Metastasis is remaining one of the major problems in cancer treatment. Like many other malignancies, urogenital tumors originating from kidney, prostate, testes, and bladder tend to metastasize to the lungs.
The aim of this retrospective study is to evaluate the operative results and prognosis of pulmonary metastasectomy in patients with primary urogenital tumors.
MethodsThis study was approved by the local ethical committee. We retrospectively analyzed the surgical and oncological results of patients who underwent lung resections for urogenital cancer metastases in our department between 2002 and 2018. Demographic data and clinicopathological features were extracted from the medical records. Survival outcomes according to cancer subtypes and early postoperative results of VATS and thoracotomy were analyzed.
Results22 out of 126 patients referred for pulmonary metastasectomy to our department had metastases from urogenital tumors. These patients consisted of 17 males and five females. Their metastasis originated from renal cell carcinoma (RCC; n=9), bladder tumor (n=7), testis tumors (n=4), and prostate cancer (n=2). There was no intraoperative complication. Postoperative complications were seen in 2 patients.
ConclusionsAlthough pulmonary metastasectomy in various types of tumors is well known and documented, the data is limited for metastases of urogenital cancers in the literature. Despite the limitations of this study, we aim to document our promising results of pulmonary metastasectomy in patients with primary urogenital tumors and wanted to emphasize the role of minimally invasive approaches.
La metástasis continua siendo uno de los principales problemas en el tratamiento del cáncer. Como muchas otras neoplasias malignas, los tumores urogenitales que se originan en el riñón, la próstata, los testículos y la vejiga tienden a hacer metástasis a los pulmones.
El objetivo de este estudio retrospectivo es evaluar los resultados quirúrgicos y el pronóstico de la metastasectomía pulmonar en pacientes con tumores urogenitales primarios.
MétodosEste estudio fue aprobado por el comité de ética local. Analizamos retrospectivamente los resultados quirúrgicos y oncológicos de los pacientes que fueron sometidos a resecciones pulmonares por metástasis de cáncer urogenital en nuestro servicio entre 2002 y 2018. Se extrajeron datos demográficos y características clínico-patológicas de las historias clínicas. Se analizaron los resultados de supervivencia según los subtipos de cáncer y los resultados postoperatorios tempranos de VATS y toracotomía.
ResultadosVeintidós de 126 pacientes remitidos para metastasectomía pulmonar a nuestro servicio tenían metástasis de tumores urogenitales. Estos pacientes consistieron en 17 varones y 5 mujeres. Su metástasis se originó a partir de carcinoma de células renales (CCR; n=9), tumor de vejiga (n=7), tumores de testículo (n=4) y cáncer de próstata (n=2). No hubo ninguna complicación intraoperatoria. Se observaron complicaciones posoperatorias en 2 pacientes.
ConclusionesAunque la metastasectomía pulmonar en varios tipos de tumores es bien conocida y documentada, los datos son limitados para las metástasis de cánceres urogenitales en la literatura. A pesar de las limitaciones de este estudio, nuestro objetivo es documentar nuestros prometedores resultados de la metastasectomía pulmonar en pacientes con tumores urogenitales primarios y queríamos enfatizar el papel de los enfoques mínimamente invasivos.
Metastasis remains one of the major problems in cancer treatment. Like many other malignancies, urogenital tumors originating from kidney, prostate, testes, and bladder tend to metastasize to the lungs.1,2 Unfortunately, the presence of metastases is usually associated with a poor prognosis. Although there is no consensus on this issue, metastasectomy may be the part of the treatment especially in cases in which the primary disease is under control.1–3 In many cases sublobar non-anatomic resections sufficient to ensure complete excision and care should be taken to sparing the lung tissue during metastasectomy.
There is no prospective, randomized study in this issue therefore the information on long-term survival in patients surgically treated for urogenital cancers’ lung metastases is limited. Due to the rarity of these cases, it is very difficult to conduct different series and prognostic studies specific to each urogenital cancer subtype. Therefore, it would be logical to use the umbrella term “urogenital cancer”. Although this umbrella term was used frequently in our study, although the number of cases was limited, we preferred to present the survival data of each urogenital cancer subtype.
This retrospective case series and literature review aim to evaluate the results of pulmonary metastasectomy in patients with primary urogenital tumors and make a contribution to the literature by clarifying the role of surgery in treatment.
MethodsPatient selectionThis study was approved by the local ethical committee. We retrospectively analyzed 126 patients who underwent lung resections for metastases in our department between January 2002 and December 2018. The patients included in the study consisted of who had received curative treatment for one of the urogenital malignancies and were in routine follow-up. Ovarian and uterine malignancies were excluded. During the study period, bilaterally synchronous metastases are also observed, however, these patients had limited pulmonary functions, and lung resections (wedge resections) were only performed for diagnostic purposes. Inclusion and exclusion criteria for the study are shown in Table 1. Twenty-two patients who met the inclusion criteria were eligible for participation in the study. Preoperative pulmonary evaluation including spirometry, diffusing capacity for carbon monoxide (DLCO) and, if necessary, VO2max and cardiopulmonary exercise tests was performed for each patient. Flexible bronchoscopy was applied to each patient in the preoperative period. A small number of patients had preoperative histological diagnosis. The decision of surgery was made mostly based on the history of urogenital cancer and the data obtained by CT and PET-CT. However, intraoperative frozen section study was performed in all cases.
Inclusion and exclusion criteria.
| Inclusion | Exclusion |
|---|---|
| Urogenital malignancies (renal cell carcinoma, urothelial carcinoma, testicular tumor, prostate carcinoma) | Ovarian and uterine malignancies |
| Patients who underwent pulmonary resection with malignant negative margin for pulmonary metastasis | Uncontrolled primary disease |
| Metachronous metastasis | Mediastinal lymph node involvement |
| Extrapulmonary metastasis |
Endobronchial ultrasound (EBUS) and/or mediastinoscopy were performed in the presence of a mediastinal lymph node larger than 1cm in thorax computed tomography (CT) or high standardized uptake values (SUV) in positron emission tomography (PET)-CT.
Demographic data and clinicopathological features were extracted from the medical records. Disease-free interval (DFI) was defined as the time between the first curative surgery and the detection of pulmonary metastasis. Surgical procedures, metastasis localizations, histopathological results, perioperative and postoperative complications were noted.
Surgical techniqueVATS or muscle sparing posterolateral thoracotomy was preferred as the surgical technique. We did not have a clear criterion for the selection of VATS, with the increasing experience over the years, VATS has become often preferred. Preoperative thorax-CT guided methylene blue marking method was used in cases where it was thought that the lesion could not be localized during the operation.
The type of lung resection to be performed was decided according to the patient's respiratory capacity, location, number and size of the metastatic lesion. Along with lung resection, complete mediastinal lymph node dissection was also performed in each patient.
Statistical analysisStatistical analysis was performed using SPSS 25.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were analyzed using Chi-square tests. Continuous variables were analyzed using the Mann–Whitney U test. Survivals were estimated using the Kaplan–Meier method.
ResultsAmong 22 patients who underwent surgery consisted of 17 males and five females. Their metastasis originated from renal cell carcinoma (RCC; n=9), bladder tumor (n=7), testis tumors (n=4), and prostate cancer (n=2). Their mean age was 57.11 years (range, 21-75). The mean age of the patients with RCC and bladder tumor at the time of diagnosing metastasis was 57.6 and 59.33 years, respectively. The mean age of patients with testis tumors is 27 (Table 2).
Characteristics of patients.
| Primary disease | Number of patients | Mean age | Operation |
|---|---|---|---|
| RCC | 9 | 58.5 | 1 pneumonectomy, 1 lobectomy, 7 wedge resectionsa |
| Bladder TCC | 7 | 65.1 | 2 lobectomy, 5 wedge resections |
| Testis ca | 4 | 33.6 | 1 pneumonectomy, 3 wedge resection |
| Prostate ca | 2 | 71.5 | 2 wedge resectionb |
| Overall | 22 | 56.7 | 2 pneumonectomy, 3 lobectomy, 17 wedge resections |
RCC: renal cell carcinoma, TCC: transitional cell carcinoma (urothelial carcinoma).
The majority of patients had no symptoms, and their metastasis was detected incidentally in their routine follow-up. Two patients with centrally localized tumors were presented with severe cough and mild hemoptysis.
The site of metastasis was left lung in fifteen, right lung in six patients. One patient had bilateral lung metastases.
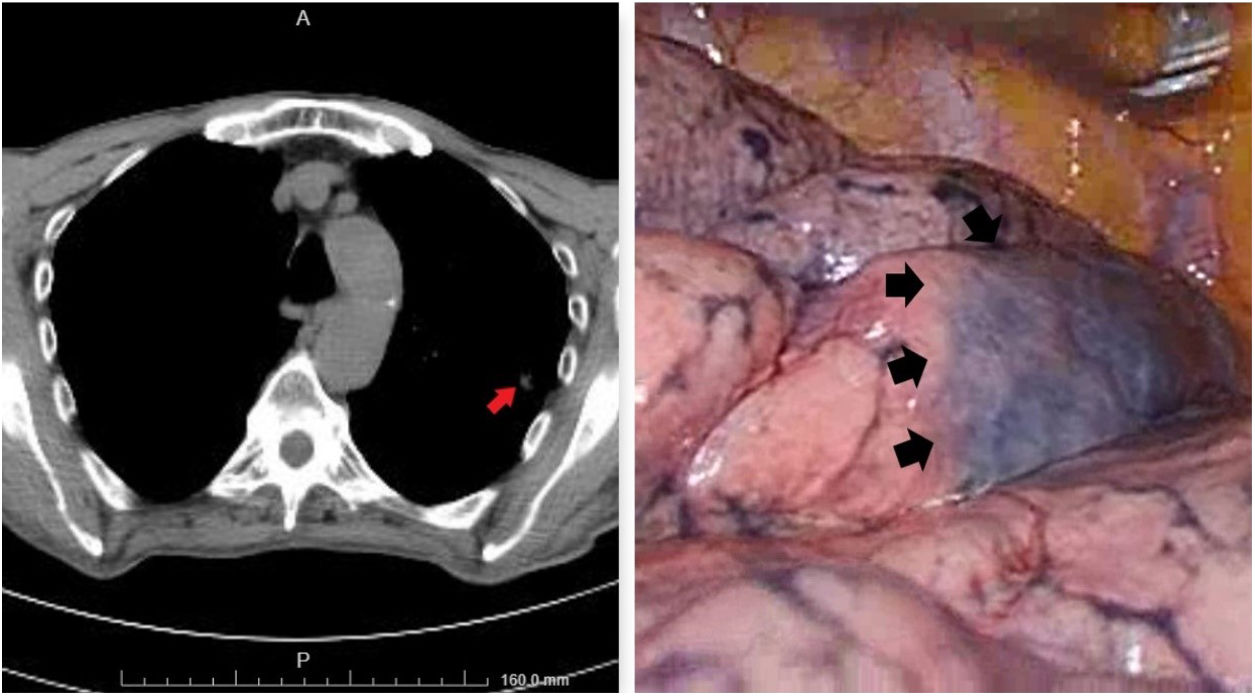
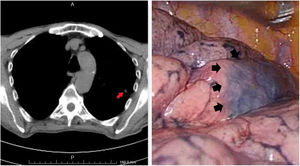
Wedge resection applied in seventeen cases with negative surgical margins. Lobectomy, instead of wedge resection, was applied in three patients because of the multiple metastases located in only one lobe. In 2 patients, the metastatic lesion was localized in the main bronchus and caused complete obstruction. Pneumonectomy had to be performed in these two patients due to suppuration and parenchymal destruction caused by obstruction. These two patients were in good performance status and tolerated the pneumonectomy well. In 13 patients, the operation was started with VATS but was converted to thoracotomy in two patients because the metastatic nodules were not detected. The preoperative thorax-CT guided methylene blue marking method was used to facilitate intraoperative detection of the parenchymal nodule in 2 of the patients who preferred VATS (Fig. 1).
Muscle-sparing lateral thoracotomy applied in seven patients. In patients operated on by VATS had shorter hospitalization and drainage time and better postoperative pain status than thoracotomy (Table 3).
Postoperative results of the VATS and thoracotomy.
| VATS (n=11) | Thoracotomy (n=11) | p value | |
|---|---|---|---|
| Mean drainage time (day) | 3.00 | 4.67 | 0.06 |
| Mean hospital stay (day) | 3.80 | 5.67 | 0.029 |
| Postoperative complication, n (%) | 1 (9.1) | 1 (9.1) | 1.00 |
| VAS scorea | |||
| Day 1 | 4.00 | 4.33 | 0.438 |
| Day 2 | 2.60 | 3.78 | 0.029 |
| Day 3 | 2.40 | 3.44 | 0.083 |
Re-operation was needed, and wedge resection was applied in one case due to the recurrent lung metastasis one year after the first surgery. Although distant metastases were observed during the follow-up period, no local recurrence or recurrent lung metastasis was observed in the lung, except for one patient.
No major complications occurred during surgery. Postoperative complications were observed in 2 patients in total. Postoperative hemorrhage was seen in one patient in the early postoperative period, and re-operation was applied.
Prolonged air leakage was observed in one patient and we managed this complication using the Heimlich valve system. In this patient, the chest tube was removed at the end of the first postoperative month. No major complication occurred in other patients on postoperative follow-up.
While neoadjuvant treatment was not given to any patient, adjuvant chemotherapy was planned for patients with prostate and testicular cancer metastasis.
The median follow-up time was 50 months (range, 20–156 months). The 1-month survival was 100%, but the 2-years survival was 66.6% (for RCC), 57.1% (Bladder tumor), 50% (testis tumor) and 0% (prostate cancer) (Table 4).
Disease free intervals and survivals of the patients.
| Primary disease | Number of patients | DFI | 1 month | 6 months | 2 years | |||
|---|---|---|---|---|---|---|---|---|
| DFS | OS | DFS | OS | DFS | OS | |||
| RCC* | 9 | 28.8 | 100.0% | 100.0% | 88.8% | 88.8% | 55.5% | 66.6% |
| Bladder TCC* | 7 | 15 | 100.0% | 100.0% | 100.0% | 100.0% | 42.8% | 57.1% |
| Testis ca | 4 | 18 | 100.0% | 100.0% | 100.0% | 100.0% | 50% | 50% |
| Prostate ca | 2 | 12 | 100.0% | 100.0% | 100.0% | 100.0% | 0% | 0% |
RCC: renal cell carcinoma, TCC: transitional cell carcinoma (urothelial carcinoma), DFI: disease free interval (month), DFS: disease free survival, OS: overall survival.
Although lung metastasis excision in various types of tumors is well known and documented, the data is limited to the role of the surgery for metastases of urogenital cancers on literature (Table 5).
Literature review.
| Authors | Study year | Number of patients | Pathology of primary disease | Median survival (months) | Ref. |
|---|---|---|---|---|---|
| Cerfolio RJ et al. | 1994 | 96 | RCCa | 36 | 7 |
| Friedel G et al. | 1999 | 77 | RCC | 37 | 6 |
| Hofmann HS et al. | 2005 | 64 | RCC | 39.2 | 8 |
| Kim JJ et al | 2011 | 15 | RCC | 34.9 | 4 |
| Kawashima A et al. | 2011 | 25 | RCC | 33.9 | 5 |
| Cowles RS et al. | 1982 | 6 | UCb | 60 | 9 |
| Siefker-Radtke AO et al. | 2004 | 31 | UC | 23 | 10 |
| Kanzaki R et al. | 2010 | 18 | UC | 52 | 13 |
| Matsuguma H et al. | 2011 | 32 | UC | 60 | 11 |
| Han WS et al. | 2012 | 16 | UC | 60 | 12 |
| Kim T et al. | 2015 | 30 | UC | 30 | 14 |
| McGuire MS et al. | 2003 | 105 | NSGCTsc | d | 18 |
| Pfannschmidt J et al. | 2006 | 52 | NSGCTsc | 75.8% (5-year survival) | 19 |
| Goto T et al. | 2010 | 1 | Prostate ca | e | 23 |
| Wallis CJ et al. | 2011 | 1 | Prostate ca | e | 24 |
| Radulescu IM et al. | 2014 | 26 | Urologic cancers: 8Genital cancers:16 | f | 2 |
| Masoum SHF et al. | 2014 | 3 |
Considering the renal cell carcinoma, it was reported that 5-years survival rates are between 21% and 60% after pulmonary metastasectomy.2–8 It was also shown that metastasectomy would have an additional contribution to prognosis, especially in patients with few and small metastases and longer DFI.5–9 Our study demonstrated 2-years survival rates 66.6% from renal cell carcinoma, after pulmonary metastasectomy.
In literature, studies have demonstrated 5-years survival rates between 15.9% and %33 from urothelial carcinoma, after pulmonary metastasectomy.9–14 It was also shown that patients who had shown great response to chemotherapy and had no evidence of early or rapid progression elsewhere had been shown to benefit more from metastasectomy. In our experience, we had four cases of metastatic urothelial carcinoma with a 57.1% 2-years survival rate. Adjuvant therapy was not administered after the pulmonary metastasectomy. There is limited data in the literature regarding the benefit of giving adjuvant treatment after complete metastasectomy in renal cell carcinoma. In these studies, there was no significant difference in survival in the group with and without systemic adjuvant therapy.15 However, pulmonary metastasectomy combined with systemic targeted therapy and/or immunotherapy could be an optimal treatment approach in the future, but it needs to be supported by clinical trials.
The lung is the most common site of metastases in patients with testicular germ cell tumors. Studies demonstrated that resection of the pulmonary metastases has a positive effect on survival rates, especially patients with lesions limited to one site. In literature, 5-years survival rates were reported between 45% and 65% after metastasectomy.16–22 McGuire MS et al. have reviewed 105 patients with NSGCTs who undergone thoracotomy because of pulmonary metastasis and the viable non-teratomatous disease in the chest or retroperitoneum was described as a poor prognostic factor.18 In another study, Pfannschmidt J et al. have reviewed 52 cases of NSGCT who undergone pulmonary metastasectomy, and 5-year survival was reported as 75.8%. The authors described incomplete resection and elevated tumor marker levels, AFP and/or hCG as poor prognostic factors.19 In our study, we had 4 cases of testicular tumors which histological types were embryonal carcinoma. These patients underwent radical inguinal orchiectomy, retroperitoneal lymph node dissection, and adjuvant chemotherapy. However, because of metachronous pulmonary metastases, surgical resection was needed, and adjuvant chemotherapy was administered. The 2-years survival was detected as 50% after pulmonary metastasectomy.
Isolated solitary pulmonary metastases are extremely rare in prostate carcinomas. In prostate carcinoma, lung metastases usually present diffuse interstitial or multinodular patterns, and there is no clue about the survival benefit of pulmonary metastasectomy.23,24 Therefore, pulmonary metastasectomy should be performed only if the patient has solitary pulmonary metastasis resistant to hormone therapy or has severe respiratory symptoms refractory to conservative management. In our experience, we had only two patient of prostate carcinoma who underwent wedge resection. These two patients died in the first six postoperative month due to disease progression.
It is also important to choose the appropriate surgical method in patients with pulmonary metastases. Video-assisted thoracoscopic surgery (VATS) has well-documented benefits over open thoracotomy like less pain, less inflammatory response, shorter hospitalization and fast recovery after surgery.25–27 The main concern regarding the use of VATS for metastasectomy is the risk of inability to performing complete resection. There isn’t any information in the literature to confirm these concerns. Several studies even reported no relation between VATS and open thoracotomy in recurrence in the ipsilateral lung.28,29 Therefore, VATS is routinely used in our clinic for pulmonary metastasectomy. Sometimes it would be difficult to localize metastatic nodules or ground-glass opacities (GGO) intraoperatively during the VATS procedure. Thankfully, various methods that make it possible to localize these kinds of lesions with the VATS technique have been described. The most commonly used methods for this purpose are methylene blue and hook wire marking.30 We routinely use CT-guided methylene blue staining techniques in patients with small nodules and GGOs. Although the lesion is localized with almost complete accuracy by these marking methods, the confirmation should be performed with a frozen section.
In highly selected patients, resection of pulmonary metastases present minimal risk and prolong survival in urogenital tumors. Previous studies described the presence of prolonged DFI, unilateral metastases, surgically resectable tumors, and less than 3 radioimagistic detectable metastases, as a positive predictive factor. With this study, we aim to evaluate the results of pulmonary metastasectomy in patients with primary urogenital tumors in our clinic.
ConclusionNowadays, with the increasing use of minimally invasive methods, operation and hospitalization times have been shortened, and second, third, or even fourth operations in the same patient have become more possible and easier for both patient and surgeon. Although there is no consensus in the management of these patients in the literature, we suggest pulmonary metastasectomy in urogenital cancers especially for patients with good respiratory conditions. We also recommend the usage of video-thoracoscopic surgery instead of thoracotomy. Small nodules and ground-glass opacities can cause problems for VATS, but as we have mentioned, this problem can be overcome by marking techniques.
Authors’ contributionsKCC and SOK analyzed and interpreted the patient data regarding the pulmonary metastasis. GB performed the statistical analyses and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participateThis study was approved by the ethical committee of The University of Health Sciences Dr. Suat Seren Chest Diseases and Chest Surgery Research and Training Center (Registration number: 49109414-604.02; Date: 11/06/2019).
Consent for publicationNot applicable.
Availability of data and materialsData are not publicly available. Data are however available from the authors upon reasonable request and with permission.
FundingThe authors received no specific funding/support for this study.
Conflict of interestsThe authors declare that they have no competing interests.
Not applicable.
This study was presented in the ERS International Congress, Madrid 2019 as a poster.