associated with the dependent variable according our previous studies. In this test, independent variables were age, smoking, increase in METs/h/wk, decrease in waist circumference, GPX, SOD, RHR and ADMA changes. P-values were calculated as two-sided; a P-value of less than 0.05 was considered statistically significant. SPSS version 19.0 (SPSS Inc., Chicago, IL) was used for all statistical analysis.
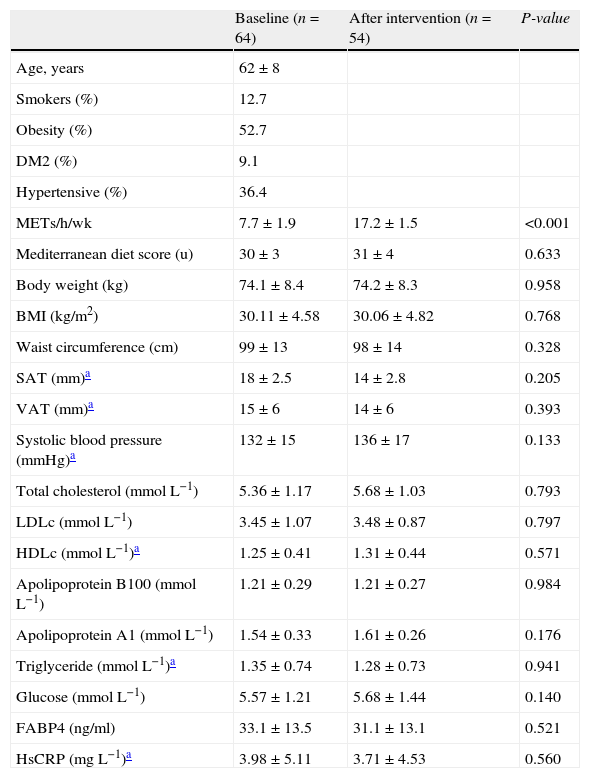
ResultsFifty-four participants completed the study protocol (Fig. 1). Mean attendance at physical activity sessions was 74%. After the intervention, an increase in the number of METs/h/wk (7.7±1.9 vs. 17.2±1.5, P<0.001) was observed. Anthropometric, diet, body fat distribution and general biochemical characteristics did not change during the intervention (Table 1).
Differences in clinical, antropometrical and biochemical data between baseline and post-intervention.
| Baseline (n=64) | After intervention (n=54) | P-value | |
| Age, years | 62±8 | ||
| Smokers (%) | 12.7 | ||
| Obesity (%) | 52.7 | ||
| DM2 (%) | 9.1 | ||
| Hypertensive (%) | 36.4 | ||
| METs/h/wk | 7.7±1.9 | 17.2±1.5 | <0.001 |
| Mediterranean diet score (u) | 30±3 | 31±4 | 0.633 |
| Body weight (kg) | 74.1±8.4 | 74.2±8.3 | 0.958 |
| BMI (kg/m2) | 30.11±4.58 | 30.06±4.82 | 0.768 |
| Waist circumference (cm) | 99±13 | 98±14 | 0.328 |
| SAT (mm)a | 18±2.5 | 14±2.8 | 0.205 |
| VAT (mm)a | 15±6 | 14±6 | 0.393 |
| Systolic blood pressure (mmHg)a | 132±15 | 136±17 | 0.133 |
| Total cholesterol (mmolL−1) | 5.36±1.17 | 5.68±1.03 | 0.793 |
| LDLc (mmolL−1) | 3.45±1.07 | 3.48±0.87 | 0.797 |
| HDLc (mmolL−1)a | 1.25±0.41 | 1.31±0.44 | 0.571 |
| Apolipoprotein B100 (mmolL−1) | 1.21±0.29 | 1.21±0.27 | 0.984 |
| Apolipoprotein A1 (mmolL−1) | 1.54±0.33 | 1.61±0.26 | 0.176 |
| Triglyceride (mmolL−1)a | 1.35±0.74 | 1.28±0.73 | 0.941 |
| Glucose (mmolL−1) | 5.57±1.21 | 5.68±1.44 | 0.140 |
| FABP4 (ng/ml) | 33.1±13.5 | 31.1±13.1 | 0.521 |
| HsCRP (mgL−1)a | 3.98±5.11 | 3.71±4.53 | 0.560 |
SAT: Subcutaneous adipose tissue; VAT: Visceral adipose tissue. FABP4: Fatty acid binding protein 4; HsCRP: High sensitive C-reactive protein.
Derived with an ANOVA or Wilcoxon test for continuous variables or chi-squared test for categorical variables.
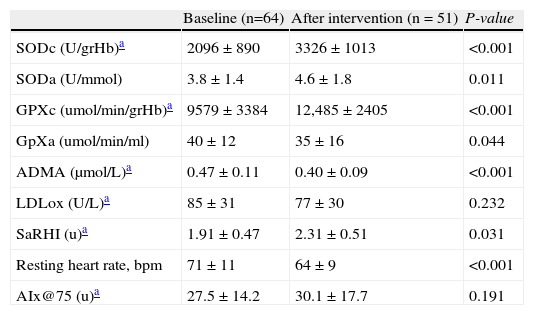
Variations in antioxidant enzymes and vascular determinants between baseline and end of the intervention are shown in Table 2. We observed a significant increase in intra-erythrocyte and plasma SOD concentrations (SODe; 2096±890 vs. 3326±1013U/grHb, P<0.001 and SODa; 3.8±1.4 vs. 4.6±1.8 Ummol, P=0.011) and both, intra-erythrocyte GPxe (9579±3384 vs. 12,488±2405μmol/min/grHb, P<0.001). The saRHI increased (1.91±0.47 vs. 2.31±0.51, P=0.031) correspondingly to a significant decrease of ADMA levels (0.47±0.11 vs. 0.40±0.09mol/min/grHb, P<0.001) and GPX activity (40±12 vs. 35±16umol/min/m, P = 0.044). LDLox did not change significantly (85±31 vs. 77±30U/L, P=0.232). RHR decreased by 8.6±19.7% after the intervention (P<0.001).
Oxidative and vascular differences between baseline and after the intervention.
| Baseline (n=64) | After intervention (n = 51) | P-value | |
| SODc (U/grHb)a | 2096±890 | 3326±1013 | <0.001 |
| SODa (U/mmol) | 3.8±1.4 | 4.6±1.8 | 0.011 |
| GPXc (umol/min/grHb)a | 9579±3384 | 12,485±2405 | <0.001 |
| GpXa (umol/min/ml) | 40±12 | 35±16 | 0.044 |
| ADMA (μmol/L)a | 0.47±0.11 | 0.40±0.09 | <0.001 |
| LDLox (U/L)a | 85±31 | 77±30 | 0.232 |
| SaRHI (u)a | 1.91±0.47 | 2.31±0.51 | 0.031 |
| Resting heart rate, bpm | 71±11 | 64±9 | <0.001 |
| AIx@75 (u)a | 27.5±14.2 | 30.1±17.7 | 0.191 |
SODc: Superoxide dismutase erythrocyte lysate levels; SODa: Superoxide dismutase plasma; GPXc: Glutathion peroxidase erythrocyte lysate levels; GPXa: Glutathion peroxidase plasma; ADMA: asymmetrical dimethylarginine; LDLox: Oxidised LDL cholesterol; saRHI: small artery reactive hyperemia index; AIx@75: Augmentation index adjusted to 75bpm.
Baseline compared with after intervention with a paired t-test or Wilcoxon test for continuous variables.
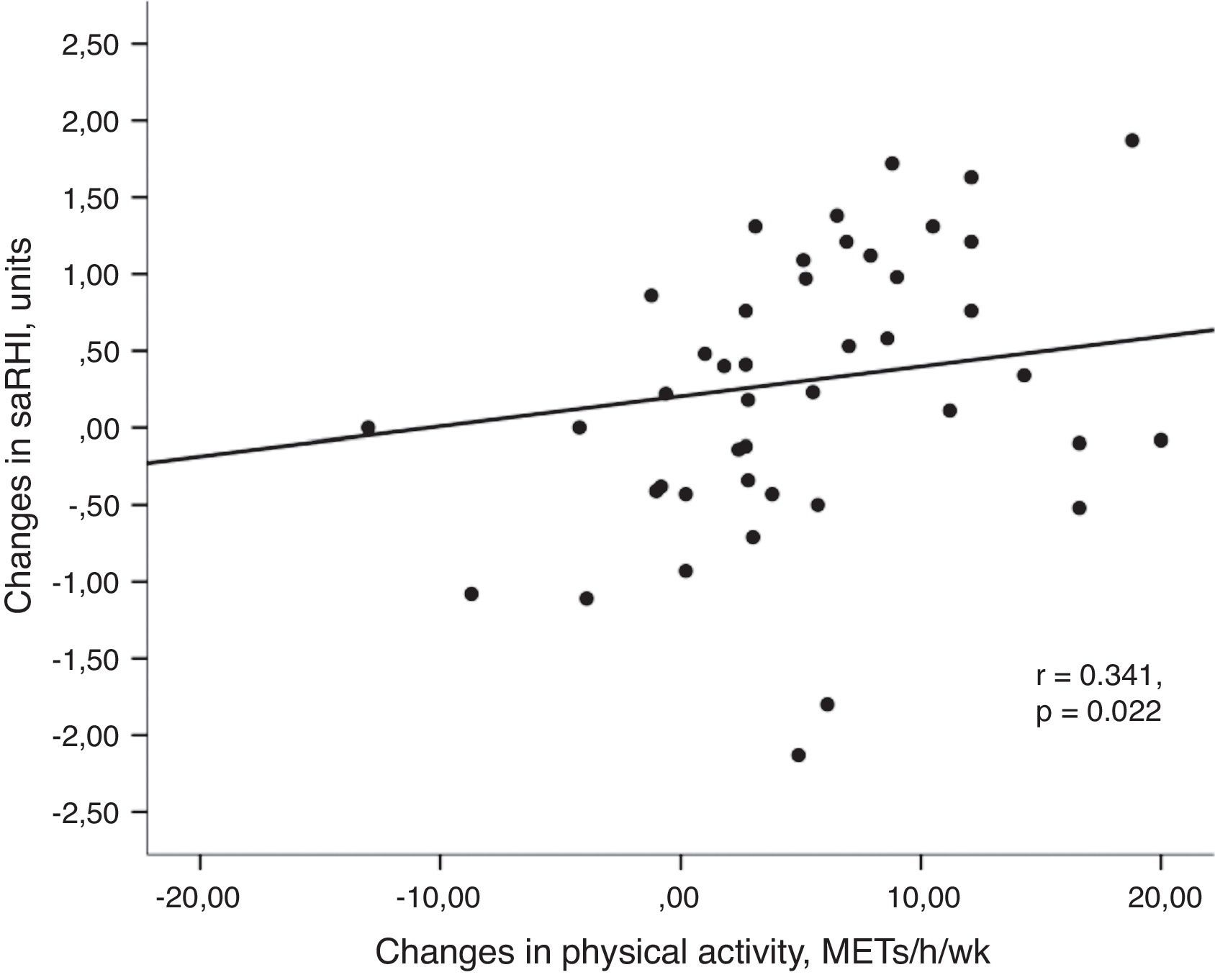
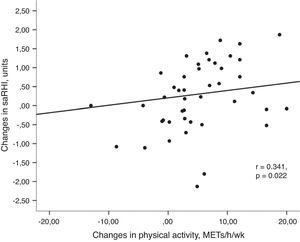
Univariant tests showed that the increase in PA was positively associated with the increase in saRHI (r=0.341, P=0.022) (Fig. 2a) and GPxe (r=0.303, P=0.047). Moreover, PA inversely correlated with changes in glucose (r=−0.302, P=0.047), body weight (r=−0.441, P=0.004) and resting heart rate (r=−0.319, P=0.041).
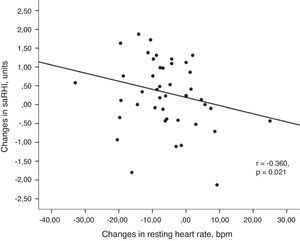
(a) Association between changes in small artery reactive hyperemia and changes in physical activity. Univariant associations derived from Spearman's correlation test. (b) Association between changes in small artery reactive hyperemia and changes in resting heart rate. Univariant associations derived from Spearman's correlation test.
Changes in saRHI inversely correlated to changes in RHR (r=−0.360, P=0.021) (Fig. 2b). Participants with greater increases in saRHI had greater increases in SODe levels compared to those with smaller increases in saRHI (2134±411 vs. 326±286U/grHb, P=0.04).
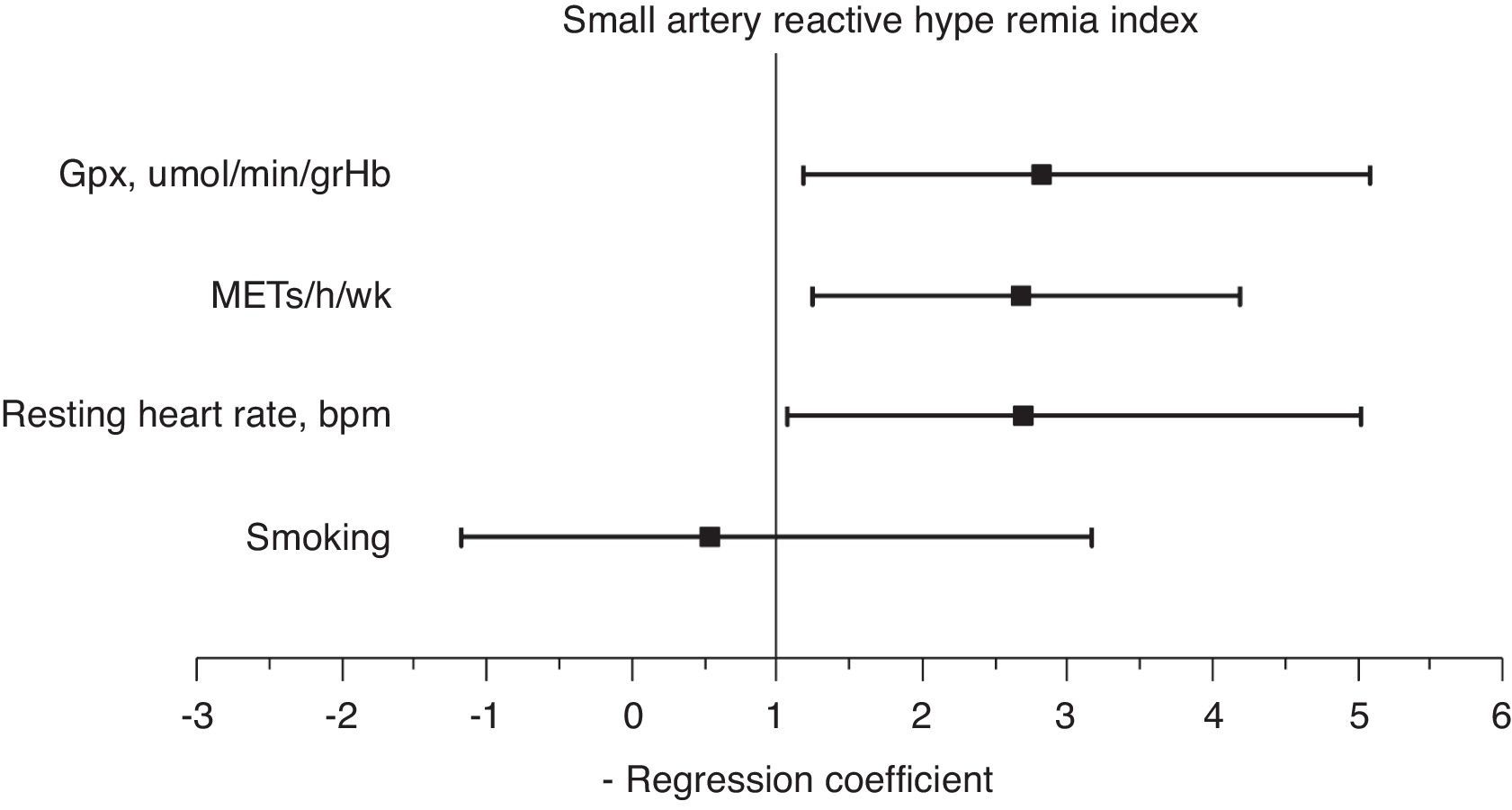
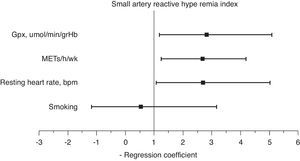
A multiple stepwise logistic regression test was performed to assess the main determinants of endothelial function improvement, using saRHI increase as the dependent variable. The independent variables included were age, smoking, increase in METs/h/wk, decrease in waist circumference, GPxe increase, SODe increase, changes in RHR and ADMA decrease. After adjusting for interactions, the best predictor model (85.4% of correctly prognosticated values and R2 Nagelkerke=0.442) included smoking, increase in METs/h/wk, changes in RHR and increase in GPxe. When this test was forced, the increase in METs/h/wk (2.65 95%CI: 1.21–4.01), changes in RHR (1.91 95%CI: 1.01–4.98) and increase in GPxe (2.61 95%CI: 1.16–5.01) remained independent predictors of saRHI improvement (Fig. 3).
DiscussionOur work shows that low-intensity level PA applied to sedentary people improves several parameters associated with cardiovascular health and that there is high adherence to such a physical activity programme. International recommendations by different scientific societies establish a minimum of 2.5–5h a week of moderate intensity walking to reduce cardiovascular risk.3,4 However, even this small amount of activity cannot be met by many elderly, handicapped or metabolically limited people, such as those with obesity. Therefore, an evaluation of the impact of exercise levels below these recommendations is warranted. A low PA intervention results in an augmented antioxidant system measured by the activity of the enzymes SOD and GPX. The increases in these enzymes suggest a better antioxidant status, although we did not observe any effect on plasma lipid oxidation as measured with oxidised LDL levels. The impact of low-level PA on peripheral small artery endothelial function was also significant. Moreover, our data also show that in parallel with saRHI increase, there was a significant decrease in ADMA levels.
Current evidence suggests that PA improves several classical cardiovascular risk factors as well as measures of new biomarkers of cardiovascular risk, including oxidative stress,20,21 endothelial wall dysfunction5,6,11,21 and high resting heart rate.22 It is well known that weight loss, especially visceral fat loss,23 but also subcutaneous fat loss,24 is one of the main determinants of these changes. By assessing body fat patterns with standardised echography methods, we observed that our results were independent of changes in body weight loss or body fat distribution. Moreover, our group has recently published that a moderate increase in physical activity decreases a marker of adipose tissue distribution like fatty acid binding protein 4 (FABP4) levels; specifically, an increase of 30METs/h/wk decreases FABP4 by 10.3 units.25 In the present study, the low increase in physical activity has not been associated with FABP4 changes.
We found that ADMA levels decreased significantly with low intensity PA. Exercise training has been documented to decrease ADMA levels,26 but no data are available for low intensity PA. ADMA acts as strong endogenous eNOS inhibitor by blocking NO synthesis from the amino acid l-arginine, which contributes to the initiation and progression of atherosclerosis.8 Therefore, the suppression of ADMA and the increase of saRHI might also reflect improved endothelial function in overweight and obese men and women undergoing low intensity PA. Even though we cannot obtain mechanistic data from this study, our results are very consistent in showing both an improvement in oxidative status and small artery endothelial function markers. These results were confirmed in multivariate analyses showing that both PA and antioxidant enzymes play a role in the observed endothelial function improvement.
Another relevant finding is the significant decrease in RHR observed after the intervention. High resting heart rate has been identified as a potential accelerator of atherosclerosis via its negative effects on the endothelium due to shear and mechanical stress. In fact, modifications of the haemodynamic environment by accelerated heart rate likely contributes to enhanced atherosclerosis in certain parts of the vascular Tree.27 It is known that physical exercise practice improves heart function by reducing RHR; however, it is important to note that small amounts of PA already have a positive impact. Interestingly, changes in RHR were among the determinants of saRHI improvement, which supports the idea that heart functionality is linked to overall endothelial function. In accordance with this observation, clinical trials with ivabradine, a selective inhibitor of the I(f) channels, showed a relationship between decreasing RHR and improved endothelial function.27,28 This association is not fully understood, but evidence derived from ivabradine studies produced two possible explanations of this effect. The first is that by slowing heart rate, shear stress is more constant and thus flow is more constant, leading to a reduced burden on the arterial wall function.29 The second is associated to the mechanical stress from each heart-beat. At a high RHR, the wall becomes stiffer; this is associated with accelerated endothelial cell turnover, premature senescence of the endothelium and high shear stresses.30 Our results support these observations and extend them to clinical practice, suggesting a benefit of low intensity PA in obese or overweight postmenopausal women.
Study limitationsOur study had some limitations. Because the study was conducted in a specific group of overweight or obese men and women, results cannot be generalised to other specific populations. Although not described, either the pharmacological agents our patients were taking, or interactions between pharmacological agents and physical activity changes, could have influenced changes in oxidative stress or endothelial function. Physical activity is measured objectively by self-reports that may lead to misclassification and bias towards weaker physical-activity or health-benefit associations. In view of the strength of our main findings, the quantitative importance of these limitations appears small.
ConclusionsThe results of the present study suggest that lower-intensity PA improves markers of cardiovascular health such as oxidative stress, RHR and peripheral small artery wall function in a group of obese and overweight men and women. These changes are independent of weight loss or fat modifications suggesting a direct impact of low amounts of PA on cardiovascular health improvement. New long-term clinical trials with other specific populations are needed to establish clinical recommendations in specific handicapped study populations at increased cardiovascular risk.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of DataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors declared no conflict of interest.
This work was supported by grants from the Spanish Atherosclerosis Society. Effect of exercise on endothelial function and abdominal adipose tissue in patients with moderate or high cardiovascular risk. Beca FEA/SEA 2010 Investigación clínico-epidemiológica (2) presented at the XXIII SEA Congress, 2010. Spanish Atherosclerosis Society to Jordi Merino.
CIBERDEM is an initiative of ISCIII, Spain. The authors’ responsibilities were as follows: JM, RF, NP, JB and LM designed the study; JM, CB, DI, DA, AV, RF and NP conducted research; MH, AC, JG performed the biochemical analyses; JM, RF, JB and LM performed the statistical tests and wrote the final manuscript. All authors have read and approved the final manuscript.