With the aim to promote the elaboration of consensus documents on state of the art topics in liver transplantation with multidisciplinary management, the Spanish Society for Liver Transplantation (SETH) organised the V Consensus Meeting with the participation of experts from all the Spanish liver transplant programmes. In this edition, the following topics were revised, and we present the summary: 1. High-risk receptors; 2. Immunosuppression scenarios; and 3. Management of the patient with hepatocarcinoma in the waiting list.
Con objeto de promover la elaboración de documentos de consenso sobre temas de actualidad en trasplante hepático de abordaje multidisciplinario, la Sociedad Española de Trasplante Hepático (SETH) ha realizado la V Reunión de Consenso con participación de expertos de todos los programas de trasplante hepático españoles. En esta edición se han abordado los siguientes temas, cuyo resumen ofrecemos a continuación: 1. Receptores de riesgo elevado; 2. Escenarios de inmunosupresión, y 3. Manejo del paciente con hepatocarcinoma en lista de espera.
With the aim of promoting the creation of consensus documents on current questions in hepatic transplant with a multidisciplinary approach, the Spanish Hepatic Transplant Society (SETH) has held the V Consensus Meeting in which experts from all of the Spanish hepatic transplant programmes participated. The following subjects were covered in this meeting, and summaries of them are offered below: 1. High-risk recipients; 2. Immunosuppression scenarios, and 3. Hepatocarcinoma patient management in the waiting list.
HIGH-RISK RECIPIENTSCoordinators: Itxarone Bilbao and Manuel de la Mata
IntroductionThe survival of liver-transplant recipients has now achieved survival rates of 70% and 60% at 5 and 10 years, respectively, in the majority of transplant centres. However, 10%–20% of patients die in the first year following transplant, due to complications in connection with failure of the hepatic graft, technical complications, infections or multiple organ failure.1
A high proportion of early post-transplant mortality accumulates in recipients with risk factors or relative contraindications. There is no well-established definition of what we describe as a high-risk recipient or one with a risk that is too high. This definition may be expressed in terms of minimum expected survival (50% at 5 years),2,3 or morbidity and the duration of hospitalisation.4 Careful and exhaustive examination of the potential candidates for hepatic transplant is essential during times of growing demand for transplant and long waiting lists.5
The probability of receiving a transplant in our context stands at around 50%.6 It seems reasonable to make an effort in selection with the aim of including those patients with the highest probability of surviving the transplant procedure, as well as regularly revising survival expectations.7
The **imbalance between the demand for transplant and the offer of donors has grown in recent years, due to the broadening of transplant indication criteria and the complexity of recipients. The latter is due to the increasing proportion of them who are elderly, with accumulated morbidity, high MELD (Model for End Stage Liver Disease) and problematic psychosocial status.
The inclusion of a recipient in the waiting list may be governed by the principle of individual justice, which grants maximum priority to the most seriously ill patients or the usefulness of the donation, according to which the benefit of survival should prevail over the expectation of life without a transplant. Other criteria, such as cost-effectiveness, should also be taken into consideration.8
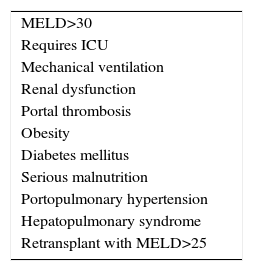
The list of risk factors that may reduce post-transplant survival is long and growing,9 due to the gradual addition of contraindications that used to be considered absolute and are now thought to be relative. There is no absolute age limit, being HIV-positive does not lead to exclusion and the duration of abstinence from alcohol has become relative. Solutions are being sought for portal thrombosis and the risks of transplanting patients who have been operated beforehand or are obese are accepted. Only in the case of very severe cardiopulmonary disease or sepsis is there sufficient consensus that hepatic transplant is absolutely contraindicated (Table 1).
Transplant in the most severely ill patients is associated with a higher probability of post-transplant death. It is necessary to identify the exclusion criteria that prevent the loss of grafts with the greatest potential for favourable evolution in other patients. Different prognostic models are available which include recipient, donor and surgical variables, which may be useful in the identification of high-risk candidates.10
Given the impossibility in a single document of revising all the risk factors, those associated with a high MELD score have been prioritised here, together with portal thrombosis, obesity and coronary cardiopathy.
High MELDNo upper limit has been set in the MELD score above which hepatic transplant would be considered useless and therefore a complete contraindication. Consensus conferences11 have taken place, while some studies conclude that even in recipients with very high MELD scores, above 30 or 40 points,12,13 there may be a benefit in terms of survival that justifies the transplant, even though this requires long periods of hospitalisation and a high economic cost. Patients with a MELD score above 35 have been reported to generate very high costs.14,15
A recent study suggests that the MELD score has little predictive power as an isolated variable in predicting post-transplant survival, and it describes how only a 10% difference in survival at 5 years is observed between patients with a MELD score of <10 and those with a MELD score of >30.4 Nevertheless, in multivariate models when combined with other variables it may help to select the candidates with a poor prognosis. This may be reproduced in other studies.16 In a systematic review of 37 studies and a total number of 53691 patients in 15 countries, it was concluded that only 2 of them describe a predictive capacity of MELD prior to transplant for postoperative survival, with a c-statistic of >0.7.17
The majority of transplant centres use the MELD score to prioritise waiting lists. This model of donor-recipient assignation was designed to favour access by the most serious patients to transplant and to reduce mortality in the waiting list.18 However, a recent communication states that the empirical assignation of points to cases that are termed exceptions to MELD, chiefly hepatocarcinoma, may delay the access to transplant of patients with hepatocellular insufficiency, leading to an increase in the MELD score or a higher rate of pretransplant mortality.19
Despite all of the above considerations, it is true that the benefit for recipients has been described to increase parallel to their MELD score.20,21 In the same way, we have to admit that this prioritised access to a graft may lead to more futile transplants and the death in the waiting list of another potential recipient with a higher survival probability.
Patients with a high MELD who die after a short period of time in the waiting list may behave in the same way as biologically very aggressive hepatocarcinomas that progress swiftly and are excluded from the waiting list. There are no verified data that would allow us to establish the period of time during which a patient with severe liver failure may conserve their vital systems with a guarantee of being able to successfully survive the transplant procedure. It is interesting that the differences between centres in terms of survival are probably greater in the sub-group with high MELD scores.12,13
The Applicability of Prognostic CriteriaThere are a good number of prognostic models which attempt to predict post-transplant survival. Some of these models centre on the recipient, while others centre on the donor. Lastly, some models include patient and graft variables. These different models and their usefulness in donor distribution have recently been revised.10
It is widely accepted that although the MELD score is useful in the prediction of pretransplant mortality, it is unable to estimate postoperative survival. The variables which influence the latter include donor quality, the surgical technique used and the perioperative management of the patient. The SOFT model, which includes recipient and donor variables, has recently been validated in recipient risk cohorts22,23 with reasonable predictive capacity.
Some of the studies undertaken offer multivariate analysis that includes variables which indicate patients with intensive care needs (dialysis, intubation), advanced age, recipients of grafts from elderly donors and with prolonged ischaemia times.24,25
One of these studies using multivariate analysis found that the need for dialysis and mechanical ventilation prior to transplant, a high MELD score and advanced age are predictive of hospitalisation during more than 60 days after transplant. This sub-group of patients with prolonged hospitalisation had significantly lower patient and graft survival, and the transplant procedure for them was found to be significantly more expensive.4
Variability in Clinical PracticeClinical practice varies widely in cases of hepatic transplant, and this is especially relevant in the recipient selection process and access to a hepatic transplant. Candidates are selected in multidisciplinary meetings where the influence of the doctors involved is clear, together with their degree of empathy with the patients, the degree to which the latter may be foreseen to follow the recommendations for treatment and the pressure brought to bear by the patients themselves, their family and the doctors in their original hospital. The different configurations of surgical teams and their readiness to accept technical challenges in increasingly complex patients is another cause of variability. Lastly, the emotional impact of the positive or negative outcomes of recent transplants cannot be denied.
How much does each one of the risk factors identified add to the mortality supposedly associated with a specific MELD score: diabetes, kidney failure, cardiopathy, and portal thrombosis? This is what the different criteria have set out to do, with limited success, and it is also what the models based on **ANN26 have attempted to do.
RECOMMENDATIONS- 1
No MELD score has been set as the upper limit that would contraindicate hepatic transplant. Degree of evidence III-B.
- 2
In multivariate analysis studies, the MELD score together with other variables connected with requirements for intensive care or donor quality may help identify recipients with a poor prognosis. Degree of evidence II-A.
- 3
Post-transplant prognostic models are of limited use in excluding patients with accumulated risk factors from access to the waiting list. Degree of evidence II-B.
- 4
The limit for advanced age as a risk factor varies between transplant units, although it has to be evaluated while taking increased life expectancy into account. Degree of evidence III-B.
Portal thrombosis is a relative contraindication for hepatic transplant, but there is no unanimity on when it becomes absolute depending on its extension.
There are 2 meta-analyses and several reviews on pre-and post-transplant management of portal thrombosis and the suitability of using anticoagulation. In an exhaustive analysis of the literature since the year 1986, only 41 articles fulfilled the quality requisites to draw conclusions and recommendations from them. All of the articles revised except for 3 are retrospective studies.27–30
The Incidence and Prevalence of Portal ThrombosisAlthough the incidence of portal thrombosis de novo in patients in the waiting list has not been fully studied, it may stand at around 7.4%.31 More is known about the prevalence of the condition (9.7%±4.5%), but in a very wide range that runs from 2.1% to 23.3%, and this affects different waiting list patient inclusion policies. The groups which consider portal thrombosis to be a contraindication reported low rates of incidence. The different criteria used when reporting events may also contribute to this difference in figures. It would therefore be desirable when publishing results for all of the authors to follow a single classification or definition of the event.
Typology and ClassificationThere are several classifications, some of which are based on morphological aspects32 while others are based on aspects of the required technical or surgical treatment.33–38 Of all these, the most widely accepted is Yerdel's classification,35 as it covers not only morphology but also refers to the presence of suitable collateral vessels that could be used in an extra-anatomical reconstruction of the portal flow. It distinguishes 4 grades. In grade i the thrombus partially affects (<50% of the opening) of the main trunk of the portal vein, with or without minimum extension of the superior mesenteric vein. Grade ii represent complete thrombosis or more than 50% of the vessel opening, with or without minimum extension of the superior mesenteric vein. Grade III includes cases of complete thrombosis of the portal vein and the proximal superior mesenteric vein, where the distal part of the same is clear. Grade iv identifies complete thrombosis of the portal vein and the superior proximal and distal mesenteric vein.
According to morphological classifications, 56.2% of thromboses reported in the literature are partial and 43.3% are total. According to Yerdel's classification, 46.4% are grade I, 36.4% are grade II, 11.1% are grade III and 14% are grade IV.
Preoperative ManagementThere are 3 interesting aspects in the preoperative management of portal thrombosis in the waiting list: selecting the ideal diagnostic imaging test, selecting the candidates from the list who will require stricter follow-up as they have a higher probability of developing portal thrombosis, and thirdly, either reversing the thrombus or at least preventing it from progressing.
Diagnostic TestsUltrasound, abdominal CT angiography and magnetic resonance are the most widely used tests. Ultrasound is a valid way of diagnosing total thrombosis of the portal vein and its intrahepatic branches, as well as informing about the direction of portal flow. CT-angiography supplies information on the state of the superior mesenteric vein, the existence of portal-systemic shunts and the state of the renal and cava veins. There is consensus between the hepatic transplant groups in our country that the princeps test for all candidates for hepatic transplant is abdominal CT-angiography, as this offers the most complete information on the size of the thrombosis as well as the surgical strategy that should be used during the hepatic transplant. Nevertheless, the sensitivity of any of the 3 tests is from 92% to 100% for grade III and IV portal thrombosis, while the corresponding figures for grade i and ii thrombosis are 14.3%–50%.27
Risk Factors That Favour Portal ThrombosisAlthough many authors try to find a strong association between certain genetic mutations associated with hypo-hypercoagulability and the development of portal thrombosis, only a few isolated works have found an association.39,40 Other risk factors described in different articles are autoimmune cirrhosis and cryptogenetic cirrhosis,41 together with alcoholic cirrhosis in males treated previously for upper digestive tract haemorrhage.35 Contrary to what could be expected, portal thrombosis is not associated with a high MELD, although this association does affect results. Therefore, the coincidence of complete portal thrombosis and a MELD score above 25 is associated with higher mortality.42
The ideal interval between imaging tests while patients are in the waiting list is unclear. Some authors have shown that increasing the frequency of imaging tests does not increase the detection rate of portal thrombosis.43 Nevertheless, all of the groups agree that an ultrasound scan every 3 months and a CT-angiography every 6 months seems to be a reasonable frequency.
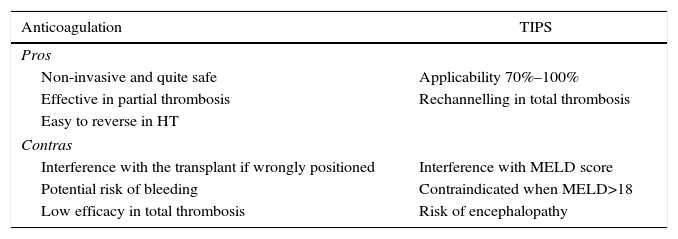
Anticoagulation vs Intrahepatic Percutaneous ShuntThe final purpose of anticoagulation as well as a percutaneous intrahepatic shunt (TIPS) is to make the thrombus regress or at least to prevent it from progressing. All of the studies which recommend either of the 2 procedures are based on few patients in non-randomised studies, so that the degree of recommendation is low (IIIB).
Anticoagulation achieves total unblocking in 40%–75% of cases of partial portal thrombosis. However, it rarely achieves unblocking in cases of complete thrombosis, although it does prevent growth of the thrombus.44–46 Anticoagulation seems to be more effective when the time between diagnosis and commencement of treatment is shorter. It is recommended that this interval be less than 6 months, although this datum is sometimes impossible to determine as the finding is incidental. Before starting anticoagulation it is obligatory to rule out severe cardiac or lung disease, together with the presence of hepatocarcinoma or a tumour thrombus, as well as a recent history of haemorrhage of the upper digestive tract. A previous endoscopy would also be indicated, with the prophylactic ligature of varices and the administration of beta-blockers, as there is a 5% risk of secondary bleeding due to anticoagulation.47
There is no consensus on the best type of anticoagulation.29,30 Low molecular weight heparin has the drawback of requiring subcutaneous injections and the lack of a fast-acting antidote when the opportunity for transplant arises. Vitamin K antagonists have the drawback of increasing the **INR and interfering with the MELD score, although they do have a powerful and fast-acting antidote. Lastly, thrombin or activated factor X inhibitors have the advantage of oral administration, but no way of reversing their action is known and there is no experience in cirrhotic patients.
TIPS is applicable in 70%–100% of cases, according to the retrospective series with the greatest number of patients.48–50 Nevertheless, the fact that none of these articles is prospective must be taken into account, and they do not undertake analysis according to intended treatment. TIPS have to be inserted in an experienced centre to prevent the wrong positioning of their distal end and technical interference with a future transplant. Nor would they be recommended in patients with a MELD score >18, due to the high probability of their clinical state and hepatic function worsening (Table 2).
Advantages and Disadvantages of Using Anticoagulation and TIPS in Candidates With Portal Thrombosis.
| Anticoagulation | TIPS |
|---|---|
| Pros | |
| Non-invasive and quite safe | Applicability 70%–100% |
| Effective in partial thrombosis | Rechannelling in total thrombosis |
| Easy to reverse in HT | |
| Contras | |
| Interference with the transplant if wrongly positioned | Interference with MELD score |
| Potential risk of bleeding | Contraindicated when MELD>18 |
| Low efficacy in total thrombosis | Risk of encephalopathy |
According to the literature,27–29 the technique used depends on the degree of the thrombosis. Thus for Yerdel degrees I, II and III the so-called anatomical techniques are used (thrombectomy or thromboendovenectomy)51 in 75% of cases, with the insertion of a venous graft from the superior mesenteric vein of the recipient into the portal vein of the donor in 8.4% of cases, or anastomosis of the donor's portal vein to a venous collateral of the recipient in 2.4% of cases. For Yerdel grade iv it is necessary to use non-anatomical techniques, such as hemitransposition in 3.3% of cases, renoportal anastomosis in 1.4% (recommended above all for patients who have a spontaneous or surgical splenorenal shunt) and portal vein arterialisation in 0.2% of the total number of cases. Selection of the different extra-anatomical techniques will depend on the presence of a splenorenal shunt and the experience of the team. Lastly, we cannot forget hepatointestinal transplant as a radical alternative when the thrombosis is very extensive.
Results After Hepatic TransplantOverall analysis of all transplanted patients with portal thrombosis1–3,52–54 shows higher mortality at 30 days (10.5%) and at one year (18.8%) in comparison with patients with a permeable portal vein that had received a hepatic transplant (7.7% and 15.4%, respectively). However, more recent series only show these differences in connection with the technique used, emphasising whether they were anatomical or not, which in turn is clearly connected with the grade of thrombosis.55 Therefore, and according to Yerdel's classification, mortality at 30 days is 2.34%, 5%, 8.6% and 27%, while mortality after one year is 13%, 35%, 33% and 50% for grades i, ii, iii and iv, respectively.
With regard to morbidity,1–3,52–54 the most solid evidence lies in the higher probability of re-thrombosis after transplant (10.3% of cases), although its incidence would also be connected with Yerdel's grades: 3.5%, 10.7%, 22.2% and 17% for grades I, II, III and IV, respectively. Early re-thrombosis may lead to the loss of the graft, so that it seems recommendable to use anticoagulation, above all in severe grades. Nevertheless, there is no clinical evidence to indicate which type of anticoagulation to use, or for how long. Other complications worthy of mention are gastrointestinal haemorrhage secondary to the persistence of portal hypertension (above all in connection with extra-anatomical techniques), ascites, kidney failure and sepsis.
Although the results of Asian series56,57 show good results with good planning of the surgical strategy used, in general Yerdel grade iv, which requires complex extra-anatomical techniques, should not be an indication for hepatic transplant from a live donor.
RECOMMENDATIONS- 1
Portal thrombosis is a risk factor for hepatic transplant, but it cannot be considered to be an absolute contraindication, not even in its most extensive forms (type iv thrombosis in Yerdel's classification). Degree of evidence I-B.
- 2
Studies using imaging techniques (Doppler ultrasound and CT-angiography) must be used in the evaluation protocol of potential hepatic transplant recipients. Degree of evidence I-A.
- 3
There is no solid evidence on anticoagulation guidelines in waiting list patients with portal thrombosis, although if administered, it is recommended that this be done soon after the moment of diagnosis. Degree of evidence II-B.
- 4
There is no evidence regarding the efficacy of TIPS implant in waiting list patients with portal thrombosis, and if this is performed highly experienced teams are required to prevent technical problems during surgery. Degree of evidence III-B.
- 5
The survival of transplanted patients with complete portal thrombosis (grade iv) is limited, but there is no consensus on whether it alone should lead to exclusion of a candidate from the waiting list. Degree of evidence III-B.
- 6
Grade iv portal thrombosis requires exhaustive planning and management in transplant units with sufficient experience in extra-anatomical reconstruction techniques. Degree of evidence III-B.
Obesity is an increasingly common pathology in the general population.58 Spain is at an intermediate endemic level, with a prevalence of from 10% to 15% among men and from 15% to 20% among women. The incidence of a BMI>30% among patients in the waiting list for hepatic transplant is from 15% to 35%.59–61 Additionally, around 45%–64% of all liver transplant patients will gradually gain weight during the first 3–5 years after transplant, and up to 30% will become obese. One meta-analysis62 analyses a total of 316 articles published in the last 20 years, of which only 13 comply with the set quality criteria. However, the recommendations extracted from this meta-analysis have a low degree of evidence, as all of them are retrospective and the definition of obesity is not homogeneous.
The Approach to Obesity in Transplant CandidatesNutritional evaluation in transplant candidates with terminal hepatopathy is difficult, as conventional nutritional parameters are not always applicable. Almost all authors agree that malnutrition and excess weight are hard to manage in liver transplant candidates.63,64 As there is no obesity classification which takes into account ascites, anasarca and dilutional hypoalbuminemia,65 the most widely accepted classification is that of the WHO,66 which defines overweight as having a BMI from 25 to 30kg/m2, moderate obesity (class i) when the BMI is 30–35kg/m2, severe obesity (class II) when the BMI is 35–40kg/m2, and morbid obesity (class iii) when the BMI>40kg/m2.
There is no agreement on a maximum degree of obesity to be accepted in a waiting list for hepatic transplant, and in fact no author considers it per se to be an absolute contraindication. The American Guide to Clinical Practice67 states that a BMI>40kg/m2 is a relative contraindication (degree of evidence II-B).
Different studies show with a degree of evidence I-B that class i or higher obese individuals according to the WHO classification have to follow nutritional and dietary advice given by nutritional specialists.5 Some authors have tried to claim that dietary and nutritional interventionism has an impact on patients in the waiting list, but they have only been able to do so in cases of malnutrition.61 In patients with a persistent BMI>45kg/m2, the results in terms of survival for men (HR: 1.52; CI 95%: 1.03–2.24; P=.035) and women (HR: 0.99; CI 95%: 0.65–1.5; P=.96), were quite similar for those who had reduced their BMI<45kg/m2.
There is neither consensus nor sufficient experience to recommend more aggressive interventionism, such as bariatric surgery while patients are in the waiting list. The studies described to date67,68 are retrospective, with a very small number of patients which in some cases have been carefully selected, with a free period between bariatric surgery and transplant longer than one year. Bariatric surgery may have a role to play in patients at very early stages of hepatopathy,69 but it would not be indicated in advanced stages of the same.70 Another option described by some authors is to carry out bariatric surgery at the end of the hepatic transplant,71 or months after the transplant.72,73 In both cases excellent planning of the procedure and type of bariatric surgery is required, to prevent complications such as excessive weight loss, poor nutrient absorption and immunosuppressors with a greater risk of rejection, or lack of access to the duodenum and biliary duct.
The Approach to Post-Hepatic Transplant ObesityThe gradual weight gain by hepatic transplant patients is a reality without any clear scientific explanation.74 According to the diabetes and kidney disease registry (NIDDK) of the hepatic transplant data base in the United States (SRTR), the weight gain amounts to 1kg per year after transplant.75,76 In our environment60 the weight gain is also 1kg per year, and it is progressive during the first 5 years and at its maximum during the first and second years.59
Post-transplant obesity affects 18%–30% of all patients, according to different authors,77 although those transplanted due to non-alcoholic steatohepatitis require strict control of obesity and metabolic syndrome, given that this pathology has the highest incidence of weight gain.75
The results of post-transplant survival of obese patients are contradictory.62,78–80 While some authors report higher mortality among obese patients, other authors show the same survival for them. This discrepancy may be explained, as M.T. Foster81 says, by the fact that, in obesity, quality is more important than quantity. On the other hand, almost all authors78 agree that obese patients subjected to hepatic transplant require longer hospitalisation, and that in the ICU80 they suffer a higher incidence of infections and metabolic syndrome.79 There are no studies which show the best immunosuppressant strategy for obese patients subjected to hepatic transplant.
To conclude, and according to Charlton's revision,75 we can reach a consensus that obesity is not in itself an absolute contraindication for liver transplant. Nevertheless, it is necessary to exhaustively evaluate patients for the presence of other comorbidities, such as diabetes mellitus, arterial hypertension, dyslipidemia and cardiovascular problems in elderly patients, given that the sum of all these variables has a negative influence on survival.82
RECOMMENDATIONS- 1
Obesity is not an absolute contraindication for hepatic transplant, but patients must be exhaustively evaluated for risk factors and comorbidity due to its negative impact on survival. Degree of evidence II-B.
- 2
It has not been proven that dietary and nutritional interventionism in waiting list patients improves the results of hepatic transplant. Degree of evidence II-B.
- 3
The results obtained to date do not make it possible to recommend a technique or specific time (pre, intra or post-transplant), for any surgical treatment of patient obesity when they are candidates for a hepatic transplant. Degree of evidence III-B.
Coronary disease has been described in 16.2%–60% of patients who are potential candidates for hepatic transplant, and it increases peri- and postoperative morbimortality.83–85 Diabetes mellitus is the most important risk factor. Screening for coronary disease must be included in the pre-transplant evaluation protocol, especially in this group of patients, in which it is recommended that coronary angiography be performed.86
In patients without risk factors the incidence of cardiac complications in the course of a hepatic transplant is low, although it is hard to know the prevalence of coronary disease solely on the basis of non-invasive tests, given that the sensitivity of these tests is not known and could only be tested using the gold standard of coronariography.87
It has been suggested that coronariography be performed in high-risk candidates, which are defined as those with more than 2 risk factors, according to the AHA/ACCF (diabetes mellitus, previous cardiopathy, left ventricle hypertrophy, age above 60 years old, smoking, arterial hypertension and dyslipidemia).88 Coronary CT is able to detect calcifications and stenosis in the coronary vessels, although there is a lack of knowledge of its diagnostic precision in comparison with coronary angiography.87,89,90 A link has been described between a cryptogenetic cirrhotic aetiology or one associated with non-alcoholic steatohepatitis and the appearance of postoperative myocardial ischaemia.91 In this study, logistical regression analysis revealed 3 risk factors associated with the appearance of acute post-transplant coronary syndrome (age, a history of ischaemic cardiopathy and pre-transplant needs for vasopressors). On the other hand, multivariate analysis identified the MELD score and the development of acute kidney failure as variables associated with overall mortality with a cardiological cause. This information can be usefully in the selection of patients for deeper cardiological study.
RECOMMENDATIONS- 1
Patients who are hepatic transplant candidates must be evaluated to rule out coronary disease. A coronariography must be considered for recipients with an accumulation of risk factors. Degree of evidence I-A.
Coordinators: José Ignacio Herrero and Evaristo Varo
In the V Consensus Meeting 4 scenarios were considered in which immunosuppression may play an important role: de novo neoplasias, hepatitis C, hepatocarcinoma (HCC) and kidney failure.
De Novo NeoplasiasThe recipient of solid organ transplants run a higher risk of developing malign neoplasia than the general population, above all over the medium to long term.92 Additionally, de novo neoplasias are a major cause of morbimortality following hepatic transplant.93,94
The role of immunosuppression in de novo neoplasias was evaluated in 2 different scenarios: (1) changes in immunosuppression to attempt to prevent the development of de novo neoplasias, and (2) changes in immunosuppression in patients who had already developed a neoplasia.
The first action to prevent the development of de novo neoplasias must be to act on the general risk factors for neoplasia, such as tobacco, alcohol or solar radiation, which are important in the general population and for transplanted patients (publications on tobacco, alcohol and solar radiation). Acting on these factors is effective in reducing the risk of neoplasia, as is shown by the fact that transplanted patients who cease smoking have a lower risk of developing a neoplasia.95
Several studies have evaluated the influence of the type of calcineurin inhibitor (CNI) that use cyclosporine or tacrolimus in the risk of developing neoplasias, although their results are contradictory. Thus, some studies suggest that there is a higher incidence of de novo neoplasias in patients treated with tacrolimus,96,97 while others find this to be the case with cyclosporine.97 Given the lack of evidence it is impossible to recommend which CNI to use to reduce the risk of de novo neoplasia post-transplant.
The oncogenic role of the antilymphocyte agents (OKT3 and ATG) has been known for years. Their use is associated with a higher risk of neoplasias in general97 and of lymphomas in particular.98 It is therefore recommended that they should not be used. This recommendation does not cover monoclonal antibodies against CD25 (basiliximab and daclizumab).
The mTOR inhibitors (mammalian target of rapamycin [mTOR])—sirolimus and everolimus—have antiproliferative capacity and they are used as chemotherapy in cancer patients. Due to this, there is high hope that immunosuppression using these drugs will give rise to a lower risk of developing neoplasia. The patients who receive immunosuppression with mTOR are at less risk of developing non-melanotic cutaneous neoplasias, in hepatic transplant99 as well as in renal transplant,100 although this does not justify their use in initial immunosuppression in all patients, given that lesions of this type are very rarely mortal.101 The effect of these drugs on the development of non-cutaneous neoplasias is far less clear.102 In clinical trials lasting several years and large numbers of patients no differences have been detected between the incidence of non-cutaneous neoplasias in patients treated with mTOR and those who continue to be treated with ICN.100
It is unclear whether the intensity of immunosuppressant treatment is associated with a higher or lower incidence of neoplasia. Some studies suggest that a more powerful immunosuppressant treatment may predispose to a higher risk of neoplasia.98,103 Moreover, some side effects of immunosuppression are dose-dependent, including renal toxicity, diabetes, dyslipidemia and arterial hypertension. Due to this, it is recommended that excessive immunosuppression be avoided.
In patients who have developed a neoplasia after transplant it is possible, in some cases, to consider changes in their immunosuppression, with a beneficial effect on their subsequent evolution. In patients with Kaposi's sarcoma replacing CNI with mTOR has a favourable influence on the disease.104 Additionally, in patients with post-transplant lymphoma, reducing immunosuppressant power is associated with better evolution.105 In all cases, reducing immunosuppression must take place in an individualised way, taking into account the time that has passed since the transplant.
In patients with non-melanotic skin cancer, replacing CNI with mTOR reduces the risk of developing a second skin cancer.106 However, the immunological risk must be evaluated, and this change in immunosuppression is not routinely considered to be justified in all patients, as non-melanotic skin cancer does not place the patient's life in jeopardy.
Lastly, although in clinical practice CNI is often replaced by mTOR in patients who have had a solid tumour,107 the lack of scientific evidence for its utility makes it impossible to make a recommendation. When this strategy is used, possible interference with antineoplastic treatment must be taken into account, given that mTOR may increase the bone marrow aplasia caused by chemotherapy. On the other hand, given that they inhibit scarring, they may give rise to complications in case of surgical treatment.
RECOMMENDATIONS1. In patients who have not developed neoplasia:
- •
Action must be taken regarding neoplasia risk factors. Degree of evidence I-A.
- •
Antilymphocytic globulins must not be used (OKT3 and ATG). Degree of evidence II-A.
- •
In general, excessive immunosuppression should be avoided. Degree of evidence I-A.
- •
It is not possible to recommend the use of one calcineurin inhibitor (cyclosporine or tacrolimus) rather than the other. Degree of evidence I-B.
- •
It is not possible to recommend the general use of sirolimus or everolimus. Degree of evidence II-A.
2. In patients who have developed neoplasia:
- •
In patients with Kaposi's sarcoma calcineurin inhibitors should be replaced by everolimus or sirolimus.
- •
In patients with post-transplant lymphoproliferative syndromes the attempt should be made to reduce immunosuppression.
It is not possible to recommend the general use of sirolimus or everolimus.
Hepatitis CThe natural history of hepatitis C following hepatic transplant is faster than it is in the general population. One of the factors that influence this faster evolution is immunosuppressant treatment. The consensus congress of the SETH in 2010108 and other revisions109 have therefore evaluated the role of different immunosuppressors in the severity of hepatitis C relapse. The conclusions of the previous consensus session are still valid: (1) it is not possible to recommend the use of a specific CNI (cyclosporine or tacrolimus) due to its influence on the post-transplant evolution of hepatitis C; (2) high doses of steroids must not be used to treat mild episodes of rejection, because they are associated with more aggressive relapses; (3) steroid-free regimes may be used, but when steroids are used they must be withdrawn progressively and not before six months after transplant, and (4) there is no evidence that mycophenolate mofetil or CD25 antibodies influence the evolution of hepatitis C relapse. In general, the overall recommendation of the 2010 consensus meeting was to avoid excessive immunosuppression, with special emphasis on avoiding the use of high doses of steroids.
Treatments for infection by the hepatitis C virus using new direct action antiviral medicines would change this situation completely.110 Their great efficacy, with sustained rates of viral response above 80%–90%, would change the prognosis of hepatitis C after transplant, given that the response to antiviral treatment after transplant is associated with a clear improvement in survival.111 Due to this, the fundamental recommendation is that hepatic transplant candidates may receive treatment using the new direct action antiviral drugs before and/or after hepatic transplant. In a situation in which direct action antiviral drugs are fully available, it may be foreseen that influence of immunosuppressant treatment on the evolution of post-transplant relapse of hepatitis C will be minimal.
RECOMMENDATIONS- 1.
Avoid the excessive use of immunosuppressant medication. Degree of evidence I-A.
- 2.
No calcineurin inhibitor has advantages vs HCV. Degree of evidence I-B.
- 3.
Doses of steroids should be scaled with progressive reduction after transplant. Degree of evidence I-A.
- 4.
The introduction of new antiviral drugs will modify the influence of immunosuppressant medication in patients with hepatitis C. Degree of evidence III.
HCC is one of the main indications for transplant in Spain, leading to more than 20% of transplants per year. The liver is also the organ transplanted the most often due to some type of cancer. The immunosuppressant management of these patients should therefore be special and especially individualised.
Retrospective studies found a direct correlation between high levels of CNI during the first post-transplant months and the risk of tumour relapse in patients transplanted according to the Milan criteria.112 This is why it is advisable to avoid overdose of the CNI, with prudent minimum levels of tacrolimus <10ng/ml and of cyclosporine <300ng/ml.113
The role of immunosuppression in HCC was evaluated in 2 different scenarios:
The Role of mTOR Inhibitors in the Prevention of Hepatocarcinoma RelapseSeveral retrospective studies and systematic reviews show that mTOR inhibitors reduce the risk of tumour relapse in HCC patients. Nevertheless, there is very little scientific evidence for this.114,115
The use of mTOR inhibitors is now generalised in clinical practice for HCC patients at high risk of relapse (alpha-fetoprotein >200ng/ml, HCC that exceed the Milan criteria in the explanted part, vascular invasion, a poorly differentiated tumour). In spite of this, this clinical practice has not been proven to have any benefit for the patient.
There is insufficient scientific evidence to recommend the general use of mTOR inhibitors to reduce the risk of HCC relapse after hepatic transplant.116
The Role of mTOR Inhibitors in the Treatment of Hepatocarcinoma RelapseThere is no evidence that the use of mTOR inhibitors improves the prognosis in patients with relapsed HCC.
In some small series the combination of mTOR inhibitors and sorafenib was safely used.117 No clinical trials demonstrate any benefit in terms of survival.
RECOMMENDATIONS- 1.
Avoid high doses of calcineurin inhibitor in the first month after transplant. Degree of evidence I-A.
- 2.
There is not sufficient evidence to recommend the use of mTOR inhibitors to prevent tumour relapse. Degree of evidence II-B.
- 3.
There is not sufficient evidence to recommend the use of mTOR inhibitors in the treatment of tumour relapse after transplant. Degree of evidence II-B.
Hepatic transplant candidates often undergo a certain degree of kidney failure. This may be functional, glomerulonephritis associated with certain hepatic diseases (such as mesangial glomerulonephritis and alcoholic cirrhosis or membranoproliferative glomerulonephritis and hepatitis C) or it may be connected with other pathologies such as nephroangiosclerosis or diabetic nephropathy.
Renal dysfunction after hepatic transplant is common and adversely affects patient survival and quality of life. Several drugs which are routinely administered after transplant may contribute to this. The CNI may cause a reversible reduction in renal blood flow and glomerular filtering. This reversible effect is due to relative vasoconstriction of the afferent glomerular arterioles. This physiological effect is related in some way with plasmatic concentration, and it is completely reversible the majority of times. The use of CNI may also be associated with progressive renal interstitial fibrosis and tubular loss, and this toxicity may accelerate if there is any underlying renal pathology, which is now a relatively frequent factor due to the increasing age of recipients. Additionally, CNI may indirectly influence renal dysfunction, inducing hypertension and alterations in glycaemia regulation.118 The mTOR inhibitors have also been shown to prolong recovery time after the damage caused by ischaemia/reperfusion, possibly due to inhibition of the epithelial and endothelial growth factors. Apart from their direct effects, mTOR inhibitors increase the toxicity of CNI through mechanisms that have yet to be elucidated. The combination of a CNI, especially cyclosporine, with an mTOR inhibitor reduces the **GFR to a greater degree than is the case with only a CNI, and in animal models this combination increases the renal fibrosis associated with CNI. Lastly, mTOR inhibitors are associated with proteinuria and significant worsening of pre-existing proteinuria. This may be associated with direct toxicity at the level of the podocytes or indirect toxicity through alteration of the glomerular vascular repair.119
Serious renal dysfunction may reach an incidence of 18% 5 years after hepatic transplant,120 and it may even be higher with the passage of time. The prevention of kidney failure should commence with the control of risk factors such as diabetes or arterial hypertension. Two basic questions must be analysed in connection with immunosuppression:
A. The moment when immunosuppression should be changed to prevent or treat kidney failure
- •
In patients with kidney failure prior to transplant or in patients at high risk of postoperative kidney failure it is advisable to reduce the dose or delay the commencement of CNI treatment, using (or not) mono- or polyclonal antibodies and/or mycophenolate mofetil (MPF).
- •
There is evidence that the above guideline is beneficial for the preservation of kidney function in the short to medium term. However, there is no consensus on its generalised use, basically due to a lack of studies on its overall effects.
B. The use of mTOR inhibitors or MPF in the prevention or treatment of kidney failure after liver transplant
- •
The guidelines in which CNI are reduced to use MPF or mTOR inhibitors (the latter after the first month) have been shown to be effective in preserving kidney function over the medium term.
- •
There is no evidence that either of these two guidelines is better than the other, although patients with significant proteinuria (>0.5g/day) should not be treated using mTOR inhibitors, except in special circumstances.
- •
Over the long term it is possible to reduce or interrupt the CNI and treat using MPF or mTOR inhibitor monotherapy, taking into account the fact that this strategy is associated with a higher risk of rejection.121–123
- 1.
In recipients with kidney failure prior to transplant or with risk factors for the development of kidney failure after transplant, it is recommended that forms of immunosuppression which protect the kidneys be used. Degree of evidence I-A.
- 2.
In renally protective forms of immunosuppression there is no difference between using MPF or mTOR inhibitors, and either may be used in monotherapy over the medium to long term. Degree of evidence II-A.
Coordinators: Emilio Ramos Rubio and Martín Prieto Castillo
This document summarises the usual clinical practice and recommendation of Spanish hepatic transplant (HT) groups in connection with “management” in the waiting list for HT of patients with HCC.
The conclusions and recommendations were established by face-to-face discussion and revision of the recent bibliography and the answers to a questionnaire prepared for this purpose.
IntroductionSubjects in connection with the management of HCC patients in the waiting list for transplant are fundamental for prioritisation, treatment when in the list and routine monitoring. Additionally, 2 more specific subjects were also added to the discussion:
- –
Conditions for withdrawal from the list and attitude to patients with tumour progression beyond the Milan criteria.
- –
Attitude to resected but transplantable patients with resected specimen histological criteria that give rise to a poor prognosis.
There is no solid evidence in the literature regarding which action protocols are the most suitable for each one of these questions. On the other hand, there is probably no unique and ideal protocol for action that would be applicable to all transplant populations or groups. This is because suitability is influenced by factors such as the number of patients in the waiting list, the percentage of patients with HCC and the waiting time in each unit.
Another aspect that must be taken into account and which hinders reaching agreement is that not all of the Spanish groups use the same HT indication criteria for patients with HCC. Some groups apply a moderate expansion of the Milan criteria for inclusion in the list, while others do not systematically exclude patients who progress beyond the said criteria while in the waiting list from HT.
Prioritisation in the Waiting ListThe application of the MELD score in prioritising patients in the waiting list has made it necessary to introduce exceptions to the same to give patients with good hepatic function the possibility of receiving a transplant. The patients with HCC represent the paradigm of this situation. The usual system consists of identifying a MELD score that ensure equal opportunities for transplant among patients with tumour and non-tumour pathology.124 This score is awarded to HCC cases at the most risk of tumour progression and therefore of exclusion from the waiting list (dropping out). This system has the drawback that a single score is not applicable to all populations, and it is also probable that the ideal score will vary over time. This aspect is also surely influenced by the significant increase in the number of patients with HCC in certain geographical areas.
The UNOS prioritisation system divides patients who fulfil the Milan criteria into two stages: T1 (a lesion <2cm) and T2 (a single lesion measuring 2cm to 5cm or 2–3 lesions all of which are ≤3cm). Currently, and following several changes in the prioritisation system, only patients in stage T2 are prioritised by the UNOS, receiving 22 points, which is equivalent to a 15% risk of mortality at 3 months. In spite of this, data suggest that patients with HC have more possibilities of receiving a graft than those with benign pathology. Mehta et al.125 believe that a sub-group of T2 patients at low risk of drop out (1.6% at 2 years) should be excluded from prioritisation. This sub-group, which represents about 20% of cases, may be identified by the following characteristics: lesion diameter 2cm to 3cm, complete response to locoregional treatment (LRT) and alpha-fetoprotein values (AFP) ≤20ng/ml.
In Europe, the consensus conference that was held in Zurich on HT in patients with HCC126 did not make any recommendation regarding the prioritisation of patients in the waiting list. In Spain, in a survey of 17 transplant groups,127 15 stated that they apply some type of prioritisation to patients with HCC. The majority used the MELD system and awarded more points to those patients with multiple tumours or single ones larger than 3cm. The score increased with increased time in the waiting list.
Instead of using the MELD score, it has been suggested that a specific score be calculated for each HCC patient using a mathematical formula. This would give rise to a continuum of scores, with the aim of expressing the real risk of progression in each patient more realistically. In the publication by Toso et al.128 the score is the result of an equation that includes the following variables: age, MELD score, diagnosis, number of HCC nodules, size and AFP values.
The authors consider that the proposed equation may not be applicable in all populations, and that it may require adjustment if waiting list conditions change. On the other hand, the MELD score and the one calculated using this equation are incompatible, so that a correlation based on the risk of drop-out would have to be established. This would calculate what is known as “dropout equivalent MELD” (deMELD), with a predictive power of tumour progression that was subsequently validated in a study by the same authors.129
As well as the prejudice to patients with no tumour pathology, one of the drawbacks of “excessive prioritisation” of HCC patients is that it may be associated with worse results in terms of survival and relapse130 after HT. Several articles have been published recently which suggest that a short time in the waiting list for HC patients is associated with a higher risk of relapse.131 In the study by Samoylova et al.132 the incidence of relapse was lower in patients who had been in the waiting list for more than 120 days (2.2% vs 3.9%; P=.002), so that the authors recommend that HC patients remain for at least 3 months in the waiting list. Nevertheless, the article by Bitterman et al.133 analysing **OPTN data does not find that a longer time in the waiting list increases the selection of tumours with more favourable histological characteristics.
RECOMMENDATIONS- 1.
Spanish groups agree that patients in the waiting list for hepatic transplant with tumours at high risk of drop-out must receive prioritisation of some type to prevent them leaving the list due to tumour progression. The most widely accepted definition of a “high risk” tumour includes solitary hepatocarcinomas larger than 3cm in diameter, together with multinodular tumours. Nevertheless, there is no consensus on whether failure in the use of at least 2 locoregional treatment should be considered a prioritisation criterion. Nor is there consensus on the attitude to low risk patients, who are prioritised by somewhat more than 50% of the groups. Degree of evidence I-A.
- 2.
It is recommended that the MELD score be used to manage prioritisation, assigning a value that favours equity in the possibility of receiving a transplant between patients with tumours and those with a benign pathology. Although it is not possible to establish a specific score that is applicable in all situations, those habitually used in practice run from 15 to 19 points. Some groups regularly increase the score when patients spend more time in the waiting list. Degree of evidence I-B.
- 3.
Considering the difficulty involved in establishing a score that ensures equity, it is recommended that the incidence of drop-outs be compared at regular intervals between patients with hepatocarcinoma and those with benign pathology. There are no data that would make it possible to establish how often results should be revised, and therefore each group should decide this based on the possible changes which occur in their own waiting list. Degree of evidence III.
- 4.
When a patient has been in the waiting list for a short time, it is considered reasonable to manage prioritisation based on clinical criteria, given that the rigid application of the MELD would probably not lead to any advantage. Degree of evidence III.
- 5.
Finally, there is no consistent evidence for recommending a minimum observation time prior to HT for patients who meet the Milan criteria. Degree of evidence II-B.
In connection with treatment in the waiting list, although there is no solid evidence that it is effective, chemoembolisation (CE) is still recommended, as is radiofrequency (RF) in the case of tumours at a high risk of relapse (UNOS T2) and when the estimated time in the waiting list will be longer than 6 months.134 This is recommendation No. 24 of the Zurich consensus conference, and it is probably more relevant in the case of patients with tumours close to 5cm in size or with high levels of AFP.135
There is no consistent evidence in favour of the neoadjuvant treatment of tumours at low risk of progression.135 Recommendation No. 23 of the Zurich consensus conference states that “given the lack of solid evidence, it is not possible to make any recommendation regarding “bridging treatment” in the waiting list for patients in stage T1 of the UNOS”. In spite of this, many groups systematically treat all patients whose time in the waiting list is foreseen to be prolonged.
According to recommendation No. 25 of the Zurich consensus conference126 no data exist that would make it possible to establish which neoadjuvant therapeutic method is the best. Nevertheless, tumour destruction procedures achieve complete necrosis in a higher percentage of cases.136–138 They therefore tend to be preferred when it is possible to use them.135 Other LRT such as external **RDT or the use of Y-90 spheres require further studies.
In a recent article by DuBay et al.139 the use of RF as a bridging therapy until HT did not give rise to any significant advantage in terms of the proportion of drop-outs or tumour relapses after HT. Evolution after HT chiefly depended on the tumour stage of the explant. However, it must be pointed out that the group of patients treated using RF had spent longer time in the waiting list.
Of the 17 Spanish groups that answered the above-mentioned survey,137 7 treated all patients with HCC in the waiting list and only 10 of those at UNOS T2. The choice of method of treatment depended on the size and number of nodules, although 2 of the groups always indicated CE.
The use of an LRT followed by a period of observation is considered indispensible to indicate HT in patients with expanded criteria. Based on this experience other authors have recommended the use of the same strategy for all HCC patients, regardless of their stage,140 except in those cases where it is not possible to use an ablative treatment.
It has recently been suggested that administering sorafenib to patients in the waiting list may delay progression of the tumour in the case of stage T2 tumours.141 Nevertheless, some evidence suggests that this strategy is associated with an increase in the incidence of biliary complications and acute rejection.142
RECOMMENDATIONS- 1.
The consensus recommendation is to treat all of the patients whose estimated waiting time will be longer than 6 months, independently of whether the patient has HCC at high or low risk of drop-out. In practice this means that the majority of patients in groups with long waiting list times will receive treatment, while the groups with short times in the waiting list will receive neoadjuvant treatment less often. Degree of evidence II-B.
- 2.
The majority of the groups support the use of tumour destruction procedures by radiofrequency or microwaves, on condition that tumour and patient characteristics make this possible. With some exceptions, CE is reserved for patients with multinodular tumours, those larger than 3cm and those which have contraindications for radiofrequency. The impossibility of using any type of LRT must not be considered a prioritisation criterion. Degree of evidence II-B.
- 3.
All of the groups agree that achieving complete long-term necrosis in single tumours less than 3cm in size should not be considered a reason for excluding a patient from the waiting list. Degree of evidence II-A.
- 4.
Finally, another conclusion accepted by all of the groups is that there is no evidence to support treatment with sorafenib in the waiting list. Degree of evidence II-B.
In connection with this aspect, there is consensus in the scientific literature on the need to use a monitoring programme using imaging techniques for the patients in the waiting list. Recommendation No. 22 of the Zurich consensus conference states that it is necessary to regularly monitor patients in the waiting list using dynamic CT or MR. However, the revision by the work group of Kneteman et al.143 shows that this recommendation is not based on specific studies, but rather on data about the precision of the different imaging techniques, on knowledge of the natural history of HCC and on the results of monitoring programmes. It is concluded that patients in the waiting list should be monitored every 3 months using 3 phase multidetector CT or MR. There is still no evidence to support recommending the use of other techniques such as PET/CT.
Several publications have also emphasised the usefulness of determining AFP levels at regular intervals. Recommendation No. 12 of the Zurich consensus conference126 concludes that AFP levels offer relevant prognostic information, although it sets no criteria for practical application.
Some evidence suggests that patients with high levels of AFP present vascular invasion and non-differentiated tumours more often, giving rise to a higher risk of tumour progression.144 This could be a reason for prioritising these patients in the waiting list. In the survey carried out among Spanish groups in 2013,127 5 groups considered a level of AFP higher than 200ng/ml to be a reason for prioritisation. However, a higher rise in AFP levels may imply a contraindication for HT or justify the need to maintain a period of observation following the use of an LRT.
In the study by Hameed et al.,145 a level of AFP>1000ng/ml that did not fall to less than 500ng/ml in spite of an LRT was found to be a negative prognostic marker in tumour biology, and it was associated with a high risk of post-transplant relapse. This circumstance may therefore be considered to be an exclusion criterion for HT. According to the authors, applying this criteria would exclude 5% of candidates and achieve a 20% reduction in the incidence of relapse. However, the most widely accepted cut-off point with prognostic value for AFP levels stands at 400ng/ml. In the revision by Merani et al.,146 patients who presented levels higher than 400ng/ml at the moment of being included in the list after which levels fell (<400ng/ml) due to the use of an LRT had survival results due to intended treatment similar to those for patients with AFP levels that were always below 400ng/ml, and better than those in whom it was not possible to reduce AFP levels.
RECOMMENDATIONS- 1.
Given the usual growth characteristics of hepatocarcinoma, it is recommended that restaging take place at regular intervals of the patients in the waiting list, at least once every 3 months. Helical CT or MR can be used for the examination. Thoracoabdominal CT makes it possible to perform a broader staging, while MR has an additional value in hepatic staging. Although some groups support the use of PET/CT, solid data are still lacking to confirm the usefulness of systematically using it. Degree of evidence I-A.
- 2.
It is recommended that AFP levels be measured every 3 months for patients in the waiting list. A “moderate” rise (200ng/ml) in these levels would represent a prioritisation criteria for 50% of the groups. Nevertheless, there is broad agreement that a rise above 400ng/ml or a rapid increase in AFP levels should lead to the suspicion of possible tumour progression. This situation should lead to restaging and closer monitoring. Finally, 50% of the groups consider that if AFP levels rise above 1000ng/ml the patient should be evaluated for temporary or definitive exclusion from the waiting list. Degree of evidence II-B.
In this situation the following strategies may be followed: (1) the definitive exclusion of the patient from the list; (2) the application of a LRT and, if the patient once again fulfils the Milan criteria, include the patient again in the HT list, and (3) keep the patient in the list in spite of the progression, on condition that certain specific limits are not surpassed.
Strategy No. 2 is the one proposed by recommendation No. 26 of the Zurich consensus conference126 as well as in the article by the workgroup of Kneteman et al.143 This strategy is based on the fact that the response to LRT is considered to be a good datum for the evaluation of the biological aggressiveness of a specific tumour. Evaluation of the response should take place by applying mRECIST criteria.136
In the survey of 17 Spanish groups published in 2013,127 11 groups excluded patients that progressed beyond the usual criteria of the group from the list. In the other 6 groups, patients were only excluded if macroscopic vascular invasion or extrahepatic disease occurred, or if rapid growth of the tumour was observed.
RECOMMENDATIONS- 1.
The consensus recommendation is not to systematically exclude these patients from the waiting list. Nevertheless, more than 50% of the groups recommend their temporary exclusion and the use of locoregional treatments. If these mean that the patient once again fulfils the Milan criteria according to mRECIST, they may be included in the list once again after a period of observation of 3–6 months. Degree of evidence III.
- 2.
The other groups also consider that locoregional treatments be used, but they do not exclude the patient from the list on condition that no extrahepatic disease is detected, that no macroscopic vascular invasion occurs, that the hepatic disease does not widely surpass the Milan criteria (a tentative suggestion was made that the limit be set at criteria “up to seven”) and that the patient maintains a good performance status. Degree of evidence III.
Resection seems to be a safe bridging treatment before HT,147 and it may be applicable in selected patients with waiting times foreseen to be longer than one year.148 However, it is currently rarely used for this purpose.
Unlike LRT, resection makes it possible to obtain a full histological study of the tumour, thereby helping to achieve a better evaluation of the risk of tumour relapse. This knowledge could potentially be of use to decide whether a patient should be solely monitored, or if they should be offered HT preventatively. Although this possibility is attractive,149,150 in practice it does not seem to be very widespread.
In some countries resected patients cannot be considered for HT unless they suffer a relapse. As a result of this, and contrary to what occurs with radiofrequency, this strategy cannot be applied. In the opinion of Majno et al.,135 this rule should be revised.
In the survey published in 2013, only 8 of the 17 groups which answered directly include patients with histological data indicating a poor prognosis in the waiting list.
RECOMMENDATIONSFrom the discussion between Spanish transplant groups it can be concluded that around 50% of the groups consider that surgery may be a bridging treatment prior to HT in selected cases. On the other hand, almost all of the groups recommend that patients with resected HCC be included in the list, if the resected part presents histological signs with a poor prognosis. These patients should not receive any prioritisation apart from that in connection with the morphological criteria of their tumour. Degree of evidence II-B.
Conflict of InterestsThe authors declare that they have no conflict of interests.
| Scientific Committee of the Spanish Transplant Society | |
| Secretary | |
| Javier Briceño Delgado | Hospital Universitario Reina Sofia |
| Board Members | |
| Itxarone Bilbao | Hospital Vall d’Hebron |
| Rubén Ciria Bru | Hospital Universitario Reina Sofia |
| José Luis Fernández Aguilar | Hospital Regional Universitario |
| Magdalena Salcedo | Hospital General Universitario Gregorio Marañón |
| Víctor Sánchez Turrión | Hospital Universitario Puerta de Hierro |
| Trinidad Serrano | Hospital Clínico Universitario Lozano Blesa |
| Attendants to the V Consensus Meeting of the Spanish Hepatic Transplant Society | |
| Group 01 High-risk recipients | |
| Coordinators | |
| Itxarone Bilbao | Hospital Universitario Vall d’Hebron |
| Manuel de la Mata | Hospital Universitario Reina Sofía |
| Participantes | |
| Manuel Abradelo | Hospital Universitario 12 de Octubre |
| Isolina Baños Pérez | Hospital Universitario Puerta de Hierro |
| Asterio Barrera | Hospital Universitario Río Hortega |
| Manuel Barrera | Hospital Universitario Nuestra Señora de la Candelaria |
| David Calatayud | Hospital Universitario Clínic |
| Delia d’Avola | Clínica Universitaria de Navarra |
| José Luis Fernández Aguilar | Hospital Regional Universitario de Málaga |
| Francisco Galeano | Hospital Universitario Infanta Cristina |
| Francisco Agustín García Gil | Hospital Clínico Universitario Lozano Blesa |
| Daniel Garrote | Hospital Universitario Virgen de las Nieves |
| Mikel Gastaca | Hospital de Cruces |
| Manuel Gómez Gutiérrez | Hospital Universitario Juan Canalejo |
| Manuel López Santamaría | Hospital Universitario La Paz Infantil |
| Javier Nuño Vázquez-Daga | Hospital Universitario Ramon y Cajal |
| Ricardo Robles | Hospital Universitario Virgen de la Arrixaca |
| Gonzalo Rodríguez Laiz | Hospital General Universitario de Alicante |
| Juan Carlos Rodríguez Sanjuan | Hospital Universitario Marqués de Valdecilla |
| Angel Rubín | Hospital Universitario y Politécnico La Fe |
| José Manuel Sousa | Hospital Universitario Virgen del Rocío |
| Santiago Tomé | Hospital Clínico Universitario de Santiago de Compostela |
| F. Javier Xiol | Hospital Universitario de Bellvitge |
| Group 02 Immunosupression Scenarios | |
| Coordinators | |
| José Ignacio Herrero | Clínica Universitaria de Navarra |
| Evaristo Varo | Hospital Clínico Universitario de Santiago |
| Participants | |
| Victoria Aguilera | Hospital Universitario y Politécnico La Fe |
| José María Álamo | Hospital Universitario Virgen del Rocío |
| Pablo Bellot | Hospital General Universitario de Alicante |
| Javier Briceño | Hospital Universitario Reina Sofía |
| Lluís Castells | Hospital Universitario Vall d’Hebron |
| Gonzalo Crespo | Hospital Universitario Clínic de Barcelona |
| Valentín Cuervas-Mons | Hospital Universitario Puerta de Hierro |
| Emilio Fábrega | Hospital Universitario Marqués de Valdecilla |
| Luisa González Diéguez | Hospital Universitario Central de Asturias |
| Javier Graus | Hospital Universitario Ramon y Cajal |
| Paloma Jara | Hospital Universitario La Paz Infantil |
| Carlos Jiménez | Hospital Universitario 12 de Octubre |
| Miguel Jiménez | Hospital Regional Universitario de Málaga |
| Laura Lladó | Hospital Universitario de Bellvitge |
| Esther Molina | Hospital Clínico Universitario de Santiago |
| Isidoro Narvaez | Hospital Universitario Infanta Cristina |
| Jorge Ortiz de Urbina | Hospital de Cruces |
| Elena Otón | Hospital Universitario Nuestra Señora de la Candelaria |
| José Antonio Pons | Hospital Universitario Virgen de la Arrixaca |
| Magdalena Salcedo | Hospital Universitario Gregorio Marañón |
| Gloria Sánchez Antolín | Hospital Universitario Río Hortega |
| Trinidad Serrano | Hospital Clínico Universitario Lozano Blesa |
| Group 03 Management of the Hepatocarcinoma List | |
| Coordinators | |
| Martín Prieto | Hospital Universitario y Politécnico La Fe |
| Emilio Ramos | Hospital Universitario de Bellvitge |
| Participants | |
| Rafael Bañares | Hospital Universitario Gregorio Marañón |
| Rafael Bárcena | Hospital Universitario Ramon y Cajal |
| Gerardo Blanco | Hospital Universitario Infanta Cristina |
| Francisco Javier Bustamante | Hospital de Cruces |
| Fernando Casafont | Hospital Universitario Marqués de Valdecilla |
| Ramon Charco | Hospital Universitario Vall d’Hebron |
| Manuel Delgado | Hospital Universitario Juan Canalejo |
| Javier Fernández Castroagudín | Hospital Clínico Universitario de Santiago de Compostela |
| Teresa Ferrer | Hospital Universitario Virgen del Rocío |
| Yiliam Fundora | Hospital Universitario Virgen de las Nieves |
| Josep Fuster | Hospital Universitario Clínic |
| Carmen García Bernardo | Hospital Universitario Central de Asturias |
| Félix García Pajares | Hospital Universitario Río Hortega |
| Rafael López Andújar | Hospital Universitario La Fe |
| Sara Lorente Pérez | Hospital Clínico Universitario Lozano Blesa |
| José Luis Montero | Hospital Universitario. Reina Sofía |
| Fernando Pardo | Clínica Universitaria de Navarra |
| Sonia Pascual | Hospital General Universitario de Alicante |
| Pablo Ramírez | Hospital Universitario Virgen de la Arrixaca |
| Juan Miguel Rodrigo | Hospital Regional Universitario de Málaga |
| Víctor Sánchez Turrión | Hospital Universitario Puerta de Hierro |
| María Arántzazu Varona Bosque | Hospital Universitario Nuestra Señora de la Candelaria |
Please cite this article as: Pardo F, Pons JA, Briceño J, en nombre de la Sociedad Española de Trasplante Hepático. V Reunión de Consenso de la Sociedad Española de Trasplante Hepático sobre receptores de riesgo elevado, escenarios actuales de inmunosupresión y manejo del hepatocarcinoma en espera de trasplante. Cir Esp. 2015;93:619–637.
Appendix 1 shows the hepatic transplant teams that took part in the V Consensus Meeting of the Hepatic Transplant Society.