Low carbohydrate diets have become increasingly popular for weight loss. Although they may improve some metabolic markers, particularly in type 2 diabetes mellitus (T2D) or metabolic syndrome (MS), their net effect on vascular function remains unclear.
ObjectiveEvaluate the relation between dietary macronutrient composition and the small artery reactive hyperaemia index (saRHI), a marker of small artery vascular function, in a cohort of MS patients.
DesignThis cross-sectional study included 160 MS patients. Diet was evaluated by a 3-day food-intake register and reduced to a novel low-carbohydrate diet score (LCDS). Physical examination, demographic, biochemical and anthropometry parameters were recorded, and saRHI was measured in each patient.
ResultsIndividuals in the lowest LCDS quartile (Q1; 45% carbohydrate, 19% protein, 31% fat) had higher saRHI values than those in the top quartile (Q4; 30% carbohydrate, 25% protein, 43% fat) (1.84±0.42 vs. 1.55±0.25, P=.012). These results were similar in T2D patients (Q1=1.779±0.311 vs. Q4=1.618±0.352, P=.011) and also in all of the MS components, except for low HDLc. Multivariate analysis demonstrated that individuals in the highest LCDS quartile, that is, consuming less carbohydrates, had a significantly negative coefficient of saRHI which was independent of confounders (HR: −0.747; 95%CI: 0.201, 0.882; P=.029).
ConclusionsThese data suggest that a dietary pattern characterized by a low amount of carbohydrate, but reciprocally higher amounts of fat and protein, is associated with poorer vascular reactivity in patients with MS and T2D.
Las dietas bajas en hidratos de carbono son muy populares para la pérdida de peso. Aunque estas puedan mejorar algunos marcadores metabólicos, en particular en la diabetes mellitus tipo2 (DM2) o en el síndrome metabólico (SM), su efecto neto sobre la función de la pared arterial sigue siendo poco clara.
ObjetivoEvaluar la relación entre la composición de macronutrientes de la dieta y el índice de hiperemia reactiva de pequeña arteria (saRHI) en una cohorte de pacientes con SM.
DiseñoEn este estudio transversal se incluyeron 160 pacientes con SM. La dieta fue evaluada mediante un registro alimentario de 3 días que se tradujo a una puntuación de dieta baja en hidratos de carbono (LCDS). Se registraron los parámetros demográficos, bioquímicos, antropométricos, y el saRHI se determinó en cada paciente.
ResultadosLos individuos en el cuartil inferior de LCDS (C1, 45% de hidratos de carbono, 19% de proteína y 31% grasa) presentaron valores más altos de saRHI en comparación a con del cuartil superior (C4, 30% de hidratos de carbono, 25% de proteínas, 43% de grasa) (1,84±0,42 vs. 1,55±0,25, p=,012). Estos resultados fueron particularmente semejantes en los pacientes con DM2 (C1=1,779±0,311 vs. C4=1,618±0,352, p=,011) y en todos los componentes del SM, excepto por los niveles bajos de cHDL. En el análisis multivariante se observó que los individuos en el cuartil superior del LCDS consumían menos hidratos de carbono, tenían un coeficiente negativo de saRHI alto independiente de los factores de confusión (HR: −0,747; IC95%: 0,201, 0,882; p=,029).
ConclusionesEstos hallazgos sugieren que un patrón alimentario caracterizado por una baja cantidad de hidratos de carbono, pero altas cantidades de proteínas y grasas, se asocia con una menor reactividad vascular de arteria pequeña en pacientes con SM.
Obesity and the closely related risk cluster of metabolic syndrome (MS) increase cardiovascular (CV) risk, in turn raising the incidence and prevalence of coronary heart disease (CHD). CHD accounts for most of the deaths in developed countries, and is presently the most pernicious threat to global public health.1,2 Improvements in just 3 lifestyle behaviors: smoking cessation, consuming a well-balanced, healthful diet and adequate physical activity, are remarkably effective in reducing the burden of noncommunicable “degenerative diseases of civilization,” particularly obesity, MS, diabetes, CHD and cancer.3,4 Although components of macronutrient intake are considered healthy over a fairly wide range, their ratios have been believed to affect final body weight, overall level of cardiovascular risk, and progression of atherosclerosis.5 Several major scientific bodies currently recommend a low-fat, high-carbohydrate, energy-deficient diet to manage weight and associated comorbidities.6–8 On the other hand, diets rich in fat and protein, but low in carbohydrates, have become popular, with advocates deemphasizing the role of caloric intake in weight gain. Several best-selling books endorse this strategy of carbohydrate reduction, and maintain that weight will be lost despite isocaloric intake, accompanied by a reduction in cardiovascular risk. For over 40 years, the relative advantages of both diets, along with the effects upon cardiovascular risk, have been debated.9 Faster initial weight loss, perhaps related to the satiating effects of high protein consumption,10 might improve insulin sensitivity and lipid profiles associated with low-carbohydrate diets.11,12 However, weight loss associated with these diets is generally not sustained beyond 1 year, and the overall effect on vascular health, closely related to cardiovascular outcomes, remains controversial.9,13 Several recent randomized clinical trials have studied the effect of macronutrient composition upon endothelial function (EF) using flow-mediated dilatation (FMD), with disparate results.12,14–24 In order to increase accuracy and predictive power, a standardized method of grading the extent of adherence to a low carbohydrate diet according to three scales of carbohydrate, fat, and protein intake, as percentages of total calories consumed, was adopted in this trial. The Low-Carbohydrate Diet Score (LCDS) provides an effective tool to classify individuals according to their relative levels of fat, protein and carbohydrate consumption, embodied in a simple number.13 The LCDS is based upon the percentage of energy consumed as carbohydrate, and reflects the concordance with low carbohydrate intake. The higher the individual score, the lower the carbohydrate, but the higher the protein and fat content, in that patient's diet. LCDS has successfully been employed to show that a low carbohydrate diet is positively associated with the risk of type 2 diabetes (T2D) in a large cohort of healthy men after 20 years of follow-up.25
Measurement of the peripheral arterial tonometry (PAT) hyperemic response, embodied in the small artery reactive hyperaemia index (saRHI), is a relatively new technique considered a toll reflecting endothelial and other variables upon microvascular function, dilation of the small arteries among them.26–28 SaRHI correlates with coronary endothelial dysfunction,29 accurately measures vascular effects when the net cardiovascular risk burden improves,30 and can predict future adverse cardiovascular events.31 Information from noninvasive PAT studies is useful to assess static or serial vascular function in a variety of patient types with raised cardiovascular risk.32 We previously reported that high adherence to therapeutic lifestyle changes, including a Mediterranean dietary pattern, was associated with improved small artery vascular reactivity, as assessed by saRHI, in conjunction with improvements in carotid intima-media thickness (CIMT).33 That work also generated a hypothesis that chronic consumption of high percentages of dietary protein and fat, along with a low intake of carbohydrate, may lead to small artery vascular dysfunction. The aim of this study was to evaluate the relation between dietary macronutrient composition, using LCDS, upon saRHI, in a group of patients at increased cardiovascular risk.
Subjects and methodsDesign and study participantsThis is a cross-sectional study including 160 men and women from 30 to 70 years of age, without a previous diagnosis of cardiovascular disease, who attended the Vascular Medicine and Metabolism Unit of University Hospital Sant Joan in Reus (Spain). Only patients who satisfied ATPIII criteria for MS were enrolled. Patients with diagnosed cardiovascular disease, chronic renal, hepatic, pulmonary or neurodegenerative problems, a history of prior neoplasia, or other serious chronic disease were excluded.
Clinical assessmentEach of our patients was evaluated with a review of lifestyle components (diet, physical activity and smoking), a complete physical examination including anthropometry, biochemical testing, and vascular assessment with saRHI. Physical activity was quantified in METs/h/week according to the Minnesota questionnaire adapted for a Spanish population.34 Tobacco status was assessed using standardized questionnaires administered by health care providers.35 A 3-day food-intake register was used to evaluate diet. The food data of dietary records were converted to energy and nutrient data by experienced dieticians and analyzed using the PCN (CESNID Nutritional Program, version 2.0, Barcelona, Spain). The percentages of energy from carbohydrate, protein, and fat were coded according to defined categories, using the LCDS method previously described by Halton et al. in their Women's Health Study Cohort.10,13 Briefly, the carbohydrate categories were scored from 10 (lowest intake) to 0 (highest intake), whereas protein and fat categories were scored from 0 (lowest intake) to 10 (highest intake). Ranks were added to create a total score with a maximum value of 30, representing the highest intake of protein and fat and the lowest intake of carbohydrate (see Reference 14, Table 1). The study was approved by the clinical and investigation committee of The Hospital Universitari Sant Joan, and all subjects provided written informed consent.
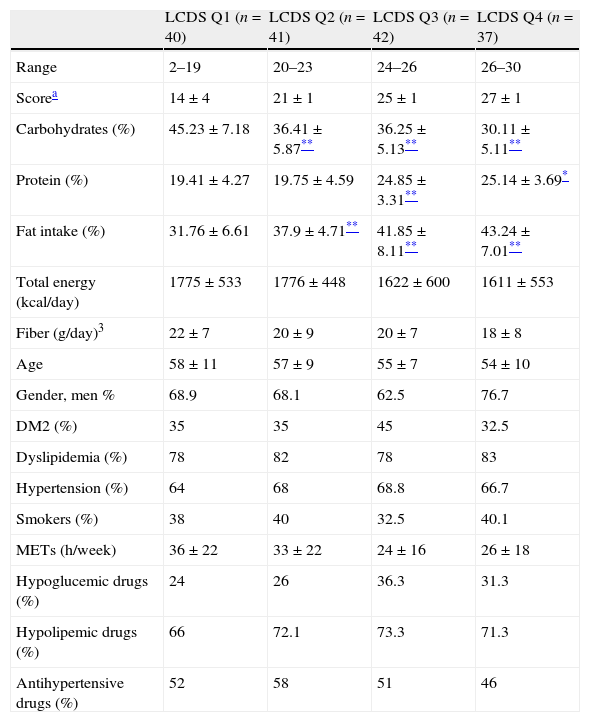
Differences in demographic and lifestyle data according to the LCDS quartiles.
| LCDS Q1 (n=40) | LCDS Q2 (n=41) | LCDS Q3 (n=42) | LCDS Q4 (n=37) | |
| Range | 2–19 | 20–23 | 24–26 | 26–30 |
| Scorea | 14±4 | 21±1 | 25±1 | 27±1 |
| Carbohydrates (%) | 45.23±7.18 | 36.41±5.87** | 36.25±5.13** | 30.11±5.11** |
| Protein (%) | 19.41±4.27 | 19.75±4.59 | 24.85±3.31** | 25.14±3.69* |
| Fat intake (%) | 31.76±6.61 | 37.9±4.71** | 41.85±8.11** | 43.24±7.01** |
| Total energy (kcal/day) | 1775±533 | 1776±448 | 1622±600 | 1611±553 |
| Fiber (g/day)3 | 22±7 | 20±9 | 20±7 | 18±8 |
| Age | 58±11 | 57±9 | 55±7 | 54±10 |
| Gender, men % | 68.9 | 68.1 | 62.5 | 76.7 |
| DM2 (%) | 35 | 35 | 45 | 32.5 |
| Dyslipidemia (%) | 78 | 82 | 78 | 83 |
| Hypertension (%) | 64 | 68 | 68.8 | 66.7 |
| Smokers (%) | 38 | 40 | 32.5 | 40.1 |
| METs (h/week) | 36±22 | 33±22 | 24±16 | 26±18 |
| Hypoglucemic drugs (%) | 24 | 26 | 36.3 | 31.3 |
| Hypolipemic drugs (%) | 66 | 72.1 | 73.3 | 71.3 |
| Antihypertensive drugs (%) | 52 | 58 | 51 | 46 |
Derived with an ANOVA or Wilcoxon test for continuous variables or chi-squared test for categorica variables.
saRHI was measured using peripheral artery tonometry (PAT) technology (EndoPAT-2000, Itamar Medical Ltd., Israel). Determinations were performed in a quiet room with a controlled temperature (22–24°C) after patients had fasted for 12h and refrained from smoking or strenuous exercise for 24h. The patients lay in a relaxed, quiet and evenly illuminated environment while the device recorded changes in pulse waves in the digital arteries. The technique involved in the PAT has been described elsewhere.29,36 Blood flow measurements from two fingertips—one from each hand, one a test, the other a control were compared after a stabilization period, and a second, comparison pair of measurements, were taken before and after 5min of brachial ischemia in the test arm. The results are processed by specific software to calculate the post-ischemia reflex vasodilatation observed when measurements from the test arm (before and after ischemia) are compared to those from the control arm. The value generated is termed saRHI. The variability of this technique in our laboratory was 17%, the intraclass correlation coefficient was 0.52, and the within-subject variation was 0.19.
Biochemical determinationsVenous blood samples were obtained after a 12h overnight fasting period and centrifuged immediately and stored at −80°C until the assays were performed. Total cholesterol, triglyceride, glucose, direct low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C), apolipoprotein B100 and hs-CRP levels were measured using enzymatic and turbidimetric assays (Spinreact, SA, Spain) adapted to the autoanalyzer Cobas Mira Plus (Roche Diagnostics, Spain). Differential expression of human inflammatory cytokines was determined using the Human Cytokine Antibody Array (RayBiotech, Norcross, GA, USA). The array is coated with six specific cytokine antibodies. It was probed with serum samples to allow for the comparisons of relative cytokine levels. The cytokine–antibody–biotin complex was visualized with the addition of a streptavidin-labeled fluorescent dye using a laser scanner.
Statistical analysisData are presented as mean±SD or as median and interquartile range for continuous variables and as frequencies for categorical variables. Normality distribution of variables was assessed with the Kolmogorov–Smirnov test. Differences between diet, anthropometric, biochemical or vascular data were analyzed using the ANOVA or Kruskall–Wallis test for continuous variables or chi-squared test for categorical ones. Univariate association was tested by Spearman correlation analysis. Logistic regression model test was performed to assess the determinants of saRHI in our study group patients. The dependent variable of interest was the highest quartile of saRHI defined as a quartile of individuals with high saRHI (2.14±0.39). Independent variables were selected on the basis of univariate analysis and from the variables known to be associated with the dependent variable according our previous studies. In this test independent variables were age, gender, T2D, atherogenic dyslipidemia defined as low HDL-C (HDL-C<1.03mmol/L in men and 1.29mmol/L in women) and high triglycerides (TG>1.5mmol/L), abdominal obesity and high blood pressure according to ATPIII criteria, high LDL-C (LDL-C≥4.14mmol/L), smoking, highest quartile of LCDS, highest quartile of physical activity (40±18METs/h/week), highest quartile of SFA intake (11.6±2.8g/day) and highest quartile of fiber intake (22±7g/day). P-values were calculated as two-sided; a P-value of less than 0.05 was considered statistically significant. SPSS version 19.0 (SPSS Inc., Chicago, IL) was used for all statistical analysis.
ResultsAssociations of LCDS with biochemical and vascular dataThe median LCDS in our study group was 23±7 points. Participants were distributed into LCDS quartiles according to their macronutrient intake percentages. Table 1. The diet of individuals in the lower LCDS was composed of 45% carbohydrate, 19% protein and 31% fat. Participants in the top quartile had an intake of 30% carbohydrate, 25% protein and 43% fat. There were no differences between study participant quartiles other than the expected nutrient profile, as indicated in Table 1.
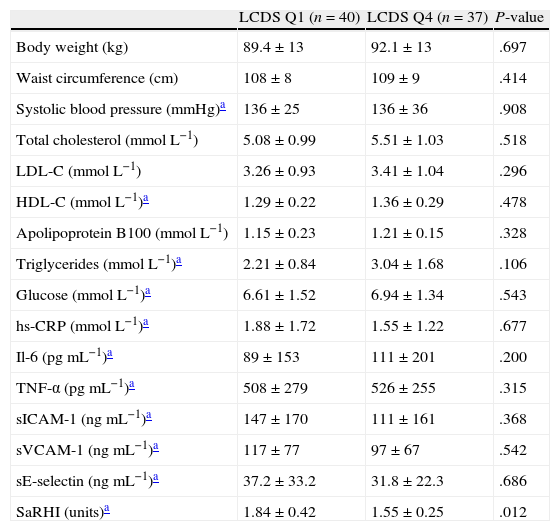
The differences in biochemical and vascular data between individuals in the lowest LCDS quartile and individuals in the highest LCDS quartile are shown in Table 2. We observed that individuals in the lowest quartile had a higher saRHI than individuals in the highest quartile (1.84±0.42 vs. 1.55±0.25, P=0.012). No significant differences were observed in the other vascular or biochemical parameters.
Anthropometric, biochemical and vascular data between lowest and highest low-carbohydrate diet score.
| LCDS Q1 (n=40) | LCDS Q4 (n=37) | P-value | |
| Body weight (kg) | 89.4±13 | 92.1±13 | .697 |
| Waist circumference (cm) | 108±8 | 109±9 | .414 |
| Systolic blood pressure (mmHg)a | 136±25 | 136±36 | .908 |
| Total cholesterol (mmolL−1) | 5.08±0.99 | 5.51±1.03 | .518 |
| LDL-C (mmolL−1) | 3.26±0.93 | 3.41±1.04 | .296 |
| HDL-C (mmolL−1)a | 1.29±0.22 | 1.36±0.29 | .478 |
| Apolipoprotein B100 (mmolL−1) | 1.15±0.23 | 1.21±0.15 | .328 |
| Triglycerides (mmolL−1)a | 2.21±0.84 | 3.04±1.68 | .106 |
| Glucose (mmolL−1)a | 6.61±1.52 | 6.94±1.34 | .543 |
| hs-CRP (mmolL−1)a | 1.88±1.72 | 1.55±1.22 | .677 |
| Il-6 (pgmL−1)a | 89±153 | 111±201 | .200 |
| TNF-α (pgmL−1)a | 508±279 | 526±255 | .315 |
| sICAM-1 (ngmL−1)a | 147±170 | 111±161 | .368 |
| sVCAM-1 (ngmL−1)a | 117±77 | 97±67 | .542 |
| sE-selectin (ngmL−1)a | 37.2±33.2 | 31.8±22.3 | .686 |
| SaRHI (units)a | 1.84±0.42 | 1.55±0.25 | .012 |
hs-CRP, high sensitive C-reactive protein; Il-6, Interleukin 6; TNF-α, tumor necrosis factor α; sICAM-1, soluble intercellular adhesion molecule 1; sVCAM-1, soluble vascular adhesion molecule 1; sE-selectin, soluble E-selectin; saRHI, small artery reactive hyperemia index.
P-value; differences regarding study groups using one-way ANOVA or Wilcoxon test.
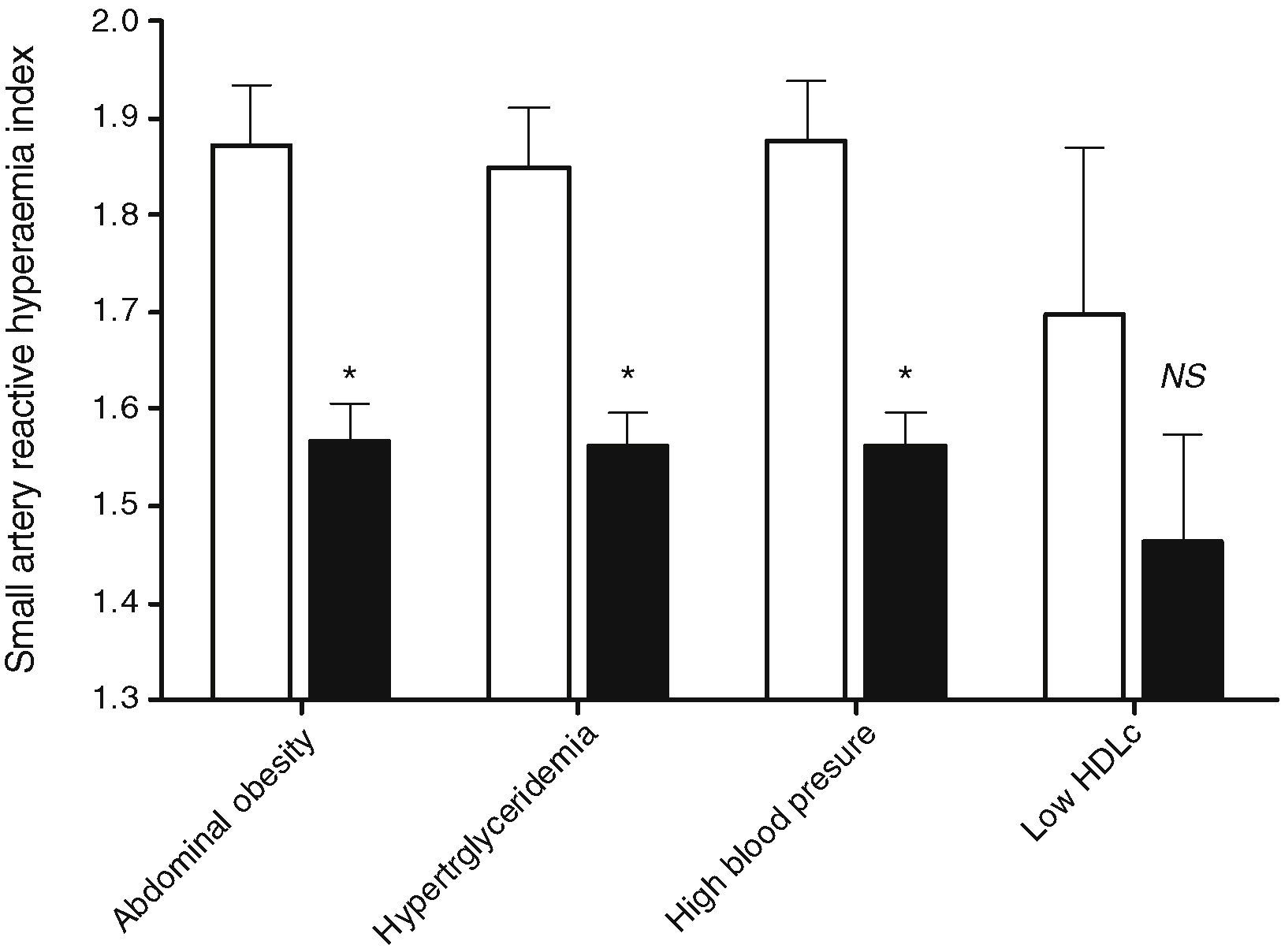
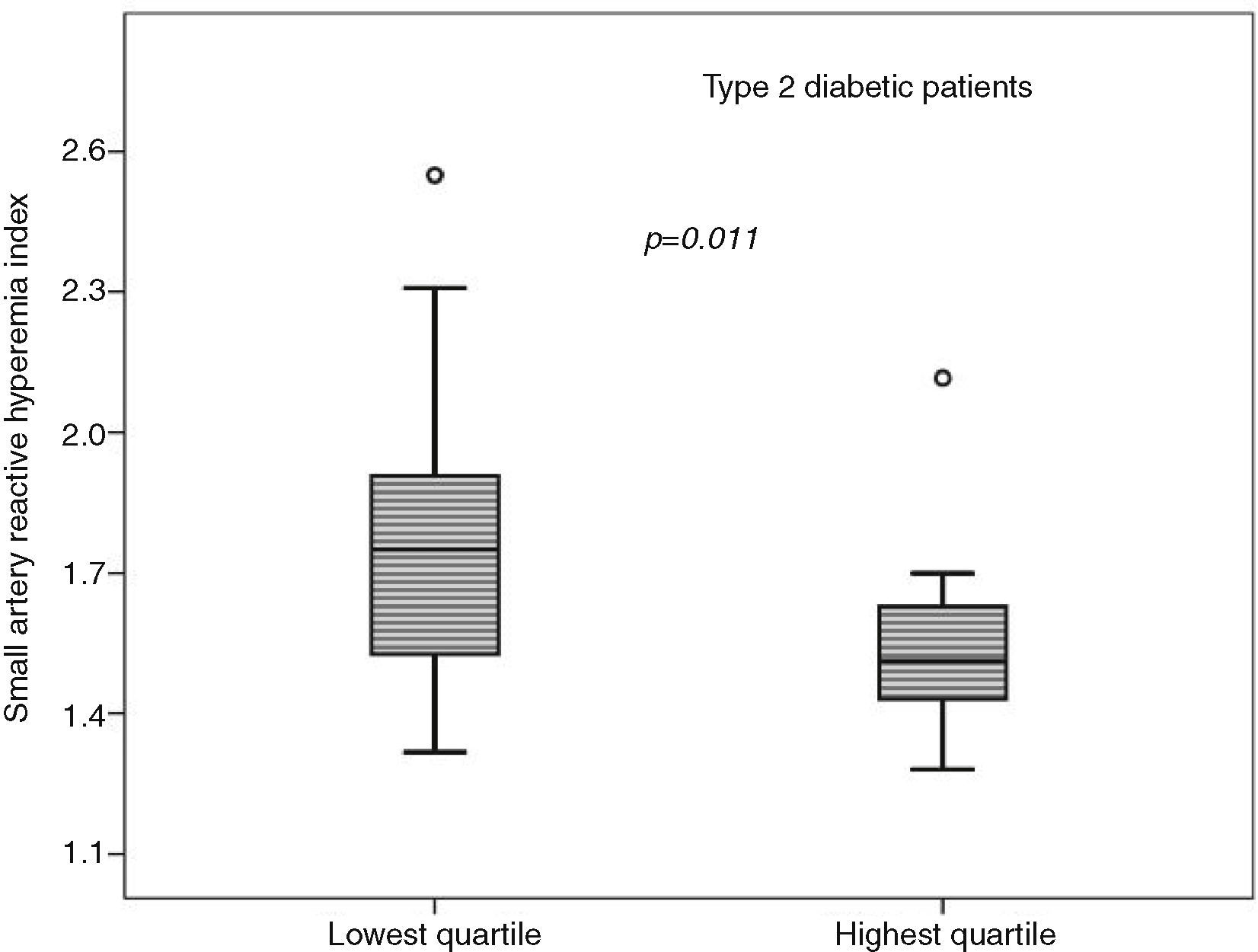
An inverse association was observed between LCDS and saRHI (r=−0.198, P=.003). When the MS components were separately evaluated, individuals in the lowest LCDS quartile had higher saRHI values than individuals in the highest quartile. The exception was in individuals with low HDL-C, in which saRHI was not different when comparing individuals according to the LCDS quartiles (1.698±0.312, n=4 vs. 1.462±0.318, n=5, P=NS), as illustrated in Fig. 1. Among the fifty-nine T2D patients, those in the lower quartile (n=14) had better saRHI values than those in the highest quartile (n=18) (1.779±0.311 vs. 1.618±0.352, P=0.011), Fig. 2. These results were not modified when pharmacological therapy was taken into account.
Differences in saRHI according to the LCDS quartile and the metabolic alteration or risk factor. Small artery reactive hyperaemia index according to low-carbohydrate diet quartiles. White bars are low-carbohydrate diet score quartile 1 patients. Black bars are low-carbohydrate diet score quartile 4 patients. Data obtained with ANOVA-test or Kruskall–Wallis test. Values are expressed as the mean±SD or median±interquartile range. *P-value<.05.
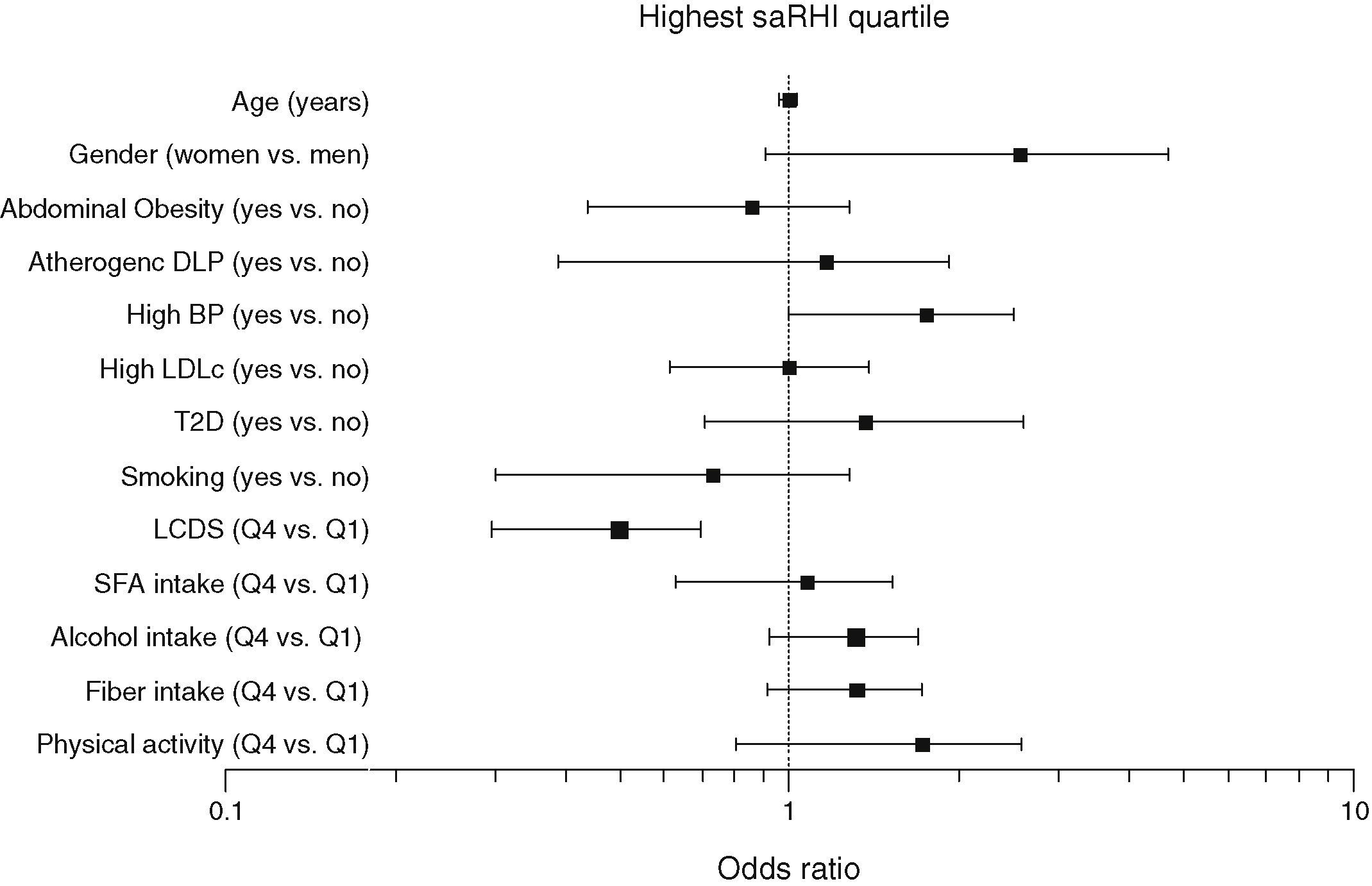
To assess the effect of LCDS on endothelial function independent of potential confounders, a multivariate stepwise test was performed. We considered the highest quartile of saRHI (2.14±0.39) as the dependent variable and age, gender, T2D, atherogenic dyslipidemia defined as low HDL-C (HDL-C<1.03mmol/L in men and 1.29mmol/L in women) and high triglycerides (TG>1.5mmol/L), abdominal obesity and high blood pressure according to ATPIII criteria, high LDL-C (LDL-C≥4.14mmol/L), smoking, highest quartile of LCDS, highest quartile of physical activity (40±18METs/h/week), highest quartile of SFA intake (11.6±2.8g/day) and highest quartile of fiber intake (22±7g/day). As seen in Fig. 3, individuals in the highest LCDS quartile, as compared with the lowest quartile, had negative probabilities of high saRHI, with a estimated prognostic value of 78% (HR: −0.747; 95%CI: 0.201, 0.882; P=.029).
Determinants of small artery reactive hyperaemia index. Multivariate stepwise binary logistic regression test. Dependent variable: highest quartile of small artery reactive hyperemia index (2.14±0.39). Independent variables: age, gender, atherogenic dyslipidemia (TG>1.5mmol/L and HDL-C<1.03mmol/L in men and 1.29mmol/L in women), abdominal obesity and high blood pressure according to ATPIII criteria, high LDL-C (LDL-C≥4.14mmol/L), smoking, highest quartile of LCDS, highest quartile of physical activity (40±18METs/h/week), highest quartile of SFA intake (11.6±2.8g/day) and highest quartile of fiber intake (22±7g/day). Estimated pronostic value: 78%. R2 Nagelkerke: 0.650.
Stratifying participants according to their unique dietary macronutrient composition (LCDS), we observed that the higher the LCDS, reflecting a lower dietary carbohydrate content, the lower the small artery reactivity in the digital vascular bed. This inverse relationship was significant and independent of the analyses used and characteristics of the metabolic risk component, and especially in T2D. A lack of a similar relationship was observed in subjects with low HDL-C might be due to the small sample size of patients with low HDL-C criteria (Q1=4 and Q4=5). While differences in endothelial function biomarkers did not reach statistical significance, they varied in the same direction. In essence, patients consuming carbohydrate-poor diets had the worst vascular and risk profiles.
The literature suggests that diets with low or very low carbohydrate content may be associated with impaired vascular function.14,20,22,24 Randomized clinical trials have shown that weight loss per se is the main determinant of improvement in endothelial function. In fact, obesity and visceral adiposity are associated with endothelial dysfunction in resistance and conduit arteries, and weight loss brought about by lowered caloric intake improves endothelial function, even in the absence of heart disease or CV risk factors.19,37
Significantly, however, in clinical trials without changes in body weight low carbohydrate/high protein diets may be associated with twice amount of arterial plaque, a reduction in the number of circulating and bone marrow endothelial progenitor cells (EPC) in murine models of atherosclerosis,38 and lower FMD in healthy and obese subjects.20,39 Moreover, we previously reported that low-carbohydrate, high-protein, high-fat diet was associated to poorer small artery endothelial function in a cohort of increased cardiovascular risk pateints.40 Our results therefore reinforce the known negative impact of low carbohydrate diets on vascular function assessed by FMD under fasting conditions, using the PAT technique.
Clinical cardiology is largely concerned with treatment of lesions in medium-sized and large arteries. However, the microcirculation and small arteries are important because they generate peripheral resistance, participate in clinical pathology of the coronary circulation, and are of significance in chronic renal disease, retinopathy and cerebrovascular disease of the white matter.41 While the European Society of Cardiology classifies RHI with other techniques to measure “endothelial function”,42 currently available data indicate each of these methods likely has a unique blend of physiological determinants, and each provides particular information about different vascular beds.38 PAT provides an additional tool for risk evaluation and prediction of outcomes. The index of cutaneous hyperemia, actually a ratio of the average pulse wave amplitude in the minute after release of the cuff to the average amplitude at baseline, depends upon characteristics of the microvasculature in the finger. FMD is highly dependent upon nitric oxide (NO) production, whereas RHI is not, even though NO has been reported to contribute to RHI.26 A recent direct comparison of methods did not reveal a strong correlation.43 However, FMD is difficult to perform compared to RHI, and the correlation between the two and other data derived are sufficient to continue clinical investigation.43,44 Indeed, saRHI correlates with coronary endothelial dysfunction,29 accurately measures vascular effects when the net cardiovascular risk burden improves,30 correlates with CIMT, and can predict future adverse cardiovascular events.30 Information from noninvasive PAT studies is useful to assess static or serial vascular function in a variety of patient types with raised cardiovascular risk.32 Our group has shown that saRHI-measured endothelial dysfunction is also associated with increased sE-selectin plasma concentrations, suggesting that at least some mechanisms are shared by both large and small artery/microvascular function.45 In patients without metabolic syndrome but with risk-related elevations in sE-Selectin and sVCAM-1, there was a strong correlation between saRHI values and levels of oxidized LDL.46 A uniting theme is that the saRHI is clearly associated to some metabolic parameters, mainly waist circumference, plasma levels of triglycerides, HDL-C and apolipoprotein A1,47 smoking, and plasma glucose.47 In asymptomatic diabetics with microalbuminuria, saRHI is also an independent predictor of CHD.48
LimitationsOur study had other limitations. One was related to the score used in the study and the cut-off values. The LCDS was performed using USA population data, and the percentiles of diet relate to healthy American white men and women. However, since the diet consumed by our study population was similar to the diet of a corresponding American population with equally high cardiovascular risk,49 the comparison is reasonable. Our study design did not control all possible confounding variables. Errors associated with completion of food diaries are possible, such as undisclosed supplemental use of omega-3 fatty acids and polyphenols. Although undescribed, either the pharmacological agents that our patients were taking themselves, or interaction with macronutrient changes, could have influenced small artery vascular reactivity. Last, a cross-sectional study cannot establish causality. In view of the strength of our main finding, the quantitative importance of these limitations appears small.
ConclusionIn a cross-sectional study carried out in metabolic syndrome patients, a dietary pattern characterized by a high low diet score (high protein and fat, low-carbohydrate content) was associated with poorer peripheral small artery vascular reactivity compared with individuals consuming a diet with a lower diet score. The association was confirmed for each MS component, except for low HDL-c criteria, and T2D patients. This relationship needs confirmation with larger cohorts in randomized clinical trials.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of DataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors declared no conflict of interest.
This work was supported by grants from ISCIII, Madrid, Spain (PI 05/1954). CIBERDEM is an initiative of ISCIII, Spain. The authors’ responsibilities were as follows: RF, NP and LM designed the study; JM, GA, RF and NP conducted research; MH performed the biochemical analyses; JM, RF and LM performed the statistical test and wrote the final manuscript. RK reviewed the manuscript, and contributed significantly to the final form of the introduction and the discussion. All authors have read and approved the final manuscript.
This work responds to a communication entitled “Negative effect of a low-carbohydrate, high protein, high fat diet on vascular reactivity in patients at increased cardiovascular risk”. Special mention 2012 (19/30) for the best abstracts presented at the XXV Congress of the SEA Reus 2012.