Because of the obesity epidemic, more obese patients are on liver transplant (LT) waiting lists. The diseases associated with obesity may increase complications and limit survival after LT. However, there is no established measure or cut-off point to determine this impact and aid decision making. The aim of the present study is to evaluate obesity in patients undergoing LT via BMI and CT-based measurement of adipose tissue (AAT). These parameters will be used to predict the risk of postoperative complications and 5-year survival.
MethodsA retrospective, single-center study was carried out at a tertiary Spanish hospital, including all patients who received LT between January 2012 and July 2019 (n = 164).
The patients were adults who underwent LT using the ‘piggyback’ technique, preserving the recipient vena cava. Visceral adipose tissue (VAT) and BMI were calculated to examine correlations with postoperative complications and 5-year survival.
ResultsNo significant association was found between postoperative complications by Comprehensive Complication Index, BMI, AAT/height, subcutaneous fat/height and VAT/height.
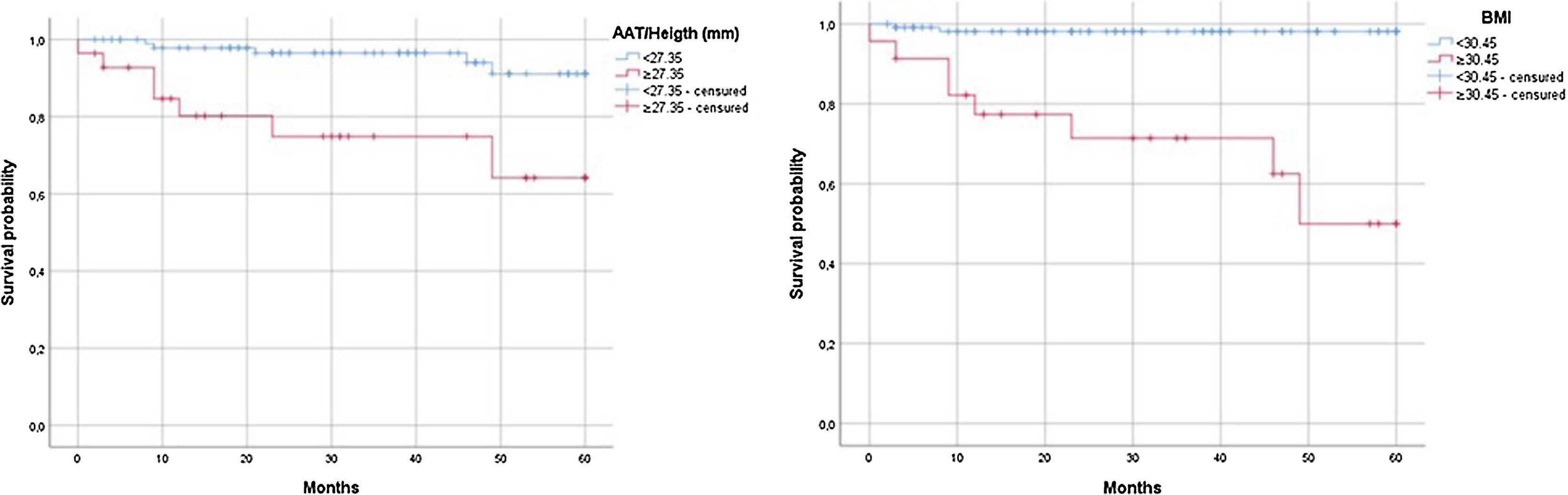
Kaplan-Meier curves for 5-year survival compared LT recipients with BMI < 30.45 versus ≥30.45, with an estimated survival of 58.97 months versus 43.11 months, respectively (P < .001) (Fig. 3) and for LT recipients with an AAT/height <27.35 mm versus ≥27.35 mm, with an estimated survival of 57.69 months versus 46.34 months (P = .001).
ConclusionsThis study does not show a higher rate of postoperative complications in obese patients. There is a significantly lower long-term survival in patients with AAT/height ≥27.35 mm and BMI ≥ 30.45. BMI is a valid estimate of obesity and is predictive of survival.
El aumento de la obesidad en listas de espera para trasplantes de hígado (TH) puede aumentar las complicaciones y limitar su supervivencia. Sin embargo, no existe un punto de corte establecido para su medición y tomar decisiones. El objetivo del estudio es evaluar la obesidad en pacientes sometidos a TH mediante IMC y tejido adiposo abdominal (AAT) en TC. Estos parámetros se utilizarán para predecir el riesgo de complicaciones posoperatorias y la supervivencia a 5 años.
MétodosEstudio unicéntrico retrospectivo en hospital español de nivel terciario, incluyendo los pacientes que recibieron un TH entre enero de 2012 y julio de 2019 (n = 164). Los pacientes eran adultos a los que se les realizó un TH mediante la técnica “piggyback”. Se calcularon el tejido adiposo visceral (VAT) y el IMC para valorar las complicaciones posoperatorias y la supervivencia a 5 años.
ResultadosNo se encontró asociación significativa entre las complicaciones postoperatorias mediante el Índice Integral de Complicaciones con IMC, AAT/talla, grasa subcutánea/talla y VAT/talla. Las curvas Kaplan-Meier a 5 años entre receptores con un IMC < 30,45 frente a ≥30,45, estimaron una supervivencia de 58,97 frente a 43,11 meses (p < 0,001) y para los receptores con un AAT/talla <27,35 mm versus ≥27,35 mm, con una supervivencia estimada de 57,69 versus 46,34 meses (p = 0,001).
ConclusionesEste estudio no muestra una mayor tasa de complicaciones postoperatorias en pacientes obesos. Hay una supervivencia a largo plazo significativamente menor en pacientes con AAT/altura ≥27,35 mm e IMC ≥ 30,45. El IMC estima correctamente la obesidad y predice la supervivencia.
Obesity is a worldwide epidemic that poses a real challenge to healthcare systems, as at least one-third of the adult population is obese.1 This translates to a growing number of obese patients on liver transplant (LT) waiting lists.2,3 In the general population, obesity has been shown to be a risk factor for cardiovascular disease, diabetes, musculoskeletal disorders, sarcopenia and cancer, which increase morbidity and mortality.4 In turn, this can lead to an increase in postoperative complications and limit long-term survival after LT, with an increased risk of death from cardiovascular events.5–7 Nevertheless, there is no standardized, objective measure of obesity nor an established range of values to measure its impact on LT or to prioritize recipients.8
Furthermore, LT recipients are prone to weight gain or obesity both before and after transplantation.9 According to the European Association for the Study of the Liver,10 patients with a BMI over 35 ought to be evaluated carefully by a multidisciplinary team before being included on the waiting list. Likewise, a BMI of 40 or greater is considered a relative contraindication for LT according to the America Association for the Study of Liver Disease.11
Nevertheless, BMI has significant limitations, as it does not account for the increase in extracellular liquids, sex, age or muscle mass.12
The negative effects of obesity are also related to the amount, type and distribution of adipose tissue,13 and visceral adipose tissue (VAT) is a predictor of mortality and cardiovascular disease.14,15
In this context, the measurement of VAT has been shown to be more predictive of cardiometabolic risk than BMI.16 Nevertheless, regarding LT, only one study has evaluated the predictive value of VAT on post-transplant risk, focusing on the risk of developing diabetes.17
Although there are various tests able to analyze fat distribution, CT could be the ideal method to measure fat mass given that it is already included in the evaluation protocol prior to LT.18 Some studies recommend that, if only one CT scan is used, the umbilical region between L4 and L5 is preferable.19
The aim of the present study is to evaluate obesity in patients undergoing LT via BMI and CT-based measurement of abdominal fat. These parameters will be used to predict the risk of postoperative complications and 5-year survival.
MethodsA retrospective, single-center study was carried out at a tertiary hospital, including all patients who underwent liver transplantation between January 2012 and July 2019 (n = 164).
The patients were adults (over 18 years of age) who underwent LT using the ‘piggyback’ technique, preserving the recipient vena cava. There were no exclusion criteria. Demographic, clinical, pathological and surgical variables, mortality and survival were recorded.
The Charlson Comorbidity Index20 and Balance of Risk (BAR) score21 were used to calculate comorbidity and patient risk. The BAR score included the age of both donor and recipient, MELD, retransplantation, previous life support, and cold ischemia time. All postoperative complications in the first 30 days were classified according to the Clavien-Dindo Score22 for later calculation of the Comprehensive Complication Index (CCI).23
VAT calculationAbdominal adipose tissue (AAT) was measured in square millimeters, and the visceral and subcutaneous compartments were subsequently evaluated separately. In order to compare results between patients and BMI measurements, the results were divided by height in millimeters (mm).
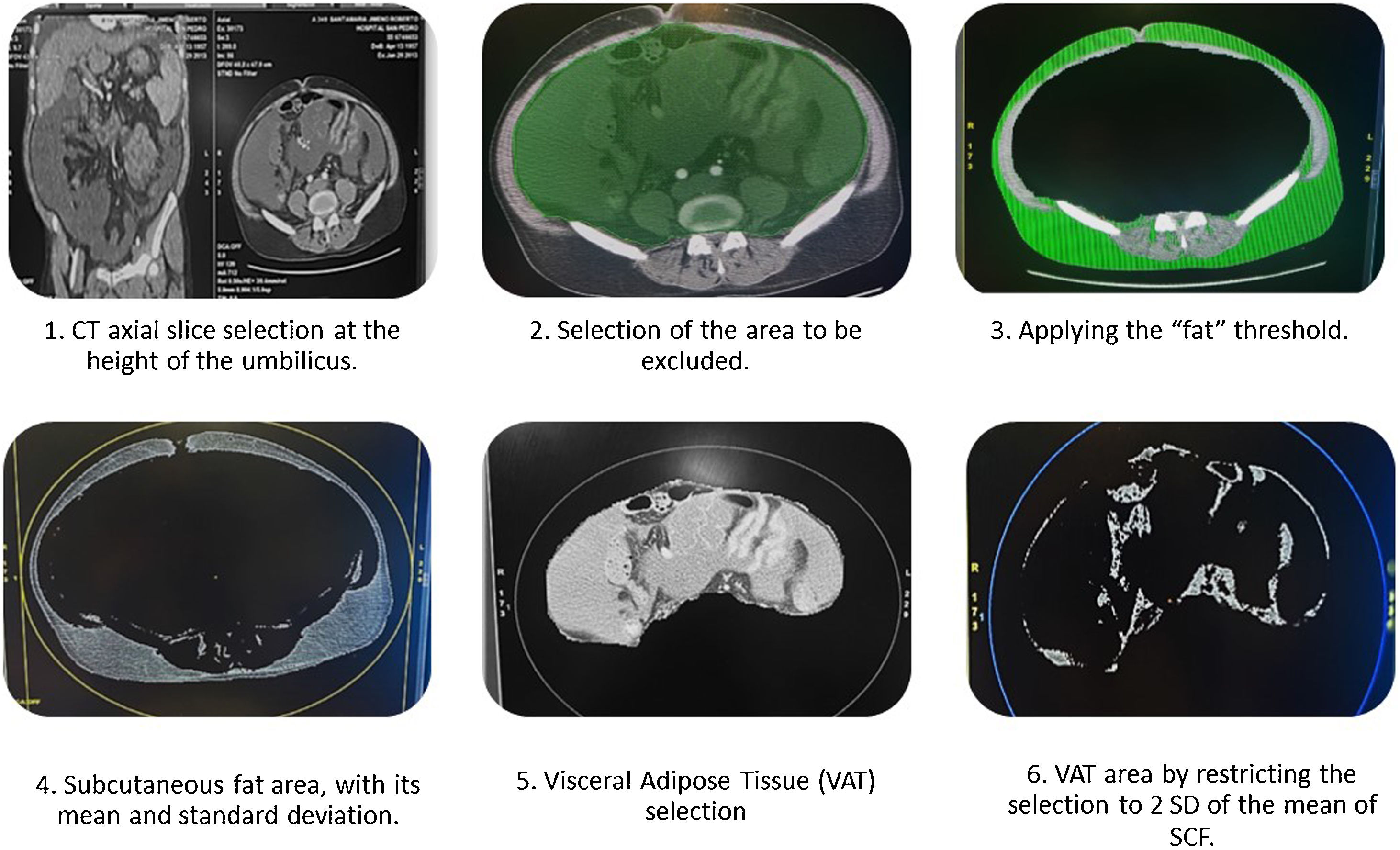
To measure abdominal fat distribution, an axial slice was selected at the level of the umbilicus (Fig. 1.1). The visceral tissue to be excluded was selected manually with the cursor using the “cut inside” tool (Fig. 1.2). Once excluded, the “threshold” tool was used to define the area by pixels and trace the area of subcutaneous fat (SCF), assigning this area an attenuation value in Hounsfield units (HU).24 In our case, this threshold was configured with values corresponding to adipose tissue, between −20 and −150, to avoid including other densities such as liquids or air (Fig. 1.3).
The resulting total volume was included in an ellipse, giving a value of abdominal adipose tissue (region of interest [ROI] in mm2) with its mean and standard deviation (SD) (Fig. 1.4).
Next, the VAT was isolated by selecting the muscle layer of the abdominal wall with the “cut outside” tool, and the adipose tissue was identified with attenuation values of between −2 and +2 SD of the mean HU of the SCF25 (Fig. 1.5). The result of this area (Fig. 1.6) is expressed in mm2 and is divided by the height of the patient to compare results between individuals.
Statistical analysisThe IBM SPSS Statistics version 24.0 program (Chicago, USA, 2012) was used, and a P-value <.05 was considered significant. The Kolmogorov–Smirnov test was used to determine the distribution of continuous variables. To find the ideal BMI and AAT cut-off points in our study population, an ROC curve was created, and the area under the curve (AUROC) was calculated. The highest Youden index was calculated (Youden index = sensitivity + specificity − 1). Five-year survival was analyzed for those patients who survived the first 30 postoperative days using Kaplan-Meier, and the comparison between distributions was made with the log-rank test. For the comparison between groups, the Mann-Whitney test was used. The multivariable study was performed by multiple logistic regression and chi-squared distribution (X2).
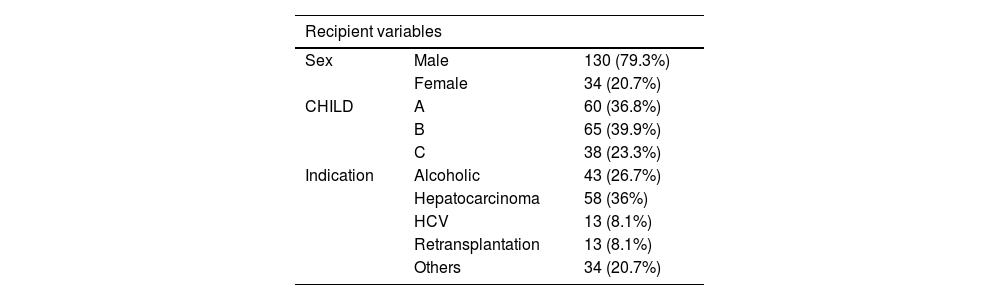
ResultsIn total, 164 patients were studied: 130 male (79.3%) and 34 female (20.7%), with a mean age of 55.34 years (σ = 9.55). Mean BMI was 26.34 kg/m2 (σ = 4.27), and the mean Charlson was 5.96 (σ = 2.06). The mean MELD score was 15.5 (σ = 6.93), and the most frequent indication for transplant was hepatocarcinoma (36%), followed by alcoholic liver disease (26.7%); there was no LT indicated for metabolic-associated fatty liver disease (MAFLD). The mean BAR score was 6.14 (σ = 3.73). Mean AAT/height was 20.85 mm (σ = 9.04) (20.9 in males and 20.64 in females, P = .899); mean VAT/height was 6.34 mm (6.66 in males versus 5 in females, P = 0.027); and mean SCF/height was 14.47 (14.07 in males versus 16.16 in females, P = .182). Mean donor age was 61.59 years (σ = 16.02), 89% due to brain death and 11% in Maastricht type III asystole, with premortem cannulation and normothermic extracorporeal membrane oxygenation (NECMO) preservation. The mean cold ischemia time was 327.03 minutes (σ = 119.2) (Table 1).
Demographic variables of recipients and donor characteristics, comparing anthropometric factors by sex.
| Recipient variables | ||
|---|---|---|
| Sex | Male | 130 (79.3%) |
| Female | 34 (20.7%) | |
| CHILD | A | 60 (36.8%) |
| B | 65 (39.9%) | |
| C | 38 (23.3%) | |
| Indication | Alcoholic | 43 (26.7%) |
| Hepatocarcinoma | 58 (36%) | |
| HCV | 13 (8.1%) | |
| Retransplantation | 13 (8.1%) | |
| Others | 34 (20.7%) | |
| Mean | Standard deviation | |
|---|---|---|
| Age (years) | 55.34 | 9.55 |
| BMI (kg/m2) | 26.34 | 4.27 |
| AAT (mm2) | 35,546.92 | 15,877.73 |
| AAT/height (mm) | 20.85 | 9.04 |
| VAT (mm2) | 10.686,14 | 5.625,19 |
| VAT/height (mm) | 6,34 | 3,31 |
| SCF (mm2) | 24,367.03 | 11,554.93 |
| SCF/height (mm) | 14.47 | 6.86 |
| Charlson index | 5.96 | 2.06 |
| MELD | 15.45 | 6.93 |
| BAR score | 6.14 | 3.73 |
| Donor variables | ||
|---|---|---|
| Age (y) | 61.59 | 16.02 |
| Cold ischemia (min) | 327.03 | 119.2 |
| Types of donors | DBD | 89% |
| DCD | 11% | |
| Cause of death | Cerebrovascular | 80% |
| Trauma | 12.7% | |
| Other | 7.3% | |
| Comparison of anthropometric factors by sex | |||
|---|---|---|---|
| Male | Female | P | |
| BMI | 26.57 | 25.93 | 0.77 |
| AAT | 35 644.23 | 35 146.85 | 0.885 |
| VAT | 11 201.04 | 8154.71 | 0.006 |
| SCF | 23 785.39 | 26 280.83 | 0.278 |
| AAT/height | 20.9 | 20.64 | 0.899 |
| VAT/height | 6.61 | 5 | 0.007 |
| SCF/height | 13.99 | 16.16 | 0.221 |
BMI: body mass index; AAT: abdominal adipose tissue; VAT: visceral adipose tissue; SCF: subcutaneous fat.
The different anthropometric measurements between sexes were compared (Table 1). The only clinically significant difference was found in the VAT, with a mean of 11 201.04 mm2 in males versus 8154.71 mm2 in females (P = .006) and the VAT/height, which was 6.61 mm in men and 5 mm in women (P = .007).
Postoperative complications were reviewed based on BMI, obtaining a mean CCI of 39.8 in the BMI < 30.45 group and 44.3 in the BMI ≥ 30.45 group (P = .432). The overall mean CCI was 41.2 (σ = 25.8), and 13 deaths occurred during the first 30 postoperative days.
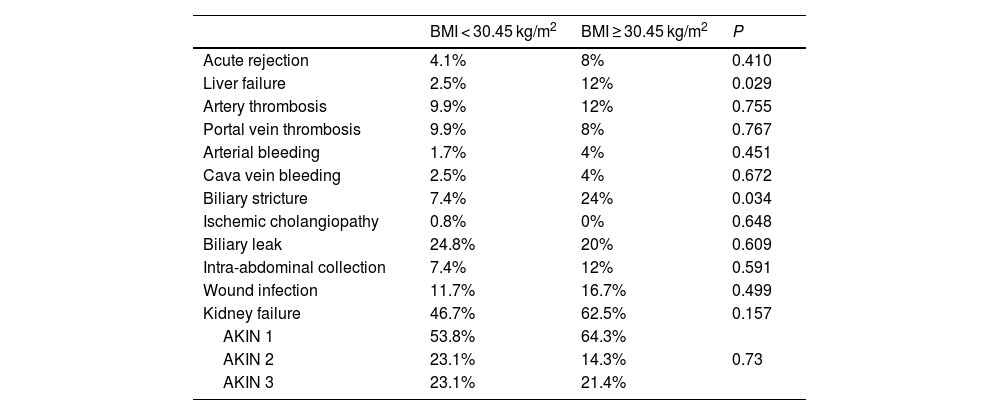
After comparing complications during the first 30 postoperative days, a greater frequency of primary liver failure (12% versus 2.5%, P = .029) and a greater number of cases of biliary stricture (24%) were observed in the BMI ≥30.45 group, with no statistically significant differences in the rest of the complications detailed in Table 3.
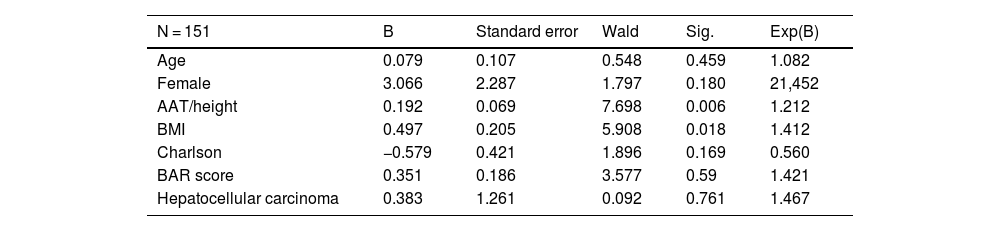
Multivariable analysis of factors associated with 5-year survival in LT patients who survive the first 30 days after surgery (13 deaths).
| N = 151 | B | Standard error | Wald | Sig. | Exp(B) |
|---|---|---|---|---|---|
| Age | 0.079 | 0.107 | 0.548 | 0.459 | 1.082 |
| Female | 3.066 | 2.287 | 1.797 | 0.180 | 21,452 |
| AAT/height | 0.192 | 0.069 | 7.698 | 0.006 | 1.212 |
| BMI | 0.497 | 0.205 | 5.908 | 0.018 | 1.412 |
| Charlson | −0.579 | 0.421 | 1.896 | 0.169 | 0.560 |
| BAR score | 0.351 | 0.186 | 3.577 | 0.59 | 1.421 |
| Hepatocellular carcinoma | 0.383 | 1.261 | 0.092 | 0.761 | 1.467 |
Summary of complications during the first 30 postoperative days comparing patients with BMI < 30 kg/m2 and BMI ≥ 30 kg/m2.
| BMI < 30.45 kg/m2 | BMI ≥ 30.45 kg/m2 | P | |
|---|---|---|---|
| Acute rejection | 4.1% | 8% | 0.410 |
| Liver failure | 2.5% | 12% | 0.029 |
| Artery thrombosis | 9.9% | 12% | 0.755 |
| Portal vein thrombosis | 9.9% | 8% | 0.767 |
| Arterial bleeding | 1.7% | 4% | 0.451 |
| Cava vein bleeding | 2.5% | 4% | 0.672 |
| Biliary stricture | 7.4% | 24% | 0.034 |
| Ischemic cholangiopathy | 0.8% | 0% | 0.648 |
| Biliary leak | 24.8% | 20% | 0.609 |
| Intra-abdominal collection | 7.4% | 12% | 0.591 |
| Wound infection | 11.7% | 16.7% | 0.499 |
| Kidney failure | 46.7% | 62.5% | 0.157 |
| AKIN 1 | 53.8% | 64.3% | |
| AKIN 2 | 23.1% | 14.3% | 0.73 |
| AKIN 3 | 23.1% | 21.4% |
AKIN: Acute Kidney Injury Network.
No significant association was found between postoperative complications by CCI and the variables BMI, AAT/height, SCF/height and VAT/height (Pearson Correlation Coefficient for BMI = −0.14, P = .871; AAT/height = 0.073, P = .403; SCF/height = 0.052, P = .549; VAT/height = 0.09, P = .304). In addition, a Cox regression model was made after excluding those patients who died within the first 30 days after LT (Table 2). The variables associated with 5-year survival were BMI (P = .018) and AAT/height (P = .0206). Sex, BAR score, CCI and the presence of hepatocarcinoma did not show significant associations.
With respect to 5-year survival, the correlation with BMI was −0.058 (P = .49), with AAT/height -0.168 (P = .05), with SCF/height −0.171 (P = .048), and −0.101 (P = 0.242) with VAT/height.
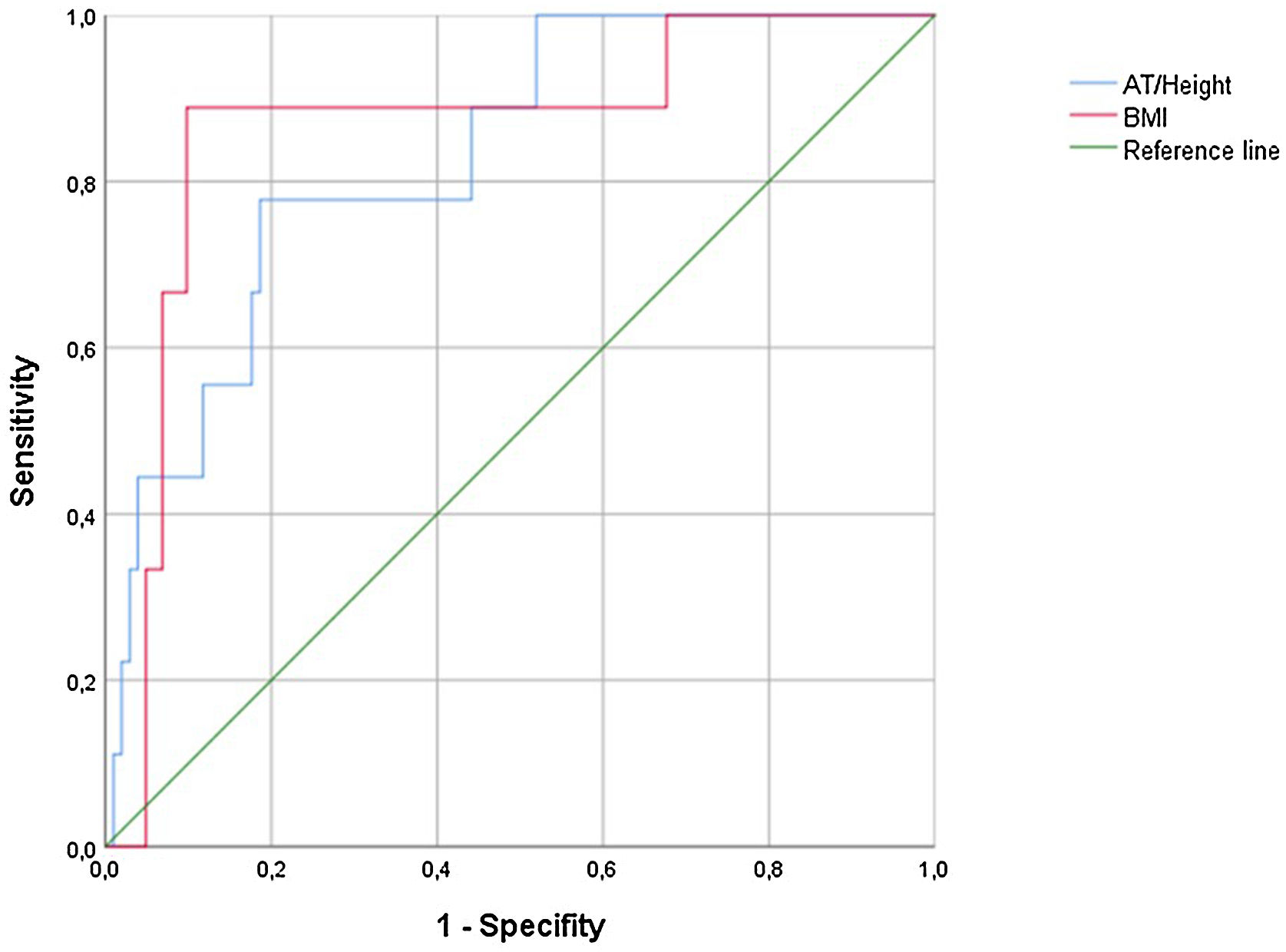
ROC curves for 5-year survival provided the following results: AAT/height = area under curve (AUC) 0.829 (0.704–0.954, P = .001); BMI: AUC = 0.864 (0.731–0.997, P < .001). The Youden index was used to calculate the cut-off point, obtaining a result of 27.35 mm with a sensitivity of 77.8% and a specificity of 81.4% for AAT/height, and a sensitivity of 88.9% and a specificity of 98% for BMI (Fig. 2).
Kaplan-Meier curves for 5-year survival compared LT recipients with BMI < 30.45 (n = 125) versus ≥30.45 (n = 125), with an estimated survival of 58.97 months versus 43.11 months respectively (P < .001) (Fig. 3) and for LT recipients with an AAT/height <27.35 mm versus ≥27.35 mm, with an estimated survival of 57.69 months versus 46.34 months (P = .001) (Fig. 3).
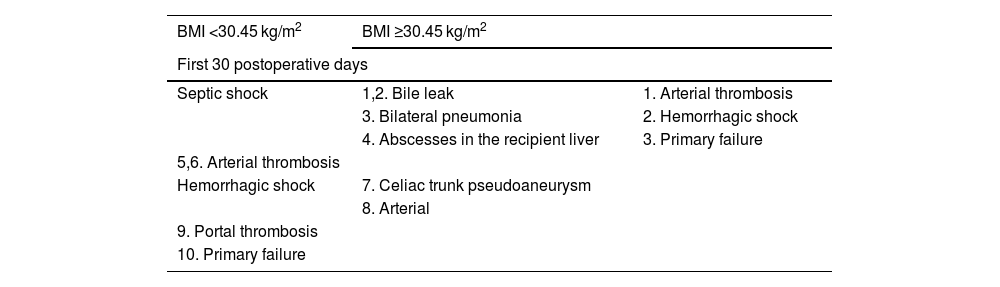
During follow-up, mortality in the BMI ≥ 30.45 group was due to septic shock in 4 patients, cancer progression in 2, after surgery for biliary stenosis in one patient (post-hepaticojejunostomy sepsis) and due to ischemic heart disease in one patient. In the BMI < 30 group, 2 patients died from septic shock (one due to an infected hematoma, and one following hemicolectomy), and one patient from chronic organ rejection (Table 4).
Cause of death during first 30 postoperative days and follow-up after the first 30 days in BMI < 30 kg/m2 and BMI ≥30 kg/m2 groups.
| BMI <30.45 kg/m2 | BMI ≥30.45 kg/m2 | |
|---|---|---|
| First 30 postoperative days | ||
| Septic shock | 1,2. Bile leak | 1. Arterial thrombosis |
| 3. Bilateral pneumonia | 2. Hemorrhagic shock | |
| 4. Abscesses in the recipient liver | 3. Primary failure | |
| 5,6. Arterial thrombosis | ||
| Hemorrhagic shock | 7. Celiac trunk pseudoaneurysm | |
| 8. Arterial | ||
| 9. Portal thrombosis | ||
| 10. Primary failure | ||
| Follow-up after first 30 days | ||
|---|---|---|
| 1. Infected hematoma | Septic shock | 1. Urinary sepsis post-kidney transplant |
| 2. Post-colon surgery | 2. Respiratory | |
| 3. Chronic rejection, portal hypertension, massive hemoptysis | 3. Pancolitis | |
| 4. Bile leak leading to collection | ||
| 5. Recurrent hepatocarcinoma | ||
| 6. Recurrent hepatocarcinoma | ||
| 7. Ischemic heart disease | ||
| 8. Following surgery for bile duct stenosis (hepaticojejunostomy) | ||
Given the growing tendency towards obesity in the population, the most rapidly increasing cause of LT in the United States is MAFLD-induced cirrhosis, with a predicted annual increase of 55.4% between 2016 and 2030.26
Currently, the American Association for the Study of Liver Disease considers BMI ≥ 40 kg/m2 a relative contraindication for LT,27 and the European Association for Liver Studies recommends that patients with a BMI > 35 should be carefully evaluated by a multidisciplinary team before being added to the waiting list.28 However, the evidence is weak and more in the range of BMI from 30 to 40 kg/m2.29,30
The present study did not find an increase in postoperative complications or 30-day mortality in obese patients, with no correlation between the CCI and AAT or BMI. Nevertheless, it seems to show a decrease in 5-year survival for transplanted patients with increased abdominal fat and higher BMI. For our population, cut-off points of AT/height of ≥27.35 mm and BMI of ≥30.45 were chosen as the most sensitive and specific in terms of effect on survival.
The 5-year results are similar to those of the metanalysis by M. Barone et al.31 and their comparison between BMI ≥ 30 versus 18.5–29.9 with OR = 1.16* [1.01, 1.33], P < .05. However, they found differences in 30-day mortality (OR = 1.36 [1.18, 1.57], P < .001) and postoperative complications (OR = 1.60 [1.21, 2.11], P < .01). It should be noted that the studies yielded heterogenous results and that only 2 of the studies reported 30-day postoperative data.
In an attempt to justify the negative effects of obesity, multiple studies have been carried out regarding the type and distribution of visceral adipose tissue.13 Adipose tissue accumulation in the abdomen, especially visceral adipose tissue, is associated with cardiovascular risk factors and atherosclerosis,32,33 causing vascular inflammation and insulin resistance.34,35 Data derived from prospective studies from the Framingham Heart Study support the role of visceral adipose tissue (VAT) as a predictor of mortality and cardiovascular disease.36,37 In comparison, subcutaneous fat (SCF) may be associated with a net neutral metabolic effect or may even be beneficial in diverse conditions.38
An important limitation of the study was BMI calculation. Although this was done preoperatively following patient optimization, the lack of a paracentesis protocol prior to BMI measurement may have influenced results because BMI does not factor in the increase in extracellular liquids, as in the case of ascites or edema.39 Including a dry-weight measurement or an estimate would more realistically help plan the possible size of the graft for matching and the actual nutritional status of the patient.40
Furthermore, BMI does not account for gender, age or body fat percentage, including the significantly different body fat percentage between men and women or the loss of muscle mass in older patients.31 Other limitations of the study are its retrospective nature and the low number of obese patients.
The “normal” amount of VAT varies by sex and ethnicity; it is higher in Caucasian men, Afro-American women, and Asians of both sexes.41 In general, a higher risk of postoperative complications as well as longer operative times and hospital stay was only observed when patients were grouped according to VAT, and not by BMI.42–45 The studies used multivariant regression analysis to show the role of VAT measurement as a predictor of postoperative complications using a cut-off point of 100 cm2. The methods used to calculate this cut-off point were not made clear, except in the study by Kozlow et al.,18 where a receiver operating characteristic (ROC) curve was used.
It has recently been suggested that specific VAT measurements via imaging techniques would be more adequate than BMI for evaluating postoperative adverse events in general surgery.46 Furthermore, said obesity could be associated with decreased muscle mass and a clear increase in negative consequences/effects in the state of sarcopenic obesity.47,48 For this reason, preoperative calculation of VAT and muscle mass has been proposed in LT candidates, this being an easily implemented measure with no additional costs, given that the CT scan is already part of the standardized preoperative protocol. Nevertheless, our study has only found a correlation between total adipose tissue and postoperative outcomes, but not for more specific visceral or subcutaneous compartments.
In spite of this, the role of BMI and the results of this study must be highlighted, showing validity and observed correlation with mortality when compared with a more objective parameter such as AAT.
In conclusion, the present study does not show a higher rate of postoperative complications in obese patients. There is a significantly lower long-term survival in patients with AAT/height ≥27.35 mm and BMI ≥ 30.45. BMI is a valid estimation of obesity and is predictive of survival.
Finally, further studies are necessary to help confirm the role in LT of obesity and other variables such as sarcopenia, diabetes and cardiovascular disease. Furthermore, persistent or newly diagnosed obesity after LT is associated with and appears to increase mortality.49 This raises the possibility of surgical or endoscopic treatment of obesity, both for the treatment of MAFLD and to reduce surgical risk in LT.50,51
Conflicts of interestThe authors declare no conflicts of interest.