Colorectal cancer (CRC) is a major health concern and it is associated with significant morbidity and mortality. Over the last decades, the relationship between cancer and nutritional and inflammatory status in oncologic patients was studied thoroughly and multiple immunonutritional scores were developed. These scores have been mainly related to the prognosis of several cancers.
An interaction between the tumour and the host is generated, triggering a systemic inflammatory reaction leading to several neuroendocrine changes. This situation favours a tendency towards anorexia and catabolism. Our hypothesis is that nutritional and inflammatory status of oncologic patients is correlated to postoperative morbidity.
MethodsThis is a prospective observational cohort study with those patients undergoing curative surgery for CRC at our institution between September 2015 and March 2017. Nutritional and inflammatory status was established using Onodera's Prognostic Nutritional Index (PNI). Complications (overall, severe, infectious and anastomotic leakage) were carefully collected during the first 30 days of the postoperative period.
ResultsAfter carrying out the multivariate analysis, PNI turned out to be a great predictive and protective factor for overall complications (RR: 0.279; 95% CI: 0.141–0.552), severe complications (RR: 0.355; 95% CI: 0.130–0.965), infectious complications (RR: 0.220; 95% CI: 0.099–0.489) and anastomotic leakage (RR: 0.151; 95% CI: 0.036–0.640).
ConclusionOur work reports that PNI is an independent predictive factor for the development of postoperative complications following curative surgery for CRC.
El cáncer colorrectal (CCR) constituye un problema sanitario relevante, asociado a una morbimortalidad significativa. A lo largo de las últimas décadas se ha estudiado en profundidad el vínculo que existe entre el cáncer y el estado nutricional e inflamatorio de los pacientes oncológicos, y se han desarrollado múltiples escalas inmunonutricionales, relacionadas principalmente con el pronóstico oncológico de varios tipos de cáncer.
Al generarse una interacción entre el tumor y el huésped, se desencadena una reacción inflamatoria sistémica que conduce a una serie de alteraciones neuroendocrinas. Dicha situación favorece una tendencia hacia la anorexia y el catabolismo. Nuestra hipótesis es que el estado nutricional e inflamatorio de los pacientes oncológicos se correlaciona con la morbilidad postoperatoria.
MétodosEste es un estudio observacional y prospectivo de cohortes, con pacientes tratados mediante la cirugía curativa para el CCR en nuestro centro, entre septiembre del 2015 y marzo del 2017. El estado nutricional e inflamatorio de los pacientes fue establecido mediante el uso del Prognostic Nutritional Index (PNI). Las complicaciones (globales, graves, infecciosas y fuga anastomótica) fueron cuidadosamente observadas y registradas durante los 30 primeros días del período postoperatorio.
ResultadosTras llevar a cabo el análisis multivariante, el PNI resultó ser un gran factor predictivo de complicaciones globales (RR: 0,279; IC 95%: 0,141-0,552), complicaciones graves (RR: 0,355; IC 95%: 0,130-0,965), complicaciones infecciosas (RR: 0,220; IC 95%: 0,099-0,489) y fuga anastomótica (RR: 0,151; IC 95%: 0,036-0,640).
ConclusionesNuestro trabajo refleja que el INP es un factor predictivo independiente para el desarrollo de complicaciones tras la cirugía curativa del CCR.
Colorectal cancer (CRC) is a major health concern, accounting for 10% of all global cancers. Despite the great progress made in both diagnosis and oncological treatment, it is associated to high morbidity and mortality rates.1 Surgery represents the main curative treatment option.2,3
Postoperative complications rate is approximately 29.7% for colon cancer and 40% for rectal cancer.4–6 Most relevant medical complications are acute pulmonary oedema (2.9%), pneumonia (2.4%–6.2%), acute renal failure (0.6%–2%), ischaemic heart disease (0.5%), and acute stroke (0.4%).7–9 Amongst surgical complications, the most important ones are paralytic ileus (7.5%), surgical site infection (3.8%–14%) and anastomotic fistula (8.5% for colon, and up to 15% for rectum).10,11 Mortality rate during the first 30 days of postoperative period is up to 6.7%.12–14 In recent years, it has been observed that the appearance of complications, during the postoperative period of curative surgery for CRC, not only affects quality of life and short-term mortality rate, but it also has a very relevant influence on the long-term prognosis of cancer. Complications are related to higher rate of local and distant recurrence, as wells as worse overall survival (OS) and disease-free survival (DFS).15–20 This is why it is very important to identify preoperatively the patients who are at high risk of developing complications.
Over the last few decades, the close relationship between nutritional and inflammatory status and cancer has been thoroughly studied. An interaction between the tumour and the host itself triggers a systemic inflammatory reaction in which several cytokines are released. Additionally, there are several neuroendocrine changes that provoke hormonal imbalances, which generates a tendency towards anorexia and catabolism (increased proteolysis and lipolysis).21,22 Consequently, with the aim of analyzing this situation in oncologic patients, several scores have been developed, calculated mainly from variables obtained from a preoperative peripheral blood analytics. One of these is Prognostic Nutritional Index (PNI), designed in 1984 by Onodera. It is an index to assess the nutritional and inflammatory status and it is calculated by a simple mathematical formula: PNI=(10×serum albumin [g/dL])+(0.005×lymphocytes/μL).23 Higher values indicate a better situation of the patient.
Our hypothesis is that nutritional and inflammatory status of patients with cancer is related to postoperative morbidity and mortality. We have thus designed a prospective cohort study to assess the capability of PNI to predict the appearance of complications after curative surgery for CRC.
Materials and MethodsThis is a prospective observational cohort study with those patients undergoing curative surgery for CRC at our Institution between September 2015 and March 2017. We excluded those undergoing urgent or non-curative surgeries as well as those who obtained pathological diagnoses others than CRC. This study is part of a public research project and it has been approved by our institution's clinical research ethics committee. We have followed all the ethical principles and data protection guidelines, according to our institution's clinical research ethics committee, to collect all the information from this study's patients.
We have collected preoperative variables that may influence the development of postoperative complications: sex, age, body mass index (BMI), cardiovascular risk factors (arterial hypertension, diabetes mellitus, dyslipidaemia, and smoking), neoadjuvant treatment with chemotherapy or radiotherapy, anaesthetic risk according to the classification of the American Society of Anaesthesiology (ASA), stent placement prior to surgery (in those cases that presented an obstructive neoplasm and who were not operated on urgently), location of the tumour (colon or rectum), time of surgery and intraoperative transfusion of packed red blood cells. In addition, we highlight that we only considered curative procedures in which we could achieve a R0 resection.
All our variables were dichotomous, except three quantitative variables (age, BMI and operative time). Anaesthetic risk was recoded in a new dichotomous variable, so that patients were classified as high risk (ASA III–IV) or low risk (ASA I–II). A peripheral blood test was obtained the day before surgery to establish albumin and lymphocyte levels and thus calculate PNI (10×serum albumin [g/dL])+(0.005×lymphocytes/μL). Optimal cut-off point for this score was calculated using receiver operating characteristics (ROC) curve, endpoint variable for this analysis was global complications. We used this value to create two risk groups, thus easing the interpretation of our results.
Complications were carefully collected during the first 30 days of postoperative period, or until hospital discharge in case of having a longer hospital stay. We established four variables to define our morbidity: overall complications, severe complications (grades III, IV and V of Clavien-Dindo classification), infectious complications and anastomotic leakage. This last one was considered as an infectious complication and it was exclusively analyzed among those patients with anastomosis and without a protective stoma.24 Anastomotic leakage was defined as the presence of intestinal content within the drainage or through the wound, the outflow of oral contrast in the computed tomography, or the direct visualization during endoscopy or surgery. We assessed the influence of PNI, preoperative and intraoperative variables on the appearance of these four complications.
Statistical analysis was performed using SPSS (version 18.0, IBM Corporation). Chi-squared test for qualitative variables and Student's T test for quantitative variables were used to carry out univariate analysis (UVA). A P<.15 was used as the cut-off point for the entry of variables in multivariate study. Logistic regression was used in multivariate analysis (MVA), in which a P<.05 was used to establish statistical significance.
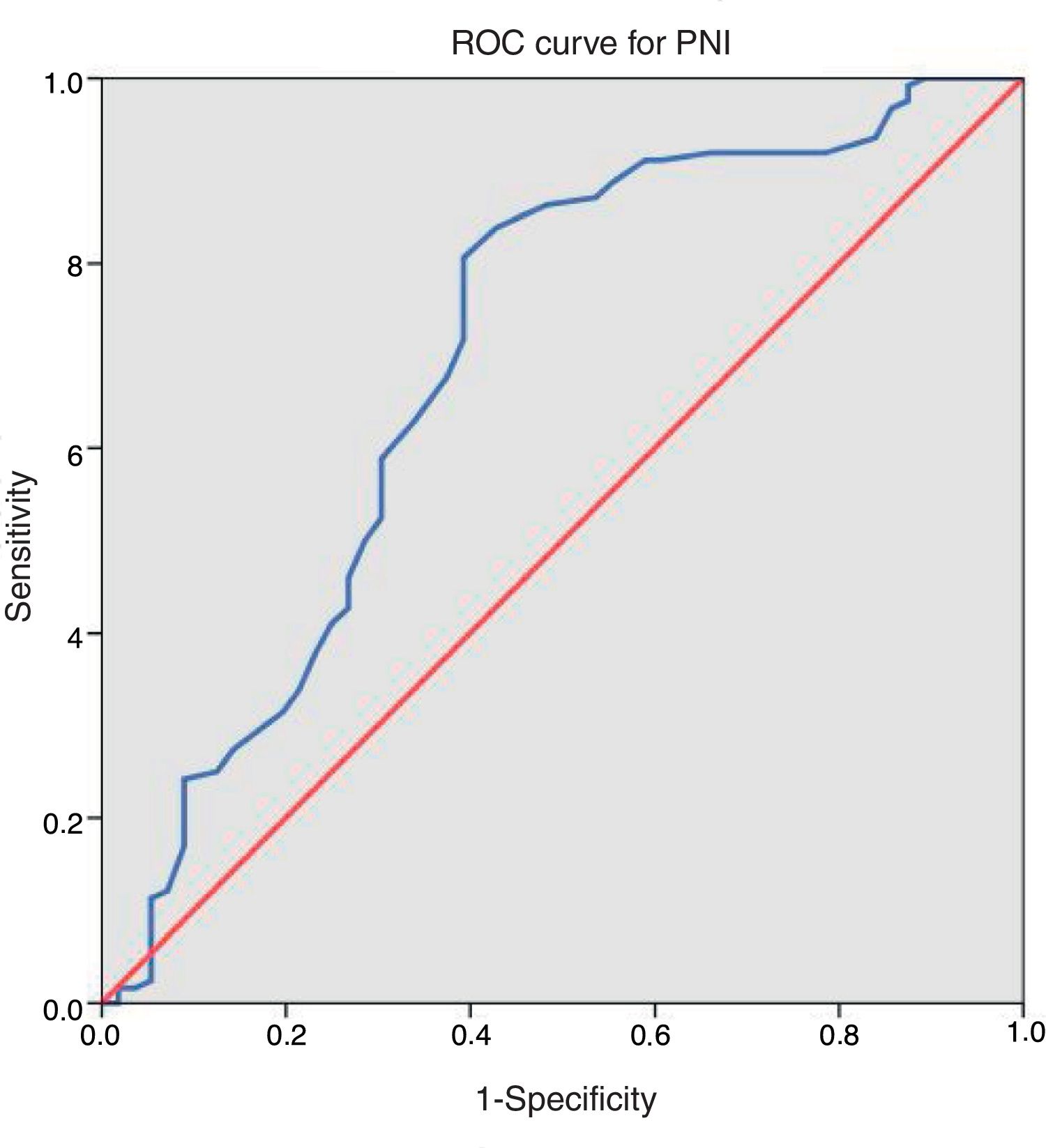
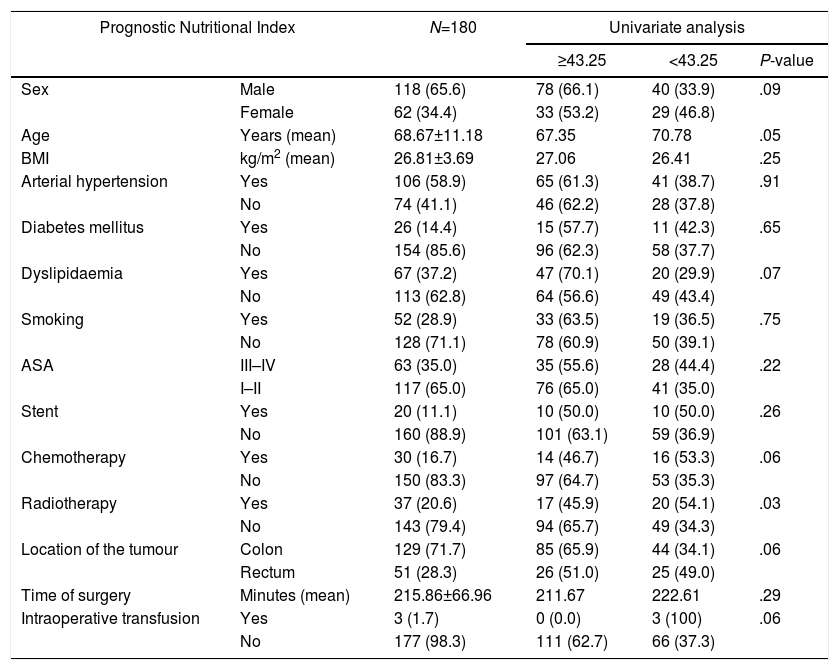
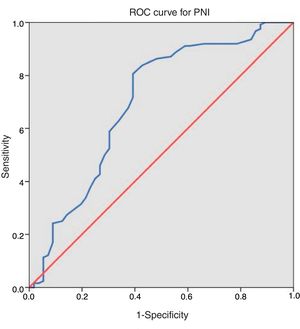
ResultsAfter applying inclusion and exclusion criteria, 180 patients were finally analyzed, from which 118 (65.6%) were men and 62 (34.4%) were women. Distribution according to ASA classification was as follows: 8 (4.4%) patients were grade I, 109 (60.6%) grade II, 61 (33.9%) grade III and 2 (1.1%) grade IV. Taking our dichotomous grouping into account, 63 (35.0%) patients had high anaesthetic risk (ASA III–IV) and 117 (65.0%) had low risk (ASA I–II). Regarding neoadjuvant treatment, 30 (16.7%) patients received chemotherapy and 37 (20.6%) received radiotherapy. Tumour was located in the colon in 129 (71.7%) cases and in the rectum in 51 (28.3%). Mean operative time was 215.86±66.96min (range 80–450). Mean value of PNI was 45.06±6.08 (range 22–64). After applying ROC curve [Fig. 1], we obtained a cut-off point of 43.25, so that 111 (61.7%) patients presented values equal or superior to this value and the remaining 69 (38.3%) below it. Distribution of risk factors in our sample of patients and between both PNI groups is described in Table 1.
Distribution of Risk Factors in Our Sample of Patients and Between Both PNI Groups.
| Prognostic Nutritional Index | N=180 | Univariate analysis | |||
|---|---|---|---|---|---|
| ≥43.25 | <43.25 | P-value | |||
| Sex | Male | 118 (65.6) | 78 (66.1) | 40 (33.9) | .09 |
| Female | 62 (34.4) | 33 (53.2) | 29 (46.8) | ||
| Age | Years (mean) | 68.67±11.18 | 67.35 | 70.78 | .05 |
| BMI | kg/m2 (mean) | 26.81±3.69 | 27.06 | 26.41 | .25 |
| Arterial hypertension | Yes | 106 (58.9) | 65 (61.3) | 41 (38.7) | .91 |
| No | 74 (41.1) | 46 (62.2) | 28 (37.8) | ||
| Diabetes mellitus | Yes | 26 (14.4) | 15 (57.7) | 11 (42.3) | .65 |
| No | 154 (85.6) | 96 (62.3) | 58 (37.7) | ||
| Dyslipidaemia | Yes | 67 (37.2) | 47 (70.1) | 20 (29.9) | .07 |
| No | 113 (62.8) | 64 (56.6) | 49 (43.4) | ||
| Smoking | Yes | 52 (28.9) | 33 (63.5) | 19 (36.5) | .75 |
| No | 128 (71.1) | 78 (60.9) | 50 (39.1) | ||
| ASA | III–IV | 63 (35.0) | 35 (55.6) | 28 (44.4) | .22 |
| I–II | 117 (65.0) | 76 (65.0) | 41 (35.0) | ||
| Stent | Yes | 20 (11.1) | 10 (50.0) | 10 (50.0) | .26 |
| No | 160 (88.9) | 101 (63.1) | 59 (36.9) | ||
| Chemotherapy | Yes | 30 (16.7) | 14 (46.7) | 16 (53.3) | .06 |
| No | 150 (83.3) | 97 (64.7) | 53 (35.3) | ||
| Radiotherapy | Yes | 37 (20.6) | 17 (45.9) | 20 (54.1) | .03 |
| No | 143 (79.4) | 94 (65.7) | 49 (34.3) | ||
| Location of the tumour | Colon | 129 (71.7) | 85 (65.9) | 44 (34.1) | .06 |
| Rectum | 51 (28.3) | 26 (51.0) | 25 (49.0) | ||
| Time of surgery | Minutes (mean) | 215.86±66.96 | 211.67 | 222.61 | .29 |
| Intraoperative transfusion | Yes | 3 (1.7) | 0 (0.0) | 3 (100) | .06 |
| No | 177 (98.3) | 111 (62.7) | 66 (37.3) | ||
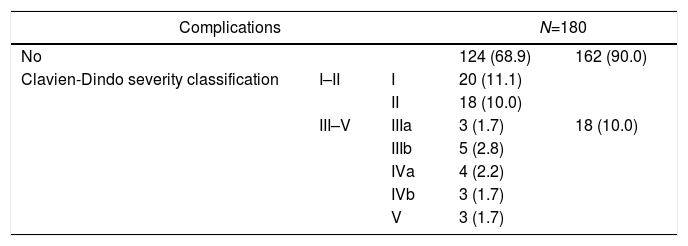
During the first 30 postoperative days, 56 (31.1%) patients exhibited some kind of complication. Distribution following the severity levels of Clavien-Dindo classification is described in Table 2. Severe complications (Clavien-Dindo classification III–V) were detected in 18 (10.0%) patients and infectious ones appeared in 34 (18.9%) of them. Analyzing anastomotic leakage specifically, this finding was observed in 11 (6.5%) patients amongst the 159 with anastomosis and without a protective stoma.
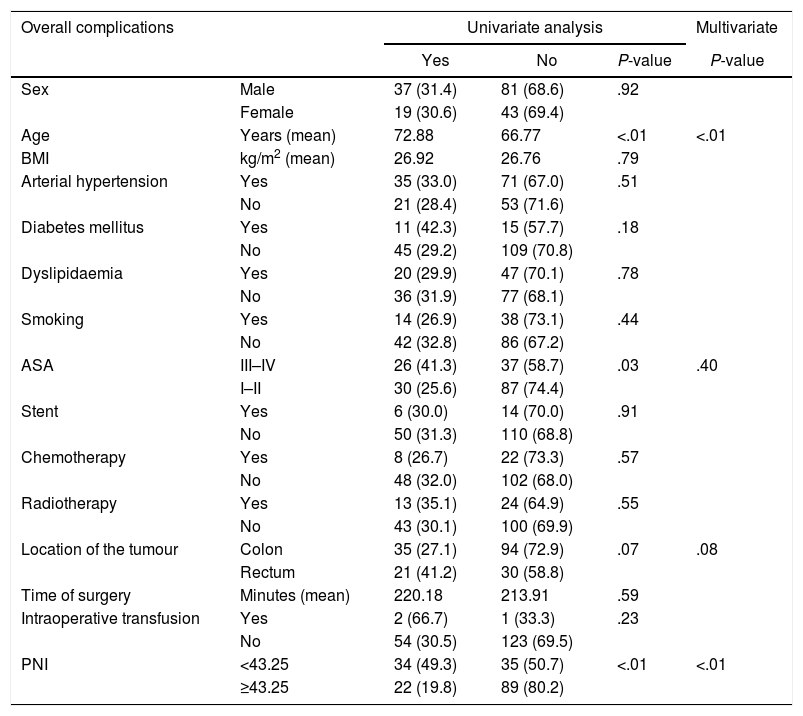
For overall complications, four variables had statistical significance: age, ASA III-IV, location of the tumour and PNI. After MVA, age and PNI obtained a P<.05. PNI was presented as a significative protective factor, with a RR of 0.279 (95% CI of 0.141–0.552) [Table 3].
Distribution of Overall Complications According to Risk Factors and Nutritional and Inflammatory Status.
| Overall complications | Univariate analysis | Multivariate | |||
|---|---|---|---|---|---|
| Yes | No | P-value | P-value | ||
| Sex | Male | 37 (31.4) | 81 (68.6) | .92 | |
| Female | 19 (30.6) | 43 (69.4) | |||
| Age | Years (mean) | 72.88 | 66.77 | <.01 | <.01 |
| BMI | kg/m2 (mean) | 26.92 | 26.76 | .79 | |
| Arterial hypertension | Yes | 35 (33.0) | 71 (67.0) | .51 | |
| No | 21 (28.4) | 53 (71.6) | |||
| Diabetes mellitus | Yes | 11 (42.3) | 15 (57.7) | .18 | |
| No | 45 (29.2) | 109 (70.8) | |||
| Dyslipidaemia | Yes | 20 (29.9) | 47 (70.1) | .78 | |
| No | 36 (31.9) | 77 (68.1) | |||
| Smoking | Yes | 14 (26.9) | 38 (73.1) | .44 | |
| No | 42 (32.8) | 86 (67.2) | |||
| ASA | III–IV | 26 (41.3) | 37 (58.7) | .03 | .40 |
| I–II | 30 (25.6) | 87 (74.4) | |||
| Stent | Yes | 6 (30.0) | 14 (70.0) | .91 | |
| No | 50 (31.3) | 110 (68.8) | |||
| Chemotherapy | Yes | 8 (26.7) | 22 (73.3) | .57 | |
| No | 48 (32.0) | 102 (68.0) | |||
| Radiotherapy | Yes | 13 (35.1) | 24 (64.9) | .55 | |
| No | 43 (30.1) | 100 (69.9) | |||
| Location of the tumour | Colon | 35 (27.1) | 94 (72.9) | .07 | .08 |
| Rectum | 21 (41.2) | 30 (58.8) | |||
| Time of surgery | Minutes (mean) | 220.18 | 213.91 | .59 | |
| Intraoperative transfusion | Yes | 2 (66.7) | 1 (33.3) | .23 | |
| No | 54 (30.5) | 123 (69.5) | |||
| PNI | <43.25 | 34 (49.3) | 35 (50.7) | <.01 | <.01 |
| ≥43.25 | 22 (19.8) | 89 (80.2) | |||
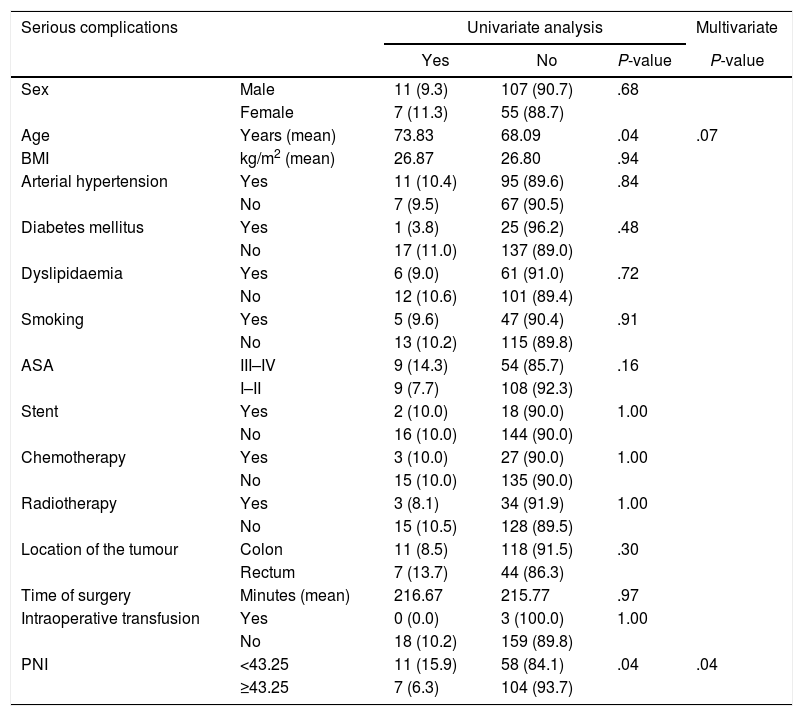
Among severe complications, two variables were relevant: age and PNI. After performing MVA, only PNI achieved statistical significance. In this case, PNI was also an important protective factor, with a RR of 0.355 (95% CI of 0.130–0.965) [Table 4].
Distribution of Severe Complications (Clavien-Dindo≥III) According to Risk Factors and Nutritional and Inflammatory Status.
| Serious complications | Univariate analysis | Multivariate | |||
|---|---|---|---|---|---|
| Yes | No | P-value | P-value | ||
| Sex | Male | 11 (9.3) | 107 (90.7) | .68 | |
| Female | 7 (11.3) | 55 (88.7) | |||
| Age | Years (mean) | 73.83 | 68.09 | .04 | .07 |
| BMI | kg/m2 (mean) | 26.87 | 26.80 | .94 | |
| Arterial hypertension | Yes | 11 (10.4) | 95 (89.6) | .84 | |
| No | 7 (9.5) | 67 (90.5) | |||
| Diabetes mellitus | Yes | 1 (3.8) | 25 (96.2) | .48 | |
| No | 17 (11.0) | 137 (89.0) | |||
| Dyslipidaemia | Yes | 6 (9.0) | 61 (91.0) | .72 | |
| No | 12 (10.6) | 101 (89.4) | |||
| Smoking | Yes | 5 (9.6) | 47 (90.4) | .91 | |
| No | 13 (10.2) | 115 (89.8) | |||
| ASA | III–IV | 9 (14.3) | 54 (85.7) | .16 | |
| I–II | 9 (7.7) | 108 (92.3) | |||
| Stent | Yes | 2 (10.0) | 18 (90.0) | 1.00 | |
| No | 16 (10.0) | 144 (90.0) | |||
| Chemotherapy | Yes | 3 (10.0) | 27 (90.0) | 1.00 | |
| No | 15 (10.0) | 135 (90.0) | |||
| Radiotherapy | Yes | 3 (8.1) | 34 (91.9) | 1.00 | |
| No | 15 (10.5) | 128 (89.5) | |||
| Location of the tumour | Colon | 11 (8.5) | 118 (91.5) | .30 | |
| Rectum | 7 (13.7) | 44 (86.3) | |||
| Time of surgery | Minutes (mean) | 216.67 | 215.77 | .97 | |
| Intraoperative transfusion | Yes | 0 (0.0) | 3 (100.0) | 1.00 | |
| No | 18 (10.2) | 159 (89.8) | |||
| PNI | <43.25 | 11 (15.9) | 58 (84.1) | .04 | .04 |
| ≥43.25 | 7 (6.3) | 104 (93.7) | |||
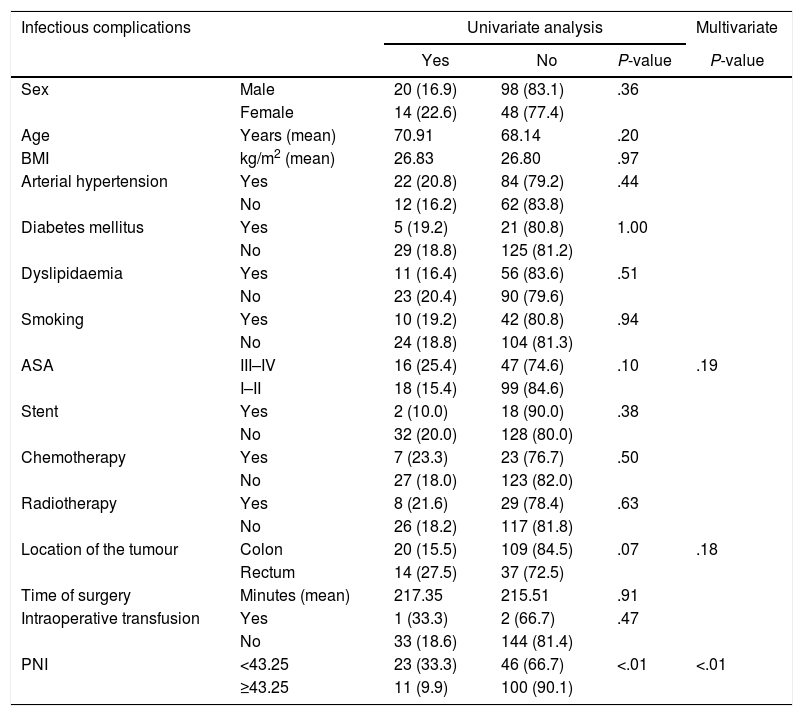
Within infectious complications, three variables reached significant values: ASA III–IV, location of the tumour and PNI. Once MVA was carried out, only PNI accomplished statistical significance, behaving PNI once again as a relevant protective factor, with a RR of 0.220 (95% CI of 0.099–0.489) [Table 5].
Distribution of Infectious Complications According to Risk Factors and Nutritional and Inflammatory Status.
| Infectious complications | Univariate analysis | Multivariate | |||
|---|---|---|---|---|---|
| Yes | No | P-value | P-value | ||
| Sex | Male | 20 (16.9) | 98 (83.1) | .36 | |
| Female | 14 (22.6) | 48 (77.4) | |||
| Age | Years (mean) | 70.91 | 68.14 | .20 | |
| BMI | kg/m2 (mean) | 26.83 | 26.80 | .97 | |
| Arterial hypertension | Yes | 22 (20.8) | 84 (79.2) | .44 | |
| No | 12 (16.2) | 62 (83.8) | |||
| Diabetes mellitus | Yes | 5 (19.2) | 21 (80.8) | 1.00 | |
| No | 29 (18.8) | 125 (81.2) | |||
| Dyslipidaemia | Yes | 11 (16.4) | 56 (83.6) | .51 | |
| No | 23 (20.4) | 90 (79.6) | |||
| Smoking | Yes | 10 (19.2) | 42 (80.8) | .94 | |
| No | 24 (18.8) | 104 (81.3) | |||
| ASA | III–IV | 16 (25.4) | 47 (74.6) | .10 | .19 |
| I–II | 18 (15.4) | 99 (84.6) | |||
| Stent | Yes | 2 (10.0) | 18 (90.0) | .38 | |
| No | 32 (20.0) | 128 (80.0) | |||
| Chemotherapy | Yes | 7 (23.3) | 23 (76.7) | .50 | |
| No | 27 (18.0) | 123 (82.0) | |||
| Radiotherapy | Yes | 8 (21.6) | 29 (78.4) | .63 | |
| No | 26 (18.2) | 117 (81.8) | |||
| Location of the tumour | Colon | 20 (15.5) | 109 (84.5) | .07 | .18 |
| Rectum | 14 (27.5) | 37 (72.5) | |||
| Time of surgery | Minutes (mean) | 217.35 | 215.51 | .91 | |
| Intraoperative transfusion | Yes | 1 (33.3) | 2 (66.7) | .47 | |
| No | 33 (18.6) | 144 (81.4) | |||
| PNI | <43.25 | 23 (33.3) | 46 (66.7) | <.01 | <.01 |
| ≥43.25 | 11 (9.9) | 100 (90.1) | |||
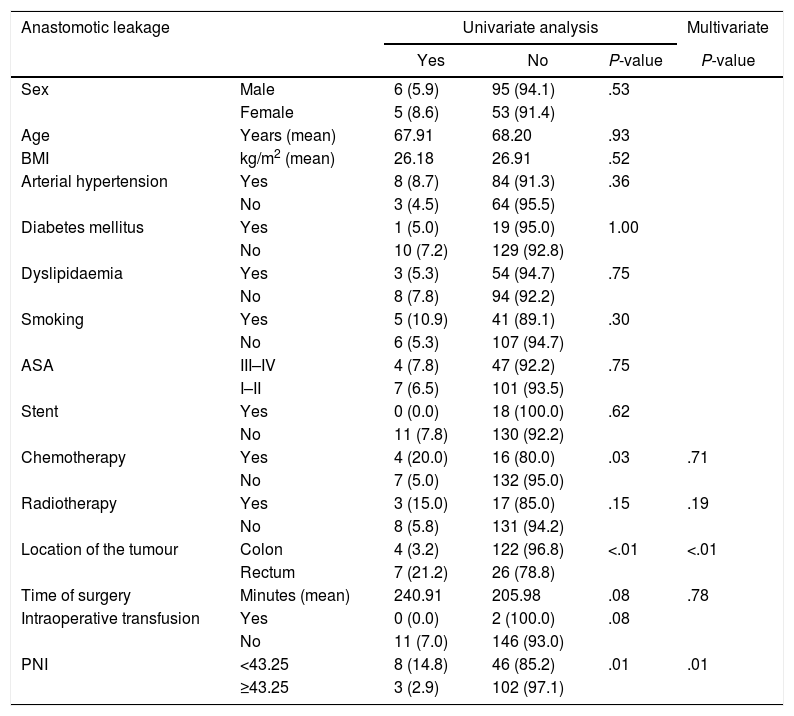
Considering anastomotic leakage individually, preoperative chemotherapy, location of the tumour and PNI proved to be remarkable variables. MVA showed that location of the tumour and PNI obtained a P<.05. Also, in this specific complication, PNI consolidates as an outstanding protective factor, with a RR of 0.151 (95% CI of 0.036–0.640) [Table 6].
Distribution of Anastomotic Leakage According to Risk Factors and Nutritional and Inflammatory Status.
| Anastomotic leakage | Univariate analysis | Multivariate | |||
|---|---|---|---|---|---|
| Yes | No | P-value | P-value | ||
| Sex | Male | 6 (5.9) | 95 (94.1) | .53 | |
| Female | 5 (8.6) | 53 (91.4) | |||
| Age | Years (mean) | 67.91 | 68.20 | .93 | |
| BMI | kg/m2 (mean) | 26.18 | 26.91 | .52 | |
| Arterial hypertension | Yes | 8 (8.7) | 84 (91.3) | .36 | |
| No | 3 (4.5) | 64 (95.5) | |||
| Diabetes mellitus | Yes | 1 (5.0) | 19 (95.0) | 1.00 | |
| No | 10 (7.2) | 129 (92.8) | |||
| Dyslipidaemia | Yes | 3 (5.3) | 54 (94.7) | .75 | |
| No | 8 (7.8) | 94 (92.2) | |||
| Smoking | Yes | 5 (10.9) | 41 (89.1) | .30 | |
| No | 6 (5.3) | 107 (94.7) | |||
| ASA | III–IV | 4 (7.8) | 47 (92.2) | .75 | |
| I–II | 7 (6.5) | 101 (93.5) | |||
| Stent | Yes | 0 (0.0) | 18 (100.0) | .62 | |
| No | 11 (7.8) | 130 (92.2) | |||
| Chemotherapy | Yes | 4 (20.0) | 16 (80.0) | .03 | .71 |
| No | 7 (5.0) | 132 (95.0) | |||
| Radiotherapy | Yes | 3 (15.0) | 17 (85.0) | .15 | .19 |
| No | 8 (5.8) | 131 (94.2) | |||
| Location of the tumour | Colon | 4 (3.2) | 122 (96.8) | <.01 | <.01 |
| Rectum | 7 (21.2) | 26 (78.8) | |||
| Time of surgery | Minutes (mean) | 240.91 | 205.98 | .08 | .78 |
| Intraoperative transfusion | Yes | 0 (0.0) | 2 (100.0) | .08 | |
| No | 11 (7.0) | 146 (93.0) | |||
| PNI | <43.25 | 8 (14.8) | 46 (85.2) | .01 | .01 |
| ≥43.25 | 3 (2.9) | 102 (97.1) | |||
CRC is a considerable medical and social problem due to its high incidence in our population. Its predominance amongst elderly patients implies higher rates of comorbidities, worse nutritional status, greater difficulty for tissue healing, worse vascularization and greater anaesthetic risk, which favours the appearance of complications.25 All this, combined with the fact that it is a contaminated anatomical area, makes that repercussions of possible postoperative complications may lead to very relevant consequences on the well-being and recovery of our patients; putting even their life at risk.
Recently, relationship between cancer and nutritional and inflammatory status in oncologic patients has been thoroughly studied. As a result of these studies, multiple immunonutritional scores have been developed (PNI, Glasgow Prognostic Score, Modified Glasgow Prognostic Score, Granulocyte/Lymphocyte Ratio, Neutrophile/Lymphocyte Ratio or Platelet/Lymphocyte ratio). These have been mainly related to the prognosis of various neoplasms, but their relationship with the appearance of complications have not been studied that much.26–29 Most of these scores are objective because they use analytical values, which also make them affordable and easy to perform. In this way, we may avoid the use of scales that are based fundamentally on subjective variables.
There are other really interesting scores related to postoperative complications, such as CR-POSSUM, which is constituted by intraoperative and physiological variables.30 However, we wanted to focus on a score formed by preoperative objective variables related to nutritional and inflammatory status. Therefore, we decided to use PNI since it constitutes an easy and helpful score to define this status. In addition, its value may be quickly calculated from a peripheral blood analytics (serum albumin and lymphocyte count). Some recent papers have also shown a relationship between nutritional status (determined mainly by serum total protein) and anastomotic leakage after colorectal surgery.31,32 We consider that albumin is a more reliable variable than serum total protein, since it is not only a nutritional but also an inflammatory status score. Values of this protein decrease due to a direct absorption by the tumour and due to the extravasation induced by tumour necrosis factor alpha.
An interaction between the tumour and the host is generated, triggering a systemic inflammatory reaction in which several cytokines such as interleukins, tumour necrosis factor α or macrophage inhibitory cytokine 1 are released.21 There is a neuroendocrine imbalance due to an increase in catabolic hormones (cortisol, myostatin) and a decrease in anabolic hormones (insulin, growth hormone, and testosterone). Secondly, acute phase reactants, such as C-reactive protein and fibrinogen, are released, favouring a tendency towards anorexia and catabolism.33 Leptin and proopiomelanocortin levels also increase, and neuropeptide Y levels decrease, which encourages the anorectic stimulus.34 There is an increase in proteolysis inducing factor and lipid mobilizing factor levels, which results in a proteolysis and lipolysis increment.35,36 As a result, several scores have been developed to analyze the nutritional and inflammatory status of cancer patients, most of them calculated from analytical variables. One of the most important immunonutritional scores is PNI, created by Onodera et al. in 1984.23 It has been mainly related to oncological prognosis and not that much to postoperative complications. Several cut-off points have been used in literature, which hinders the extrapolation of results and conclusions. Therefore, we decided to use ROC curve to establish the optimal cut-off value for our sample of patients, considering global complications as reference variable. Possibly, new studies with a larger population will be needed to establish a cut-off point which would be more globally interpretable. It has been statistically related to advanced disease in terms of local invasion, presence of pathological lymphadenopathies or distant metastases.37,38 In addition, it has demonstrated to have an independent and statistically significant influence on the prognosis of various cancers, resulting in worse OS and DFS with lower values.39–41
In 2013, Mohri et al. published a retrospective study with 265 patients in which they studied the relationship between PNI, cancer prognosis and complications rate after surgery for CRC.42 They found this score was statistically linked to worse survival and higher rate of overall and severe complications (defined, as in our work, such as those with Clavien-Dindo III–V degrees).
A similar paper was published by Tokunaga et al. in 2015.43 This was a retrospective study with 556 patients in which PNI was associated to complications and OS after surgery for CRC. They found that PNI was associated to higher postoperative morbidity and worse prognosis, in both cases with statistical significance after MVA.
Recently, Cao et al. presented a retrospective study with 228 patients, where a low PNI was related, in a significant way, to higher rate of postoperative complications, and especially to severe ones.44
Our work uses a similar approach, as it tries to relate nutritional and inflammatory status of oncologic patients (as evidenced by PNI) to higher morbidity after curative surgery for CRC. In our prospective series, PNI has been associated with the appearance of overall, severe (those that are ≥III of the Clavien-Dindo classification) and infectious complications during the first 30 days postoperatively. In addition, it has been correlated to anastomotic leakage. These relationships have exhibited statistical significance even after MVA, showing PNI behaves as an important protective factor. Nevertheless, these findings will have to be validated in prospective studies with a larger population, in order to improve the interpretability and external validation.
Findings obtained in this study show that preoperative nutritional and inflammatory status is linked to postoperative morbidity after CRC curative surgery. We are interested in being able to predict which patients have an increased risk of suffering complications, trying to reduce them. Even more if we consider that appearance of complications (particularly infectious ones), during the postoperative period of CRC surgery, is related to worse oncological prognosis, in terms of higher rate of local and remote recurrence, and lower OS and DFS.15–20
PNI represents an additional useful tool when estimating the state in which our patients reach the surgery, what can help us evaluate each one individually according to their risk of developing complications. For high risk patients, it may be taken into account the possibility of delaying a procedure, whenever it is possible, with the intention of improving the nutritional status. This could be accomplished with the administration of supplements rich in proteins. In addition, we might regard a more conservative way of facing the postoperative period, and the possibility of a derivative stoma to protect a colorectal anastomosis. However, this possible clinical management should only be considered when these results are validated in a much larger population.
Our work has obvious limitations, such as the small number of patients and the fact that these come from a single hospital. Another limitation is that we did not analyze surgeon factor. We work as a team, agreeing indications and surgical techniques; therefore, we discarded this variable from our analysis. Further work will be needed to adequately assess the relationship between nutritional and inflammatory status and the development of complications in this type of surgery. Our results will have to be validated in new prospective and multicentric studies with a larger population.
Our work reflects that PNI is, in our sample of patients, an independent predictive and protective factor for the development of postoperative complications, especially for those severe and infectious (and specifically for anastomotic leakage), following curative surgery for CRC. Most papers analyzing nutritional and inflammatory scores are retrospective. They focus on the relationship between these scores and cancer prognosis, but not on the appearance of complications.45 This study is pioneer in this field as it has a prospective design and it focuses on postoperative morbidity after CRC curative surgery.
Funding InformationWe have received funding from a governmental public health agency (Gerencia Regional de Salud de la Junta de Castilla y León) through a research project with title ‘Influencia del estado nutricional e inflamatorio preoperatorio en la morbilidad y mortalidad postoperatoria de la cirugía curativa del cáncer de colon y recto’ and file number ‘GRS 1316/A/16’.
Author Contribution- 1.
Martín Bailón-Cuadrado, MD PhD: conceiving and designing the study, collecting the data, writing the manuscript and approving the final version.
- 2.
Baltasar Pérez-Saborido, MD PhD: conceiving and designing the study, writing the manuscript and approving the final version.
- 3.
Javier Sánchez-González, MD PhD: conceiving and designing the study, collecting the data, writing the manuscript and approving the final version.
- 4.
Mario Rodríguez-López, MD PhD: conceiving and designing the study, writing the manuscript and approving the final version.
- 5.
Rosalía Velasco-López, MD: collecting the data, writing the manuscript and approving the final version.
- 6.
José C. Sarmentero-Prieto, MD PhD: conceiving and designing the study, writing the manuscript and approving the final version.
- 7.
José I. Blanco-Álvarez, MD PhD: conceiving and designing the study, writing the manuscript and approving the final version.
- 8.
David Pacheco-Sánchez, MD PhD: conceiving and designing the study, writing the manuscript and approving the final version.
- 1.
Martín Bailón-Cuadrado, MD PhD: no conflict of interest.
- 2.
Baltasar Pérez-Saborido, MD PhD: no conflict of interest.
- 3.
Javier Sánchez-González, MD PhD: no conflict of interest.
- 4.
Mario Rodríguez-López, MD PhD: no conflict of interest.
- 5.
Rosalía Velasco-López, MD: no conflict of interest.
- 6.
José C. Sarmentero-Prieto, MD PhD: no conflict of interest.
- 7.
José I. Blanco-Álvarez, MD PhD: no conflict of interest.
- 8.
David Pacheco-Sánchez, MD PhD: no conflict of interest.
We want to thank all the professionals who have contributed to the management of all these patients, and especially our nurses, who take care of them from the first to the last day.
Please cite this article as: Bailón-Cuadrado M, Pérez-Saborido B, Sánchez-González J, Rodríguez-López M, Velasco-López R, Sarmentero-Prieto JC, et al. El Prognostic Nutritional Index predice la morbilidad postoperatoria tras la cirugía curativa del cáncer colorrectal. Cir Esp. 2019;97:71–80.