During oncoplastic procedures, the vascularization and perfusion of the skin flaps is modified, thus increasing the possibility of skin necrosis.
The objective of this study is to evaluate the effectiveness of indocyanine color green angiography (ICG-A) to determine intraoperative skin necrosis after oncoplastic surgery or skin-sparing or nipple-skin sparing mastectomy (NSSM).
Patients and methodProspective observational study to evaluate the sensitivity, specificity and positive and negative predictive values of the ICG-A in women with high-risk breast cancer.
Results98 women and 156 breasts were included in the study. A total of 20 women (20.4%) presented an image of ischemia in the ICG-A. 21 women (21.4%) presented ischemic events in the postoperative period, 71.4% of these events had been detected in the third ICG-A. Three of these patients (3.1%) presented a serious complication that required reintervention. The sensitivity and specificity of the ICG-A was 71.4% and 93.5%, respectively.
ConclusionsICG-A has high specificity and negative predictive value for detecting areas of low perfusion. In breast units with highly complex surgery, it can be useful to plan extreme surgeries and identify skin areas of low perfusion.
Durante los procedimientos oncoplásticos se modifica la vascularización y perfusión de los colgajos cutáneos, incrementando así la posibilidad de necrosis cutánea.
El objetivo de este estudio es evaluar la eficacia de la angiografía con verde de indocianina (A-VIC) para determinar necrosis cutánea intraoperatoria tras una cirugía oncoplástica o una mastectomía preservadora de piel o piel-pezón (MPPP).
Pacientes y métodoEstudio prospectivo observacional para evaluar la sensibilidad, especificidad y valores predictivos positivo y negativo de la A-VIC en mujeres con cáncer de mama y alto riesgo.
ResultadosSe incluyeron 98 mujeres en el estudio y 156 mamas. Un total de 20 mujeres (20,4%) presentaron imagen de isquemia en la A-VIC. 21 mujeres (21,4%) presentaron eventos isquémicos en el postoperatorio, el 71,4% de estos eventos habían sido detectados en la tercera A-VIC. Tres de estas pacientes (3,1%) presentaron una complicación grave que precisó reintervención. La sensibilidad y especificidad de la A-VIC fue de 71,4% y 93,5%, respectivamente.
ConclusionesLa A-VIC tiene una alta especificidad y valor predictivo negativo (VPN) para detectar áreas de baja perfusión. En unidades de mama con cirugía de alta complejidad puede ser útil para planificar cirugías extremas e identificar áreas cutáneas de baja perfusión.
Oncoplastic surgery and breast-sparing mastectomies are two technical procedures that have improved the oncological and cosmetic outcomes after conservative surgery and immediate reconstruction in women with breast cancer or at high risk for breast cancer. During the performance of these surgical techniques, a significant alteration in the perfusion of the breast skin envelope occurs, which can produce areas of vascular damage to the skin and skin necrosis. Skin necrosis is the most significant adverse event in oncoplastic and reconstructive breast surgery, as it causes delays in adjuvant treatments to surgery, impairment of cosmetic outcome, and sometimes loss of the implant.1,2
ICG-A has been proposed as a diagnostic alternative to determine the vascular perfusion of the breast skin envelope during surgery. This would lead to the removal of tissue at risk of necrosis to avoid this complication during the postoperative period3–6 months. However, the literature does not currently allow an adequate assessment of this diagnostic procedure due to the absence of prospective studies that have evaluated its sensitivity, specificity and predictive values.
The aim of this prospective study was to describe ICG-A in breast skin flaps in women with breast cancer or at high risk for breast cancer undergoing oncoplastic surgery or skin-sparing mastectomy (SSM) or skin-nipple mastectomy. The results of this study are intended to evaluate the sensitivity, specificity and predictive values of this technique for predicting adverse events during the postoperative period).
MethodProspective observational study to evaluate the efficacy of ICG-A in women with breast cancer and at high risk operated between May 2023 and May 2024. The study was assessed and approved by the ethical committee of our hospital (gBREAST222022/398) and registered on the web ClinicalTrials.gov (NCT05910931).7
Inclusion and exclusion criteriaThe study population includes two groups of patients:
- a
Oncoplastic procedures for breast conservation. This group was made up of women with breast cancer in whom the tumour committee recommended a breast conservation surgical procedure. The techniques included for this study were vertical and horizontal mammoplasty and local flaps. Patients who underwent lumpectomy/quadrantectomy, lateral resections and round-block technique were excluded.
- b
SSM or NSSM with immediate breast reconstruction. This group was made up of patients diagnosed with breast carcinoma who required a mastectomy, as well as those women with a risk-reducing mastectomy for breast cancer. The surgical techniques included in this group were SSM types 1 and 4 of Carlson8 and NSSM through inframammary access with a vertical pattern. Patients with delayed reconstruction and/or retropectoral reconstruction or without reconstruction were excluded.
- a
Oncoplastic surgery technique. In patients who underwent vertical mammoplasty, a Wise vertical pattern was designed and the pedicle was selected based on the location of the tumour resection and the distance from the nipple-areola complex (NAC) to its new location.
- b
Mastectomy technique. A mastectomy adapted to the breast was performed, optimising the preservation of the original elements of the breast (inframammary fold, skin envelope, fatty transitions, NAC) according to the anatomical and oncological possibilities of each patient. The thickness of the skin flap of the mastectomy was evaluated according to the Rancati classification.9 In all NSSM, the retroareolar tissue was cleaned once the breast was removed using the Folli technique.10 Reconstruction was performed by placing a polyurethane implant in the prepectoral position.
- c
ICG-A technique. The 25 mg ampoule of indocyanine green (ICG) powder was diluted in 10 cc of distilled water. Intravenous boluses of 2.5 cc of ICG followed by 10 cc of saline solution were used. For visualisation of cutaneous perfusion, the SPY System platform with SPY-Q software (Stryker®) was used, which by means of laser light emission stimulates the ICG to emit infrared energy within 2 minutes after intravenous injection.
During the surgical intervention, three injections of ICG solution were performed to evaluate three angiographies:
- -
A first injection with the patient asleep before starting the intervention to visualise the vascular anatomy of each patient and assess the pedicles and incisions.
- -
A second bolus after breast resection to assess the viability of the skin flaps and glandular pedicles.
- -
A third injection to obtain an angiogram after implant placement and wound closure.
During the angiographies, the surgeons compared their findings with clinical examination. Skin resection was only indicated when the intraoperative clinical finding was highly suspicious of ischaemia, regardless of the angiographic findings. If the angiography raised suspicion of ischaemia, but the clinical examination did not corroborate it, skin resection was not performed. All interventions were performed by the 5 surgeons of the unit and the angiographies were recorded on video for viewing in case of postoperative ischaemia.
DefinitionsSuperficial skin necrosis. Loss of epidermis, partial dermis and/or formation of eschar that did not extend or expose the subcutaneous fat.
Deep skin necrosis. Loss of the dermis and epidermis of the skin with exposure of the subcutaneous fat.
Severe surgical complication. A complication that required surgical intervention to resolve.
Statistical analysisJustification of sample size. Assuming a sensitivity of 60% for intraoperative clinical examination and the intention to achieve a sensitivity of 90% with angiography, with a confidence level of 95% and a statistical power of 80%, estimating losses of 15%, the sample size was estimated at 49 angiographies.
A descriptive analysis of the variables included in the study was performed. All quantitative variables are expressed with their mean and standard deviation. Qualitative variables are expressed as proportions and their respective confidence intervals. Data collection and statistical analysis were performed using version 24 of the IBM SPSS statistical program.
The following formulas were used to validate diagnostic tests:
- •
Positive predictive value (PPV) = true positives (TP)/TP + false positives (FP)
- •
Negative predictive values (NPV) = true negatives (TN)/TN + false negatives (FN)
- •
Sensitivity = TP/total patients
- •
Specificity = TVN/total non patients
- •
FN = patients with a negative test/all patients
- •
FP = patients with a positive test/all patients
During the study period, 98 women met the inclusion criteria. Mastectomy with prepectoral reconstruction was performed in 75 women, and oncoplastic surgery was performed in 23 patients. In 58 women, the procedure was bilateral, resulting in the analysis of 156 breasts (Fig. 1).
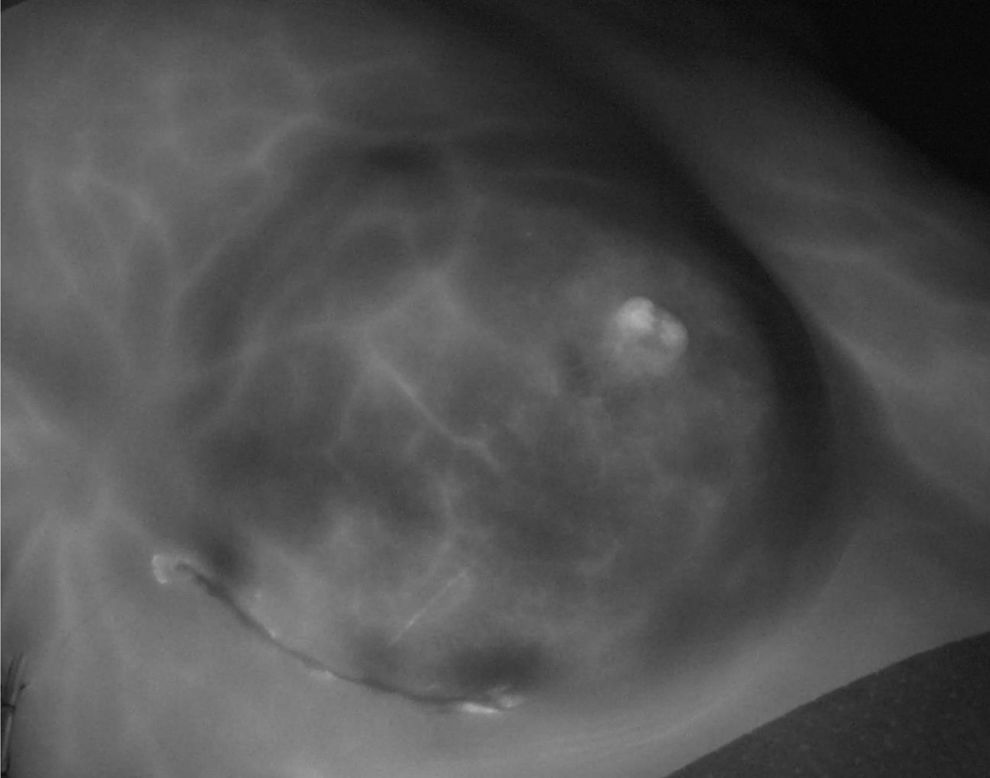
In all patients, the first angiogram was performed after the incision design had been made, showing adequate vascularisation of the breast skin in 100% of the women, even in the eight women (8.2%) included who had a history of conservative surgery and radiotherapy. In 8 patients (8.2%), the initial planning was modified due to vascularisation findings during presurgical angiography. In 22 women (22.4%), the angiographies showed a false image of necrosis, defined as the appearance of areas without contrast uptake delimited by contrast-enhancing vessels (Fig. 2). In 22 patients (22.4%) low perfusion of the ICG was detected after the second angiogram, which continued after closure (third angiogram) in 16 (72.7%) of them. A total of 20 women (20.4%) presented an image of ischaemia in the final angiogram (Fig. 3).
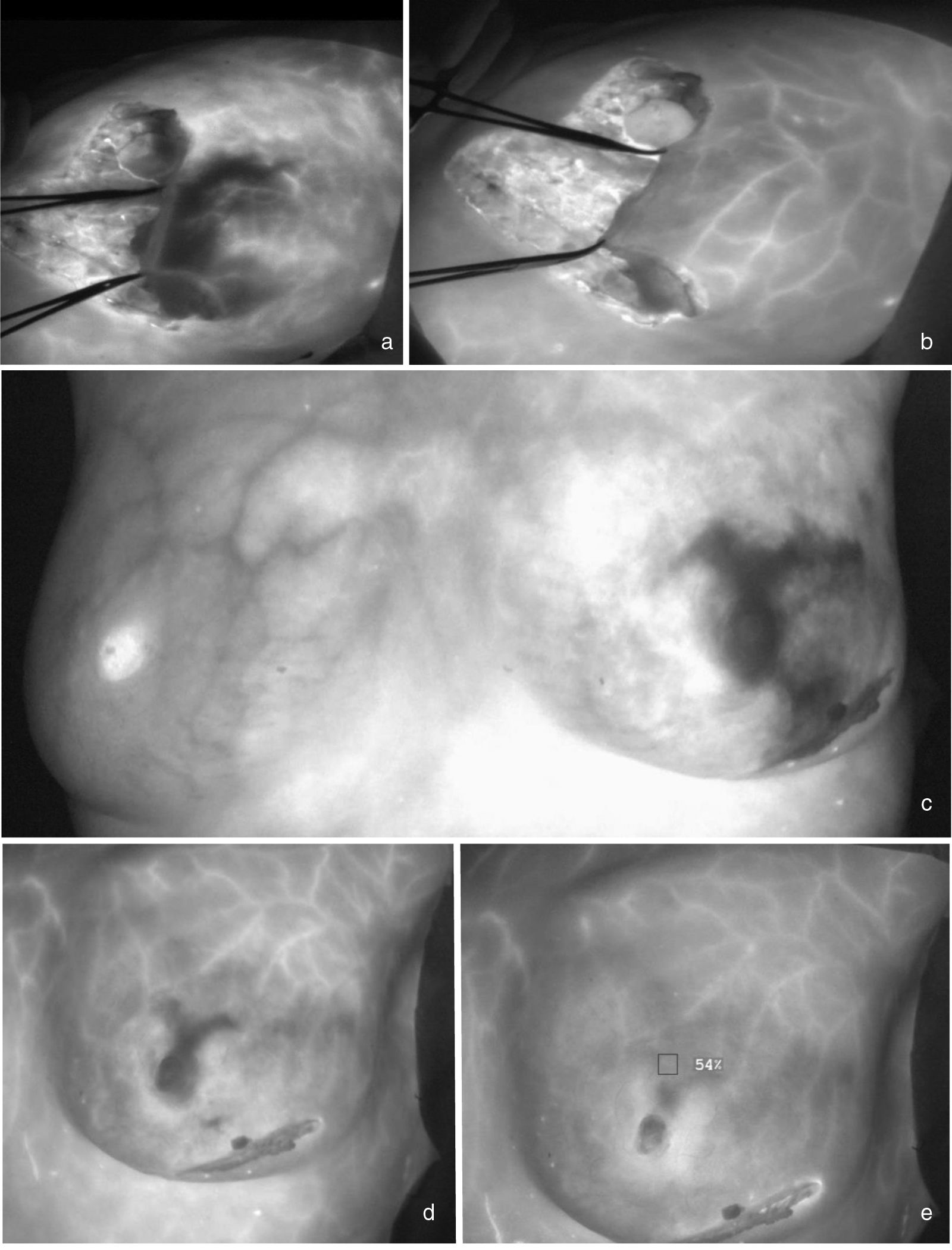
Early ischaemia with late recovery. Fig. 3a shows a woman with an extreme reduction mammoplasty who presents distal hypoperfusion in the lateral flap 30 seconds after the ICG bolus. At 140 seconds, recovery of perfusion in this area is observed (3b). Fig. 3c shows a patient with a skin- and nipple-sparing mastectomy on the left side who presents an extensive area of ischaemia in the upper and lower poles of the breast that recovers two minutes after the ICG bolus (3d and 3e).
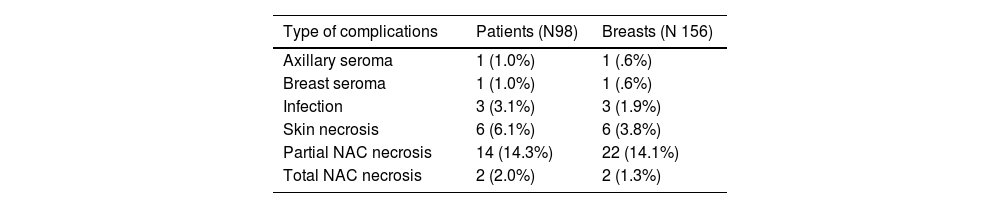
During the postoperative period, 27 women (27.6%) presented some complication, of which 21 (21.4%) were ischaemic events. In 15 of the patients with ischaemic events (71.4%), the third angiogram detected some area of poor perfusion (Table 1). In all of them, the angiographic image corresponded to the anatomical image of cutaneous ischaemia (Fig. 4). 85.7% of the ischaemic events were superficial necrosis that did not require reintervention. Three patients presented serious complications (3.1%), none of them in conservative surgery. All serious complications were detected by ICG-A. These patients required surgical intervention. One patient with NSSM through the inframammary fold and removal of the NAC due to neoplastic infiltration presented wound dehiscence secondary to necrosis, requiring replacement of the implant with an expander. Two patients with NSSM through a vertical pattern of the upper pedicle required intervention. In one of them, poorly vascularised tissue was resected and the wound closed and in the other patient, a thoracoepigastric flap was performed.
Complications during the postoperative period in study patients.
| Type of complications | Patients (N98) | Breasts (N 156) |
|---|---|---|
| Axillary seroma | 1 (1.0%) | 1 (.6%) |
| Breast seroma | 1 (1.0%) | 1 (.6%) |
| Infection | 3 (3.1%) | 3 (1.9%) |
| Skin necrosis | 6 (6.1%) | 6 (3.8%) |
| Partial NAC necrosis | 14 (14.3%) | 22 (14.1%) |
| Total NAC necrosis | 2 (2.0%) | 2 (1.3%) |
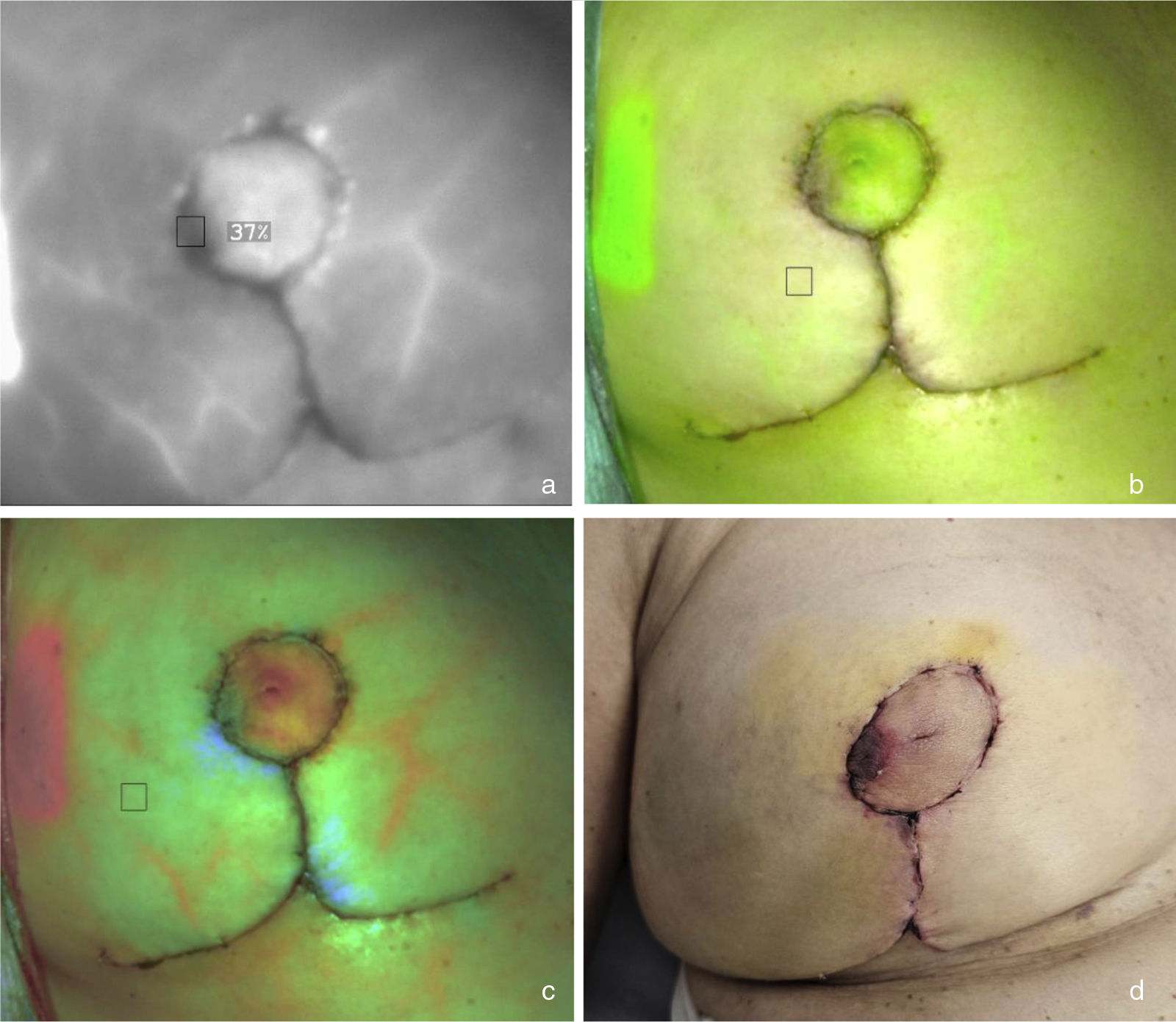
Ischaemia in the lateral area of the right areola. ICG angiography identifies an area of vascular distress in the lateral periphery of the right areola visible in fluorescence mode (absence of contrast; 4a), superposition mode (absence of green; 4b) and segmented fluorescence by colour (absence of red; 4c). During the postoperative period, the patient suffered superficial epidermiolysis in this area that did not require surgical intervention (4d).
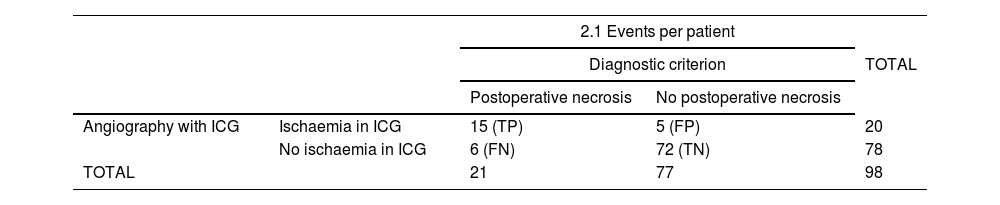
Statistical indicators per patient. The PPV of the ICG-AC was 75%, NPV 92%, sensitivity 71.4% and specificity 93.5% (Table 2.1). The FN rate was 28.6% and the FP rate 6.5%. The PPV was 77.8% if only high-risk necrosis surgeries are included (irradiated breast, vertical oncoplasty with inferior pedicle, extreme oncoplasty or type I, IV and skin- and nipple-sparing mastectomies with vertical incision).
Comparison of the results obtained in the intraoperative angiographies and the postoperative events.
| 2.1 Events per patient | ||||
|---|---|---|---|---|
| Diagnostic criterion | TOTAL | |||
| Postoperative necrosis | No postoperative necrosis | |||
| Angiography with ICG | Ischaemia in ICG | 15 (TP) | 5 (FP) | 20 |
| No ischaemia in ICG | 6 (FN) | 72 (TN) | 78 | |
| TOTAL | 21 | 77 | 98 | |
| 2.2 Events per breast | ||||
|---|---|---|---|---|
| Diagnostic criterion | TOTAL | |||
| Postoperative necrosis | No postoperative necrosis | |||
| Angiography with ICG | Ischaemia in ICG | 18 (TP) | 6 (FP) | 24 |
| No ischaemia in ICG | 11 (FN) | 121 (TN) | 132 | |
| TOTAL | 29 | 127 | 156 | |
FN: false negatives; FP: false positives; TN: true negatives; TP: true positives.
Statistical indicators per breast. The PPV of ICG-A was 75%, NPV 91.7%, sensitivity 62% and specificity 95.2% (Table 2.2). The FN rate was 37.9% and the FP rate 4.7%.
DiscussionSeveral studies have evaluated ICG-A as a diagnostic method for perfusion of mastectomy skin flaps. Most of these studies have recently been included in a Cochrane Library review11 which aimed to evaluate the ability of ICG-A to prevent necrosis in mastectomy skin flaps in women undergoing immediate reconstruction following NSSM. This review found nine studies that compared the number of postoperative complications in women undergoing ICG assessment of breast skin compared with clinical assessment. These studies evaluated a total of 1589 women with 2199 breast reconstructions and reported the number of complications per patient or per breast. The main findings of this review regarding patients were that ICG may reduce reoperation rates and that there is uncertainty as to whether ICG reduces rates of breast skin necrosis, infection, hematoma and seroma. The main results regarding the breast were that ICG can reduce breast skin necrosis, reintervention and infection rates, and that there is uncertainty about whether ICG has an effect on haematoma and seroma rates. The evidence from the studies evaluated during this review was considered to be of very low quality since there are no prospective randomised studies. This review emphasizes the need for prospective studies in oncoplastic and reconstructive breast surgery.12,13
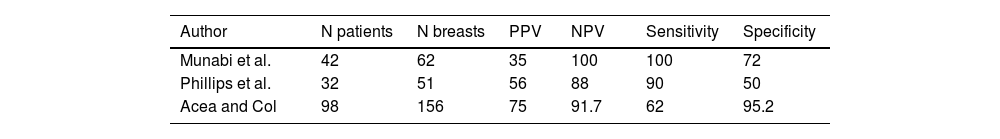
To our knowledge, only two prospective non-randomised studies14,15 have evaluated the sensitivity, specificity, and predictive values of this technique. The study by Phillips et al.14 evaluated this procedure in 51 immediate reconstructions with expansors for the prediction of postoperative skin necrosis. The sensitivity, specificity, PPV, and NPV were 90%, 50%, 56%, and 88%, respectively. In the study by Munabi et al.15 these values were 88%, 83%, 44%, and 98%, respectively. In this last study, the authors found that smoking and epinephrine injection decreased the specificity of this diagnostic method from 98% to 83%. These studies have two limitations. The first is that they were performed in patients with breast reconstruction using expansor-rectropectoral surgery. Currently, this type of reconstruction has been replaced by prepectoral reconstruction with direct implantation and therefore we lack information on this new surgical modality. Also, there are no studies that have evaluated ICG-A in women with oncoplastic procedures. Table 3 summarises the results of these authors and of the present study. The disparity in predictive values, sensitivity and specificity may be related to the number of patients/breasts in each study, the technical complexity used in each series, and the subjectivity in determining low perfusion during angiography.
The main objective of our study was to provide information on the sensitivity, specificity and predictive values of ICG-A to identify those patients in whom this diagnostic procedure provided added value in the planning and execution of their surgery. In this sense, our study confirmed that highly complex breast surgery is the best scenario for the use of ICG-A because it constitutes a high-risk context for cutaneous ischaemia.
Type 2 oncoplastic surgery (oncoreductive mammoplasty) and NSSM therefore benefit from this technology to identify areas of hypoperfusion that will require clinical evaluation during the postoperative period. In our experience, ICG-A is not suitable for performing a cutaneous resection during the intraoperative period because the extent and depth of the ischaemia will be determined during the postoperative period, and because most necroses will be superficial and will not require surgical intervention. Only those patients in whom both angiography and clinical inspection during surgery clearly show irreversible skin ischaemia benefit from intraoperative skin resection. For the rest of the patients, postoperative monitoring is advisable to determine both the extent of ischaemia and the pathological results of the process and thus specify a reintervention (if necessary) that considers both aspects. Furthermore, the high NPV of the technique (91.7% in our study) has its practical application in extreme breast surgery because it allows the planned planning to be maintained once the vascularisation of the skin or the NAC has been confirmed. Thus, in patients with extreme oncoplasty, with wide dissection of the skin flap or the NAC, or with a reduction mastectomy with an inferior pedicle, ICG-A can demonstrate the perfusion of the skin flap or the NAC and maintain the initial planning. For all these reasons, the ICG-A is aimed at surgical teams that use advanced techniques in oncoplastic and reconstructive breast surgery because both type 1 oncoplastic surgery and non-sparing mastectomy techniques present a low risk of skin necrosis.
This study has provided us with four lessons on the use and interpretation of ICG in breast surgery angiography. Firstly, the opportunity to identify the vascularisation of the NAC. Visualisation of the venous return during the first angiography, prior to surgery, allows us to identify the role of the perforators of the upper thoracic wall in the vascularisation of each of the NACs. This fact is important for the design of pedicles adapted to each NAC in vertical mammoplasties with upper pedicle, gynecomastias through periareolar approaches and mastectomies with preservation of the NAC using a vertical pattern. Secondly, the possibility arises of differentiating areas of low perfusion caused by poor vascularisation and those situations with correct irrigation, but with poor fluorescence of the IGC. This second situation has occurred in patients in whom the skin flap is too thin, either due to excessive dissection of the flap or the absence of subcutaneous thickness.
This false infravascularisation can be detected by the presence of subcutaneous vessels with dye that demonstrate the existence of vascular perfusion. Thirdly, this study has provided information on the effect of wound closure on cutaneous and NAC perfusion. Some authors16 have highlighted the impact of the pressure of cutaneous closure on cutaneous circulation and its relationship with necrotic events during the postoperative period. In none of the patients evaluated in this study was a harmful effect of cutaneous closure on cutaneous perfusion observed: patients with good perfusion after mastectomy also had it after closure, and those with perfusion defects after mastectomy had the same intensity and extension of the defect during closure. Finally, the study has facilitated the visualisation of the effect of previous scars on cutaneous circulation, demonstrating that there is no vascular barrier in them. On the contrary, in most of them vascular permeation is present.
This study has several limitations. First, the low incidence of adverse events (cutaneous necrosis) limits a more precise assessment of this procedure to analyse the relationship between intraoperative and postoperative findings. Second, the low number of patients with type 4 preserving mastectomies limits the experience in a group of patients with a higher risk of necrosis/dehiscence in the vertical wound.
To conclude, ICG-A is a useful technique for planning and evaluating cutaneous perfusion in oncoplastic and reconstructive breast surgery. Its high specificity (95.2%) helps to identify areas with defective perfusion. Most of these areas will be superficial necrosis that will not require reintervention and therefore their removal during surgery is not recommended. Its high NPV (91.7%) allows the initial planning to be maintained in extreme surgeries of high complexity. The use of this technology focuses on surgical teams with experience to perform breast surgery of high technical complexity.
FundingNo funding was received for this study.
Conflict of interestThe authors have no conflict of interests to declare.