In breast cancer surgery, there are techniques for sentinel lymph node biopsy (SLNB) that do not require Nuclear Medicine, such SentiMag®, which uses ferromagnetic particles. The main purpose of this analysis is to study the degree of concordance in SLNB between SentiMag® and the standard method (Tc99 radiotracer). The secondary objective is to identify factors that impact in sentinel node detection rate and matching detection rate between both probes.
MethodsObservational and retrospective study performed from January to December 2021 focused on patients undergoing breast surgery and SLNB who were injected with both tracers, the ferromagnetic SentiMag® and Tc99 radiotracer. Once the diagnostic accuracy tests were performed, a further evaluation of the detection rate for each probe and the concordance between probes were accomplished. After those results, a deeper analysis of differences in detection rates for each probe and concordance between probes were assessed for various factors: neoadjuvant therapy, BMI, mitotic index, and triple-negative immunohistochemical profile.
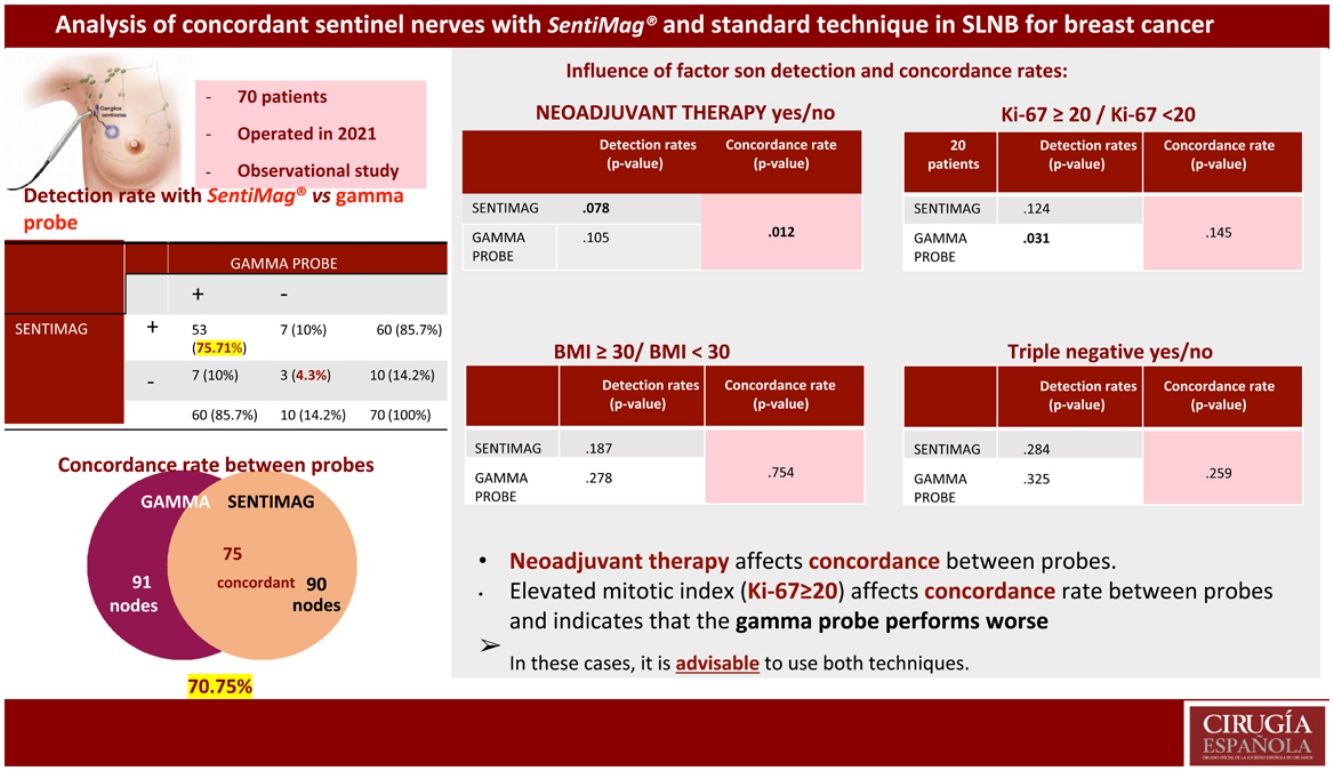
ResultsThe clinical study had a sample size of 70 patients. The overall false-negative rate (FNR) was 4.3%. The detection rate was the same for each technique (85.7%). A total of 106 nodes were biopsied, with a concordance rate of 70.75%. Significant differences were found in concordant nodes according to neoadjuvant therapy (p-value 0.012). For the Ki-67 factor (<20 or ≥20), significant differences were found in detected nodes (p-value 0.031 gamma probe; p-value 0.124 SentiMag®).
ConclusionsThe detection rates of SentiMag® and the gamma probe are equivalent. The application of the dual technique minimizes the FNR. A high mitotic index affects the detection rate of the gamma probe, and neoadjuvant therapy negatively impacts the concordance rate.
Existen técnicas para la biopsia selectiva del ganglio centinela (BSGC) en cáncer de mama que no precisan Medicina Nuclear como SentiMag® que emplea partículas ferromagnéticas. El objetivo principal es estudiar el grado de concordancia en la BSGC con SentiMag® y con el método estándar (radiotrazador Tc99). Los objetivos secundarios son identificar factores que repercutan en la detección y la concordancia entre sondas.
MétodosEstudio observacional y retrospectivo desde enero hasta diciembre de 2021. Se incluyeron pacientes sometidas a cirugía mamaria y BSGC a las que se les inyectó el radiotrazador y trazador ferromagnético. Se realizó un análisis de pruebas diagnósticas y se analizó la tasa de detección por cada sonda y la tasa de concordancia entre sondas. Además, se evaluaron las diferencias en tasas de detección por cada sonda y en concordancia entre sondas para diferentes factores: neoadyuvancia, IMC, índice mitótico y perfil inmunohistoquímico triple negativo.
Resultados70 pacientes fueron incluidas. La tasa de falsos negativos (TFN) global fue 4,3%. La detección para cada técnica fue equivalente (85,7%). Fueron biopsiados 106 ganglios con una tasa de concordancia del 70,75%. Al analizar la tasa de concordancia de los ganglios centinela en pacientes con y sin neoadyuvancia, se hallaron diferencias significativas (p-valor 0,012). Para el factor Ki-67 (<20 o ≥20) se obtuvieron diferencias significativas en la tasa de detección de ganglios centinela (p-valor 0,031 sonda gamma; p-valor 0,124 SentiMag®).
ConclusionesLa tasa de detección de SentiMag® y sonda gamma es equivalente. La aplicación de la doble técnica minimiza la TFN. La neoadyuvancia repercute negativamente en la tasa de concordancia. El índice mitótico elevado (Ki-67 ≥ 20) afecta a la tasa de concordancia entre sondas y apunta un peor desempeño de sonda gamma.
The radioactive tracer, a 99 mTc-labeled nanocolloid, is the most commonly used technique for SN (sentinel node) detection in breast cancer (BC). Preoperative lymphoscintigraphy is necessary to determine the location and number of SNs in the lymphatic regions at risk. Although the results of the isotopic tracer are excellent,1 it has two disadvantages: it irradiates (the patient and the health personnel) and it is not available in all centres due to the unavailability of nuclear medicine, as is the case in most regional hospitals.2
In the last decade new techniques have been developed to locate the SN. SentiMag® is the most studied superparamagnetic method for marking and locating the SN. It operates based on magnetic fields and therefore avoids irradiation. It does not depend on the nuclear medicine service and surgeries can be planned any day of the week. In addition, the magnetic tracer can be injected from the time of surgery up to 7 days prior to it, thus optimising surgical time and offering greater organisational autonomy.
Studies such as that by Mok CW3 claim that the SentiMag® method is not inferior to the standard technique. Other more recent studies confirm these results.4,5 In hospitals that do not have nuclear medicine, their having a valid technique for locating the SN offers important advantages:
- -
It facilitates the logistic autonomy of the centre and provides the patient greater psychological well-being, since the magnetic tracer can be injected on the same day of the intervention, avoiding transfer to the referral centre.
- -
It avoids preoperative diagnostic tests such as lymphoscintigraphy and the irradiation inherent to the radioisotope for the patient and their relatives, and for the health professional.
Our null hypothesis was that the ferromagnetic tracer and SentiMag® are equivalent detection methods to the radiotracer in BC patients undergoing SLNB.
The main objective was to evaluate the performance of SentiMag® in SN detection and to study the degree of concordance with the SentiMag® method and with the standard method (radiotracer) in axillary staging of BC. The secondary objectives were to study the clinical, radiological, or immunohistochemical factors that may influence the detection and concordance rate in SLNB performed with SentiMag® and with radiotracer in patients with BC.
Material and methodWe conducted a descriptive observational study. Data were collected retrospectively from January 1 to December 31, 2021.
Patients over 18 years of age diagnosed with breast cancer at the Vega Baja Hospital of Orihuela with negative axillary imaging and/or puncture, candidates for SLNB as recommended in the Consensus of 2022 of the Spanish Society of Senology and Breast Pathology on the axillary management of breast cancer were included. Patients with and without neoadjuvant therapy were included in the study. Patients with metastatic disease, with magnetic seeds, allergic to ferric compounds, or with metallic implants in the thorax were excluded.
Study variablesThe detection rate per probe and the concordance rate between probes (%) were analysed. Sensitivity, specificity, and predictive values of SN detection by magnetic tracer versus the standard technique were also analysed.
The other variables analysed were clinical (age, smoking, BMI, size, laterality, tumour multifocality, neoadjuvant therapy, and response), anatomo-pathological (anatomo-pathological subtype, intrinsic and Ki-67 (%)), and surgical (type of surgery, total number of nodes biopsied, detected with SentiMag®, detected with gamma probe and number of concordant nodes).
This work was approved by the research unit of the Vega Baja Hospital after the corresponding analysis by the ethics and clinical research committee.
Action protocol and measurement methodsAll patients who were candidates for surgical treatment and SLNB underwent two simultaneous lymph node detection techniques: the standard gamma probe detection technique and the magnetic localisation technique.
The day before surgery the patients were routinely transferred to the nuclear medicine referral centre, and given a peritumoral subdermal injection of an isotopic solution labelled with Tc99 and then underwent lymphoscintigraphy.
During anaesthetic induction prior to surgery and after performing an intercostal nerve block, 2 ml of Magtrace® tracer was injected periareolar followed by breast massage for 20 minutes.
Two probes were used simultaneously during axillary surgery, the gamma probe to detect the radiolabelled tracer and the magnetic probe (SentiMag®) to identify the SN with the ferromagnetic tracer. Nodes with a reading of more than 10% of the node with the highest numerical reading were considered positive by both methods.
During the operation, the number of nodes removed with each probe and the number of concordant nodes between probes were recorded. An intraoperative OSNA study was performed considering the total sum of copies. Lymphadenectomy was performed in patients with macrometastases and tumour burden >10,000 copies and in patients undergoing NACT at any tumour burden.6
Statistical analysisIBM-SPSS version 29 was used for the statistical analyses. A significance level was set at .05 with a 95% confidence interval.
A test of normality was used to decide the measures of central tendency and dispersion. The Kolmogorov-Smirnov test was used for quantitative variables: nodes detected with SentiMag® and gamma probe, as well as concordant nodes.
The guidelines used to design and draft this work comply with the STAndards for the Reporting of Diagnostic accuracy studies (STARD).7
ResultsAll patients who underwent surgery in the first year of experience with SentiMag® were included, as the aim is to analyse the preliminary results obtained, with the possibility of making further comparisons after progressing along the learning curve. Four patients were excluded due to inadequate registration of data in the computerised surgical protocol.
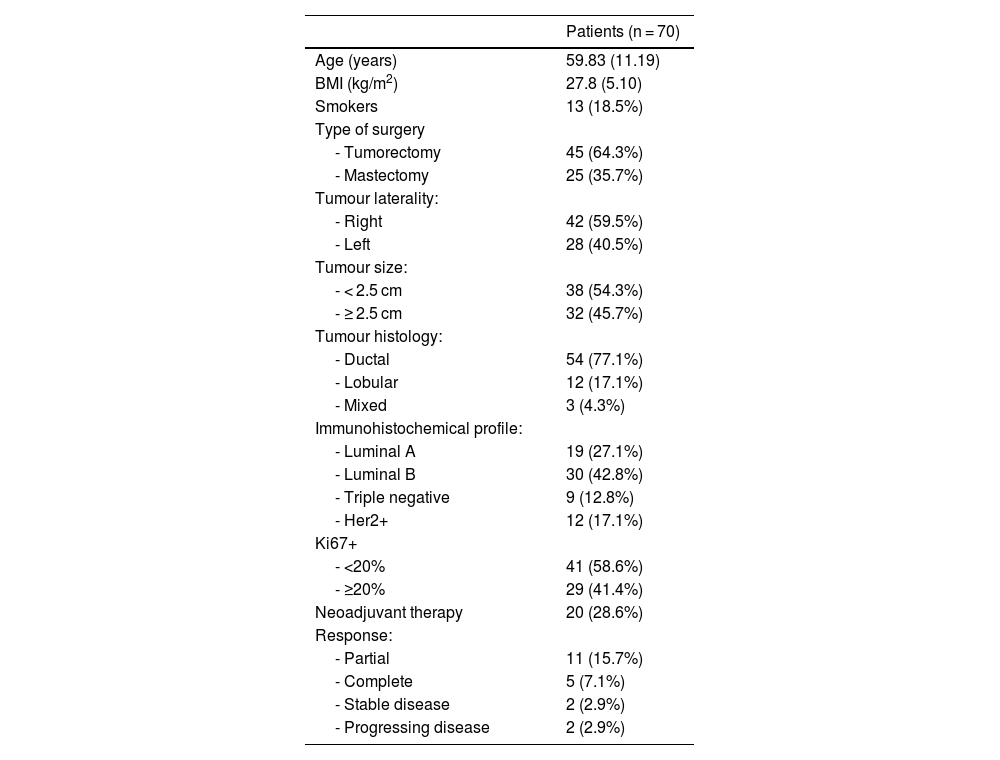
The mean age of the patients was 59.83 ± 11.19 years. The mean BMI was 27.76 with a standard deviation of 5.11, with a minimum of 18 and a maximum of 41.
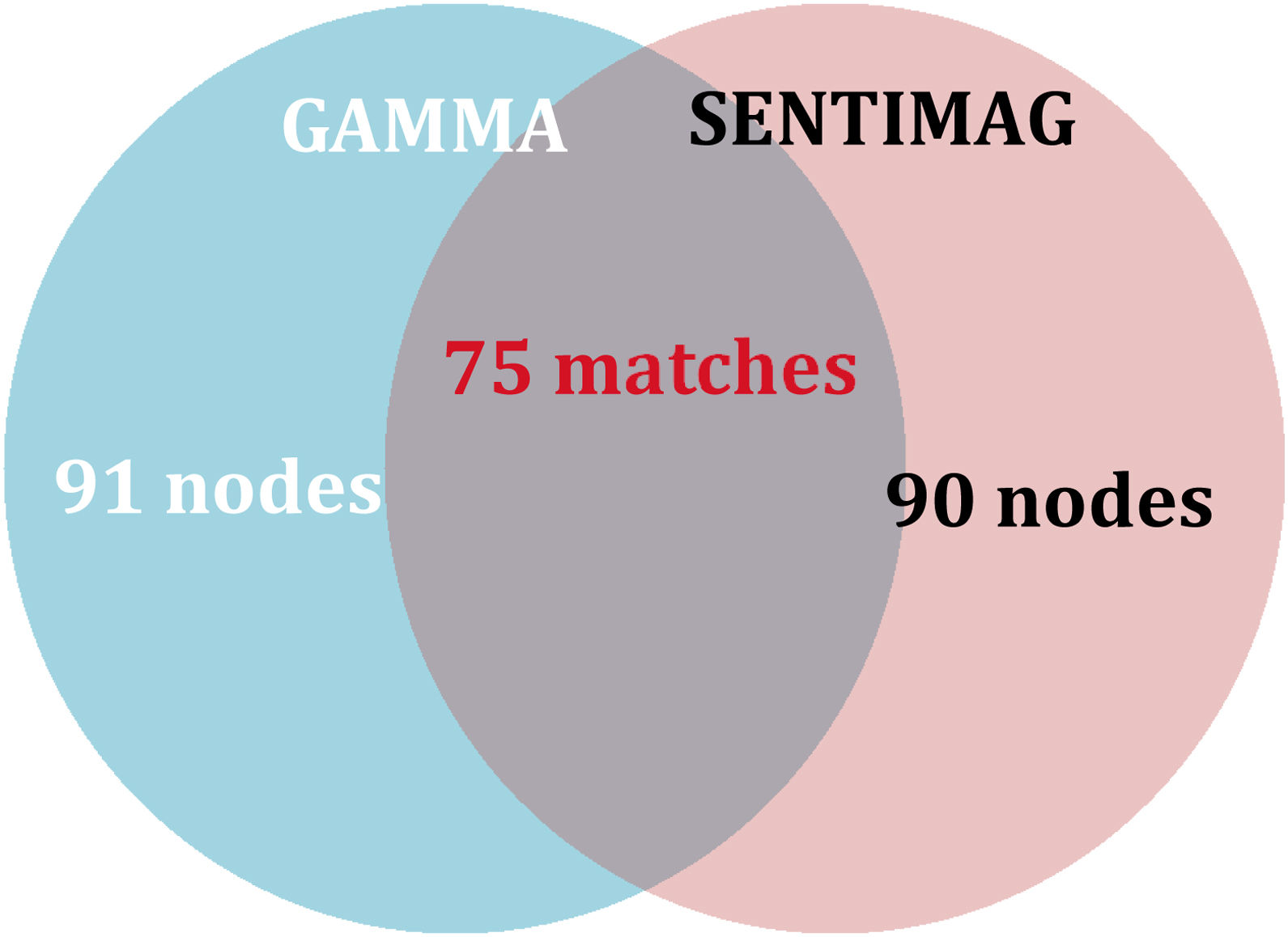
A total of 106 nodes were biopsied intraoperatively. The maximum number of nodes biopsied per patient was 4 with a median of 1 node per patient and an IQR of 1 for both the gamma probe and SentiMag®. The gamma probe and SentiMag® detected a total of 91 and 90 nodes, respectively. Patient characteristics are summarised in Table 1.
Clinical and pathological characteristics of the patients.
| Patients (n = 70) | |
|---|---|
| Age (years) | 59.83 (11.19) |
| BMI (kg/m2) | 27.8 (5.10) |
| Smokers | 13 (18.5%) |
| Type of surgery | |
| - Tumorectomy | 45 (64.3%) |
| - Mastectomy | 25 (35.7%) |
| Tumour laterality: | |
| - Right | 42 (59.5%) |
| - Left | 28 (40.5%) |
| Tumour size: | |
| - < 2.5 cm | 38 (54.3%) |
| - ≥ 2.5 cm | 32 (45.7%) |
| Tumour histology: | |
| - Ductal | 54 (77.1%) |
| - Lobular | 12 (17.1%) |
| - Mixed | 3 (4.3%) |
| Immunohistochemical profile: | |
| - Luminal A | 19 (27.1%) |
| - Luminal B | 30 (42.8%) |
| - Triple negative | 9 (12.8%) |
| - Her2+ | 12 (17.1%) |
| Ki67+ | |
| - <20% | 41 (58.6%) |
| - ≥20% | 29 (41.4%) |
| Neoadjuvant therapy | 20 (28.6%) |
| Response: | |
| - Partial | 11 (15.7%) |
| - Complete | 5 (7.1%) |
| - Stable disease | 2 (2.9%) |
| - Progressing disease | 2 (2.9%) |
Quantitative variables are represented by the mean (standard deviation). Qualitative variables are represented by absolute frequency (percentage).
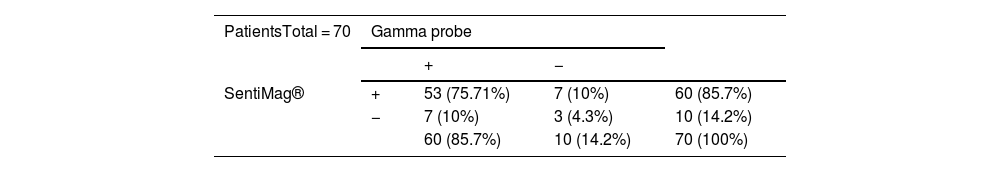
Of the patients, 75.71% had concordant sentinel nodes. If negative results are also taken into account, the concordance rate between techniques is 80%. The sensitivity and positive predictive value of SentiMag® was 88.3%.
The detection rate with each probe was 85.7%, rising to 95.7% with combined use. Furthermore, the overall FNR was 4.28%, much lower than using SentiMag® alone (11.6%). Table 2 shows the number of patients in whom SN was detected and the distribution of SN detected by each probe.
Number of patients in whom sentinel lymph nodes were detected with each of the probes.
| PatientsTotal = 70 | Gamma probe | |||
|---|---|---|---|---|
| + | − | |||
| SentiMag® | + | 53 (75.71%) | 7 (10%) | 60 (85.7%) |
| − | 7 (10%) | 3 (4.3%) | 10 (14.2%) | |
| 60 (85.7%) | 10 (14.2%) | 70 (100%) | ||
The symbol (+) represents presence of detection and the symbol (−) absence of detection. The raw number is represented (percentage).
Spearman’s Rho was calculated between the lymph nodes detected with gamma probe and with SentiMag®, obtaining a result of .75 and 1 respectively, with a p-value < .001.
As for the histological result of the lymph nodes, the SN detected in 2 (2.8%) patients with gamma probe without detection by SentiMag® was positive for malignancy. However, one SN detected in a SentiMag® patient, without detection by gamma probe, was positive for malignancy.
The concordance rate between nodes detected with each probe was 70.75%.
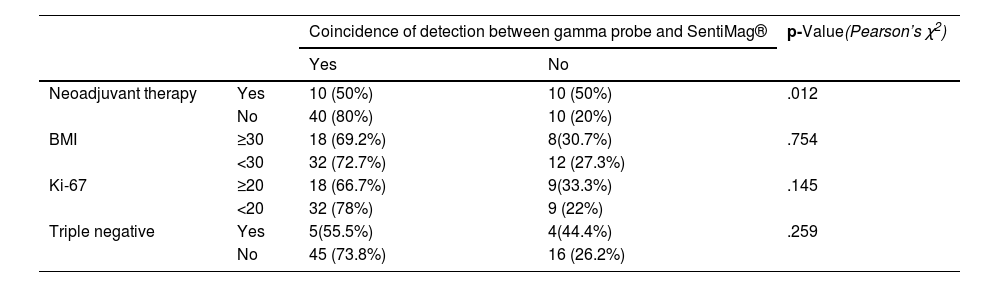
Regarding the results of the secondary endpoints, the differences in the detection rate between patients who required neoadjuvant therapy did not reach statistical significance. However, there are significant differences in the percentage of concordant nodes among the patients who required neoadjuvant therapy, with a p-value of .012.
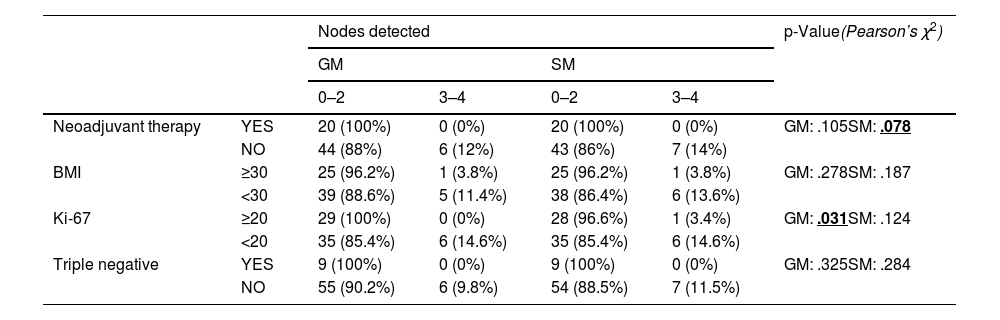
Neither were there significant differences between probes in detection or concordance in patients with a BMI ≥ 30 compared to those with a BMI < 30.
In relation to the mitotic index, statistically significant differences were obtained in the detection rate of the gamma probe, with worse results in Ki-67 ≥ 20. However, with SentiMag® no differences in detection were observed between each group established (high mitotic index vs. low mitotic index).
Using both probes for triple negative patients vs. other molecular subtypes, no significant differences were observed.
Table 3 shows the number of nodes detected by each probe according to each factor analysed and Table 4 shows the number of concordant SN for each factor analysed.
Nodes detected with each probe according to each factor analysed.
| Nodes detected | p-Value(Pearson’s χ2) | |||||
|---|---|---|---|---|---|---|
| GM | SM | |||||
| 0–2 | 3–4 | 0–2 | 3–4 | |||
| Neoadjuvant therapy | YES | 20 (100%) | 0 (0%) | 20 (100%) | 0 (0%) | GM: .105SM: .078 |
| NO | 44 (88%) | 6 (12%) | 43 (86%) | 7 (14%) | ||
| BMI | ≥30 | 25 (96.2%) | 1 (3.8%) | 25 (96.2%) | 1 (3.8%) | GM: .278SM: .187 |
| <30 | 39 (88.6%) | 5 (11.4%) | 38 (86.4%) | 6 (13.6%) | ||
| Ki-67 | ≥20 | 29 (100%) | 0 (0%) | 28 (96.6%) | 1 (3.4%) | GM: .031SM: .124 |
| <20 | 35 (85.4%) | 6 (14.6%) | 35 (85.4%) | 6 (14.6%) | ||
| Triple negative | YES | 9 (100%) | 0 (0%) | 9 (100%) | 0 (0%) | GM: .325SM: .284 |
| NO | 55 (90.2%) | 6 (9.8%) | 54 (88.5%) | 7 (11.5%) | ||
Gamma probe (GM) and SentiMag® (SM). The p-value is included for each factor.
Coincidence of detection between both probes for the clinical factors and tumour characteristics analysed.
| Coincidence of detection between gamma probe and SentiMag® | p-Value(Pearson’s χ2) | |||
|---|---|---|---|---|
| Yes | No | |||
| Neoadjuvant therapy | Yes | 10 (50%) | 10 (50%) | .012 |
| No | 40 (80%) | 10 (20%) | ||
| BMI | ≥30 | 18 (69.2%) | 8(30.7%) | .754 |
| <30 | 32 (72.7%) | 12 (27.3%) | ||
| Ki-67 | ≥20 | 18 (66.7%) | 9(33.3%) | .145 |
| <20 | 32 (78%) | 9 (22%) | ||
| Triple negative | Yes | 5(55.5%) | 4(44.4%) | .259 |
| No | 45 (73.8%) | 16 (26.2%) | ||
The raw number of matched nodes between probes and percentage is included. The p-value is included for each factor.
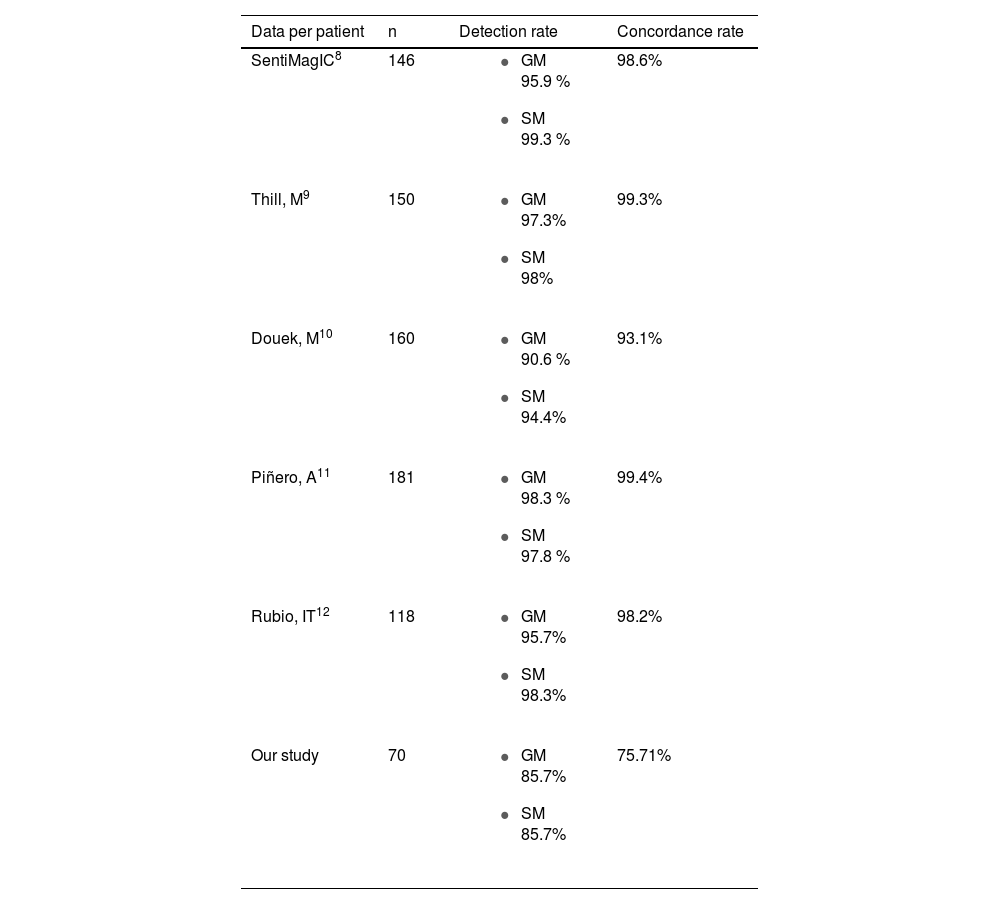
In this study, the detection rate is lower (85.7%) than that reported in other studies. Table 5 shows the detection rates obtained with SentiMag® and gamma probe in other studies, in which both methods have detection rates close to or above 95%.8–12 However, the overall detection rate with both techniques is 95.7%, which means that the combined use of both techniques reduces the FNR of SentiMag® by 7.3%.
Studies comparing gamma probe and SentiMag® and their respective concordance rate per patient.
| Data per patient | n | Detection rate | Concordance rate |
|---|---|---|---|
| SentiMagIC8 | 146 |
| 98.6% |
| Thill, M9 | 150 |
| 99.3% |
| Douek, M10 | 160 |
| 93.1% |
| Piñero, A11 | 181 |
| 99.4% |
| Rubio, IT12 | 118 |
| 98.2% |
| Our study | 70 |
| 75.71% |
Detection and concordance rate expressed as a percentage.
The concordance rate per patient (75.71%) is lower than expected (Table 5). Ninety nodes were detected with SentiMag® and 91 with the gamma probe, with 75 concordant nodes, giving a concordance rate per node of 70.75% (Fig. 1).
In comparison with studies such as that of Piñero et al. in which it exceeds 80%, in our work it was significantly lower.8,11,14 These results are conditioned by the technique used in this sample of patients, which corresponds to the initial experience with SentiMag®. It is likely that, after extending the study time with a larger sample, we will obtain more reliable results that are closer to their results.
Therefore, at this stage, the combined use of both techniques is the preferred method in our setting.
We observed that gamma probe detection rates were not significantly affected by neoadjuvant therapy. However, the detection rate of SentiMag® does show poorer performance in the case of neoadjuvant therapy (p = .078), although this was not significant. Significant differences were also found in the number of concordant lymph nodes detected among patients who had required NACT, with a worse match rate in these cases.
Other studies have stated that the FNR in patients who have required neoadjuvant therapy is higher.11 A recent systematic review analysed the performance of SLNB after NACT in patients with and without axillary involvement at diagnosis. The FNR was 14% and 6%, respectively. Following these results, they recommend SLNB including biopsy of the affected lymph node, pre-marked using dual-tracer, and at least 3 sentinel lymph nodes.13
Furthermore, many publications support the hypothesis of ipsilateral lymphatic blockage, triggered by tumour burden, radiotherapy, or previous surgery, which explains contralateral lymphatic spread14–16 and may impair tracer migration in axillary staging. Therefore, the dual tracer technique, in selected cases, may help increase the effectiveness of SLNB.
Regarding the results obtained in the clinical variables analysed, no differences were found in detection rates or concordance according to BMI, and therefore SentiMag® can be considered equivalent to the radiotracer, regardless of BMI. Similarly, in the Houpeau et al. study, there was no significant difference in SN detection between patients with BMI < 25 or ≥ 25.17
There are studies that relate elevated mitotic index to an increased risk of skip metastasis.18 In this study, significant differences were found in the detection rate of nodes detected with the gamma probe, which may mean poorer performance in patients with Ki ≥ 20 compared to SentiMag®. However, in the analysis of the concordance between probes according to mitotic index, no significant differences were found. These results support the use of the dual technique for SN detection because neither of the two probes is FN-free.
There are no studies demonstrating that SentiMag® performs worse than the standard technique in a given immunohistochemical profile of the tumour. In our study, no differences were found in the detection rate and concordance between probes in triple-negative carcinoma, which usually has a worse prognosis.
Based on our initial results and the patient sample obtained, SentiMag® has been shown to be a non-inferior technique for SN detection compared to the gamma probe. Both probes have a detection rate per patient that is equivalent and exceeds 85%. However, the results obtained with SentiMag® may be subject to errors inherent to the associated learning curve. In order to improve the reliability of the information provided by SLNB with SentiMag® and consider its isolated use at some point, we are analysing subsequent results, once the learning curve of the technique has been overcome, for future studies.
With regard to the limitations of this study, it is a retrospective study susceptible to bias: patients in whom an isolated technique was used were excluded. Likewise, patients whose surgical report contained insufficient information were excluded. Secondly, the removed lymph nodes were not marked with the probe which they were identified, so that the result of the intraoperative OSNA analysis could not be attributed to each node. Finally, we analysed a smaller sample than other studies and the data reflect our initial experience with SentiMag®, with which, over time, the mode of administering the magnetic tracer (Magtrace®) has been modified, and presumably the uptake is better. This should be confirmed in future analyses, and therefore the results of this study should be considered preliminary and should be contrasted in long-term studies.
ConclusionsSentiMag® is a non-inferior detection technique compared to the radiotracer in our setting, but the high rate of false negatives (over 10% universally accepted) when used on its own means that at this time it is not possible to avoid the use of radiotracer in our centre, both techniques being required to obtain a good overall detection rate.
Neoadjuvant therapy affects the concordance between probes, which confirms the need to use the dual technique in these patients especially.
BMI and triple-negative immunohistochemical profile do not affect the detection rate or the inter-probe concordance rate. However, high mitotic index (Ki-67 ≥ 20) does affect the concordance rate between probes and indicates a worse performance of the gamma probe in these patients, making it advisable to use both tracers.
FundingNo funding was received for this research study.
Many thanks to Breast Surgery team in Hospital Vega Baja, the mastermind behind this study. Also, thanks to the university collaborator Dr. Diego Flores from Universidad de Murcia.