One of the most severe complications after esophagectomy is anastomotic dehiscence. The use of collagen sponges could be an effective way to resolve this complication. Our objective was to perform an experimental model of esophageal anastomosis in rats to study these mechanisms.
MethodsA total of 50 Sprague-Dawley rats were used divided into 2 groups, Tachosil® group (n=25) and control group (n=25). After the section of the abdominal esophagus a single-layer esophago-gastric anastomosis was performed reinforced with 1cm of Tachosil® wrapping the anastomosis in group 1.
A functional study was performed using manometry as well as histopathological and immunohistochemical studies for angiogenic, fibrogenic and growth factors.
ResultsThe mortality in our series was 8% in the collagen dressing group, compared to 36% in the control group. When esophageal manometry was performed, the dehiscence pressure was higher in the reinforced anastomosis, On microscopical analysis, in the collagen dressing group a profuse inflammatory reaction with abundant neutrophils and macrophages surrounded by a connective matrix with fibroblasts and blood vessels was observed, the expression of VEGF, FGF1 and FGF2 was noticeably higher in the collagen dressing group.
ConclusionsThese results show that the application of collagen dressing facilitates tissue reparation phenomena, and therefore could be very useful as a reinforcement of esophago-gastric anastomosis to prevent dehiscence.
Una de las complicaciones más graves tras la cirugía de resección esofagogástrica es la dehiscencia de la anastomosis. El uso de apósitos adhesivos podría constituir una ayuda eficaz para resolver esta complicación. Nuestro objetivo ha sido realizar un estudio experimental encaminado a estudiar dichos mecanismos en un modelo de anastomosis esofágica en rata.
MétodosSe han utilizado un total de 50 ratas Sprague-Dawley divididas en 2 grupos, grupo Tachosil® (n=25) y grupo control (n=25). Tras la sección del esófago abdominal se realizó una anastomosis esófago-gástrica monoplano, reforzando con una tira de 1cm de Tachosil® envolviendo la anastomosis en el primer grupo.
Se realizó un estudio funcional mediante manometría, así como un estudio histopatológico e inmunohistoquímico para factores angiogénicos, fibrogénicos y proliferativos.
ResultadosLa mortalidad en nuestra serie alcanzó un 8% en el grupo en el que fue aplicado apósito de colágeno, frente a un 36% del grupo control. Al realizar la manometría esofágica, la presión de dehiscencia fue mayor en las anastomosis reforzadas. En el estudio microscópico, en el grupo en el que se aplicó apósito de colágeno se apreció una profusa reacción inflamatoria con abundantes PMN y macrófagos rodeados por una matriz conectiva con fibroblastos y vasos sanguíneos. La expresión de VEGF y FGF1 y FGF2 fue sensiblemente mayor en las anastomosis con apósito de colágeno.
ConclusionesEstos resultados indican que la aplicación de apósito de colágeno facilita los fenómenos de reparación tisular, por lo que podría ser de gran utilidad como refuerzo de las anastomosis esofagogástricas para la prevención de dehiscencias.
One of the most serious complications after esophagogastric resection surgery is anastomotic dehiscence, with a prevalence that ranges between 3% and 12% in the main published series.1 The use of adhesive patches could be an effective and reliable aid to resolve this complication.2 Collagen dressings (Nycomed, Takeda, Zurich, Switzerland) are collagen sponges containing human fibrin and thrombin that, when in contact with physiological fluids, transform into a firm and mechanically stable network of fibrin with positive properties.3 There have been some experimental studies about the use of collagen dressings in gastrointestinal surgery4 with good results in reducing the rate of dehiscence.5 Although the usefulness of collagen dressings in experimental esophageal surgery has been confirmed,6 the physiological mechanisms of tissue repair that occur after the application of the sponge in this area have not been studied in detail.
Our objective was to perform a clinical, functional and histopathological study aimed at studying these mechanisms in an experimental model of esophageal anastomosis in rats.
MethodsTo achieve the proposed goal, a total of 50 Sprague-Dawley (SD) male rats were used, weighing 210–240g at 4 weeks of age. The animals were divided into 2 groups, the Tachosil® group (n=25) and control group (n=25), according to the application or not of Tachosil® once the suture was completed.
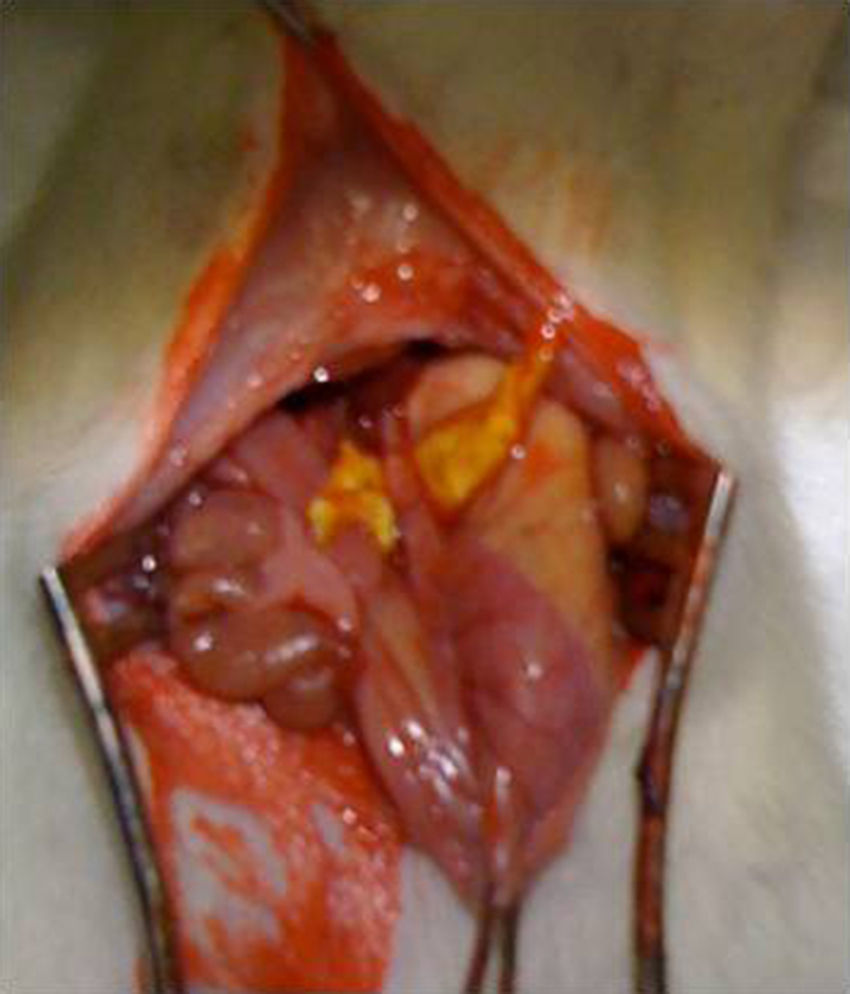
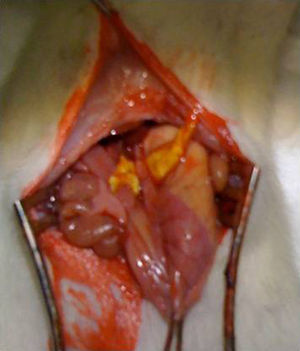
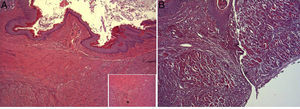
Surgical intervention: after anesthesia of the animal with a combination of intraperitoneal ketamine–xylazine (50mg/kg and 10mg/kg, respectively), a 3-cm midline laparotomy was performed and the abdominal esophagus was dissected at the cardias. The single-plane esophagogastric anastomosis was performed manually with 3 PDS 6/0 sutures. In the Tachosil® group, the anastomosis was reinforced with a 1-cm strip of Tachosil® (Fig. 1), whereas in the control group no reinforcement was used. The animals were kept under daily observation with a solution of water and buprenorphine (0.1mg/kg) and food ad libitum during the entire duration of the experiment. This study was carried out with the approval of the Animal Experimentation Ethics Committee of the Murcian Institute of Biosanitary Research.
Sacrifice and sampling: in order to analyze the healing process in the short and mid-term of animals that survived more than 7 days, half were euthanized on day 8 and the other half at 30 days post-op by inhaled anesthetic induction with isoflurane at saturation.
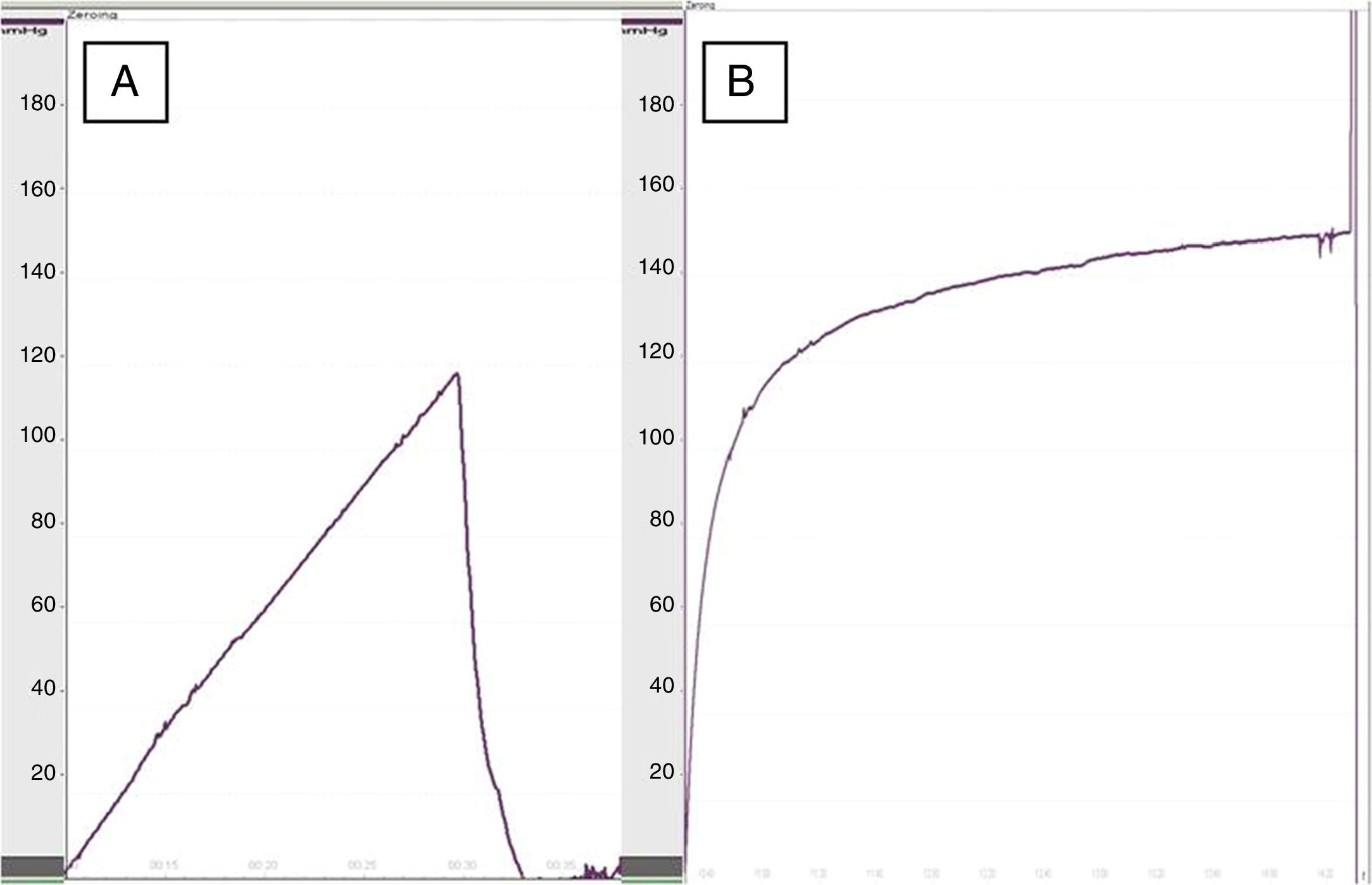
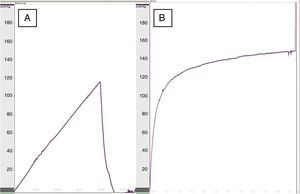
Functional study: for the manometric study, the anatomical segment between the esophagus and duodenum of the rat was used. The esophagus was cut transversally to 1.5cm above the level of the diaphragm and was resected en bloc along with the stomach and the proximal duodenum, washing the gastric contents with saline solution. Afterwards, the duodenum was closed with 3/0 silk thread and the stomach was filled with 1% methylene blue. Manometry was performed using a polyvinyl probe inserted into the lumen of the esophagus and a 4-channel polygraph (Synectics Medical, Stockholm, Sweden).7 The dehiscence pressure was defined as the maximum intraluminal pressure above which the methylene blue leaked through the anastomotic suture.
The data obtained were analyzed using SPSS 20 statistical software (Chicago, Illinois).
Immunohistopathological study: the histopathological study was performed using 3-μm thick sections stained with hematoxylin–eosin and Masson's trichrome stains. The immunohistochemical study was performed using the ABC technique, using antibodies against angiogenic (endothelial cell growth factor [ECGF], Abcam, Cambridge, United Kingdom), fibrogenic (fibroblast growth factor 1 and 2 (FGF-1 and FGF-2, Santa Cruz Biotechnology, Heidelberg, Germany) and proliferative factors (quantification of Ki-67 protein expression, Master Diagnostica, Granada, Spain). After deparaffinization and rehydration, antigenic unmasking was performed with citrate buffer, pH 6.0 (Dako Diagnostics, Sant Just Desvern, Spain) and inhibition of endogenous peroxidase. This was followed by overnight incubation with the primary antibody at 4°C, incubation with the biotinylated polymer complex (EnVision, Dako Diagnostics, Sant Just Desvern, Spain), and reaction with 3,3′ diaminobenzidine. Positive immunoreaction was identified by a brown cytoplasmic (ECGF, FGF-1 and FGF-2) and nuclear (Ki-67) halo.
ResultsThe mortality rate observed in our series reached 8% (n=2) in the group in which the collagen dressing sponge was applied, versus 36% (n=9) in the control group. The main cause of death in the control group was anastomotic dehiscence (n=7), followed by hemoperitoneum (n=2). In the group with collagen dressing, 2 cases of hemoperitoneum were observed in the first 24h post-op. Excluding these cases of early bleeding, we studied 23 rats in the control group and 23 from the collagen sponge group.
When we performed esophageal manometry, the pressure of dehiscence in the group treated with collagen dressings was 119.5±22mmHg versus 93.5±30mmHg in the control group (P<.05) (Fig. 2), which demonstrated greater resistance in the re-enforced anastomoses. No significant differences were observed in dehiscence pressure between the rats of the same groups at 8 at 30 days.
In the macroscopic study of the specimens, epithelial regeneration of the mucosa had taken place in 87% (n=20) of the animals treated with collagen dressings on the anastomoses, versus 44% (n=10) of those without sponges. Perianastomotic abscesses were observed in 26% (n=6) of the control group and in 13% (n=3) of the rats with collagen dressings; diffuse peritonitis was not observed in any of the cases. Complete dehiscence was observed in 30% (n=7) of the group without collagen sponges, whereas no cases were observed in the collagen dressing group.
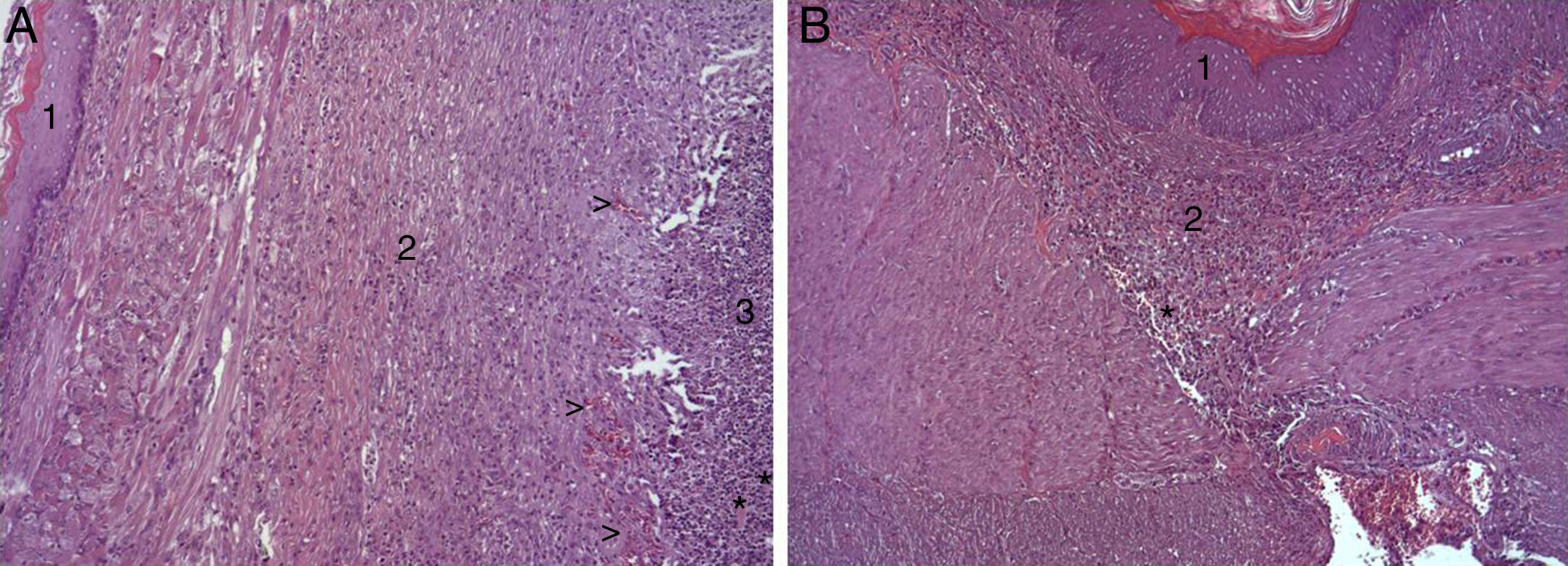
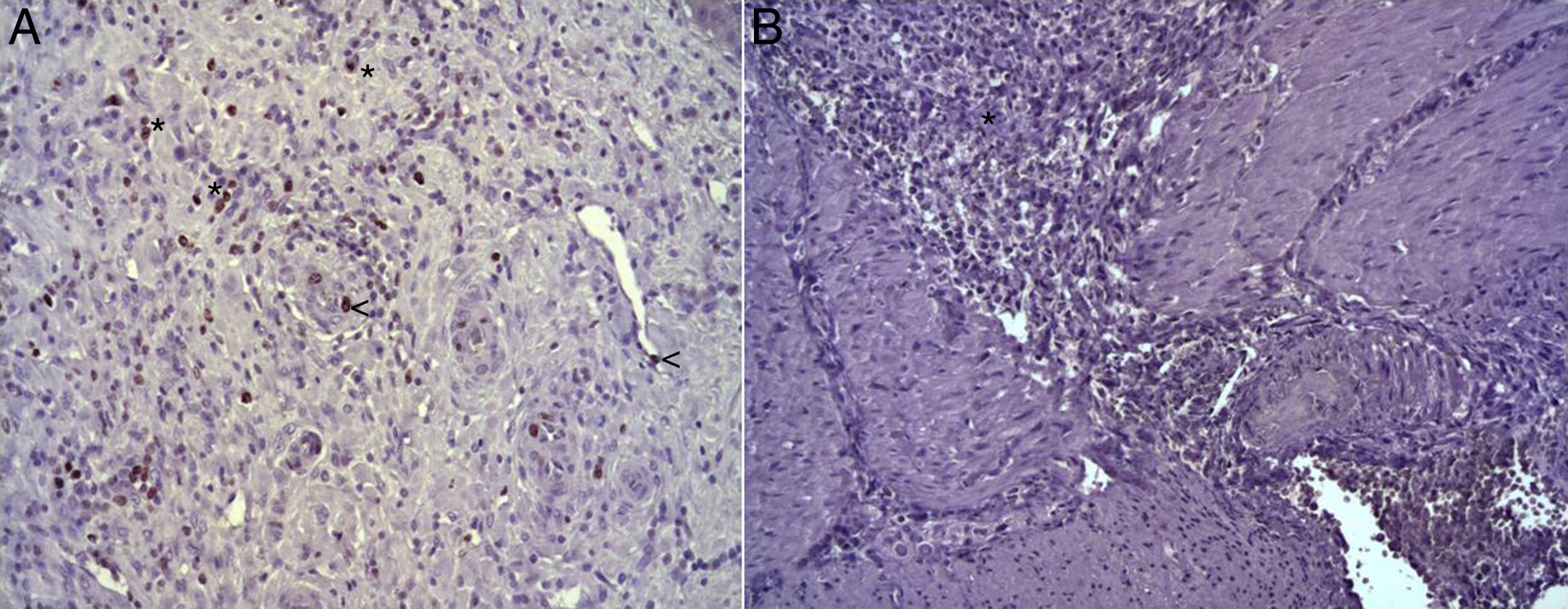
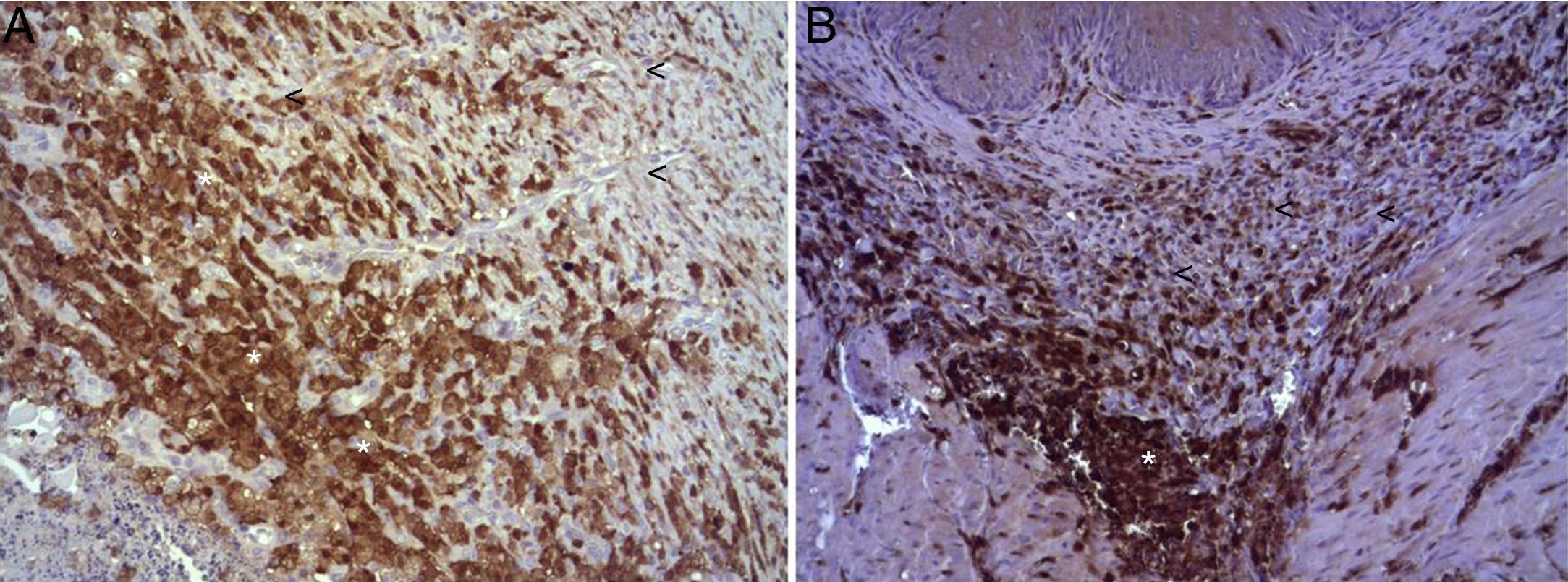
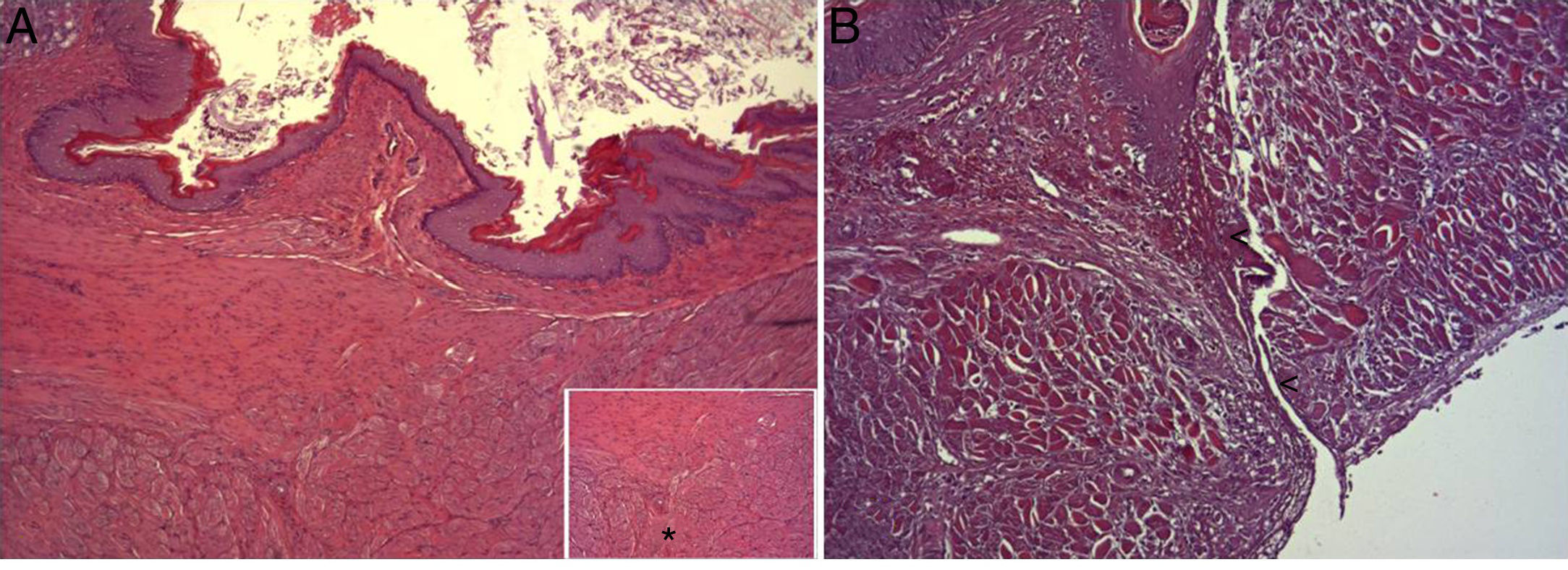
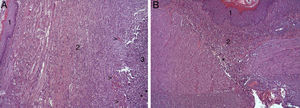
In the microscopic study, in the group where collagen dressings were applied as sealants, we observed a profuse foreign-body inflammatory reaction in the dissection area that affected the esophageal wall, with a center of fibrin material remains and abundant polymorphonuclear neutrophils and macrophages surrounded by a connective tissue matrix with numerous fibroblasts and blood vessels (Fig. 3A). The Ki-67 expression analysis revealed the proliferation of fibroblasts and, occasionally, endothelial cells (Fig. 4A). Abundant ECGF-positive macrophages were identified (Fig. 5A) as well as strong FGF1 and FGF2 expression associated with fibroblasts and inflammatory infiltrate cells. The inflammatory reaction in the control group was exclusively limited to the area of the intervention, with the presence of a diffuse infiltrate rich in polymorphonuclear neutrophils and macrophages, with little or no connective proliferation and no evident signs of neovascularization (Fig. 3B). The immunohistochemistry analysis revealed little or no cellular proliferation (Fig. 4B), while the expression of ECGF, FGF1 and FGF2 was noticeably less pronounced compared with the collagen dressing group (Fig. 5B). The immunohistopathologic analysis on day 30 in the animals with collagen dressings revealed complete epithelial, connective tissue and muscle regeneration of the esophageal wall with no signs of fibrosis (Fig. 6A), whereas signs of healing were observed in the control group associated with the dissection (Fig. 6B). In neither of the 2 groups was Ki-67, ECGF, FGF1 or FGF2 expression observed.
(A) Representative images of the most relevant histopathologic changes observed after the application of the collagen patch 8 days after surgery. After complete epithelial regeneration (1), a profuse inflammatory reaction was observed in the dissection area, characterized by an abundant connective (2) and vascular proliferation arrowheads (arrowheads), with a central area rich in PMN and macrophages (3), together with remains of fibrin protein material and cellular debris (asterisks). Hematoxylin–eosin×100; (B) representative image of the most relevant histopathological changes observed in the control animals 8 days after the intervention. After complete epithelial regeneration (1), the presence of a diffuse inflammatory infiltrate rich in macrophages and PMN is observed in the dissection area (2), with an area of cellular debris (asterisk). Hematoxylin–eosin×100.
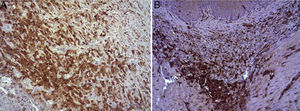
(A) Expression of Ki-67 protein in the esophagus of animals in which the collagen sponge had been used, 8 days after the intervention. Abundant positive fibroblasts are observed (asterisks) and, occasionally, endothelial cells (arrowheads). Immunohistochemistry ABC anti-Ki67×200; (B) expression of the Ki-67 protein in the esophagus of the control animals 8 days after the intervention. No positive cells were observed in the area of inflammatory infiltrate (asterisk). Inmunohistochemistry ABC anti-Ki67×200.
(A) Expression of ECGF in the esophagus of animals in which the collagen sponge was applied 8 days after the intervention. Abundant positive macrophages are observed (asterisks), together with numerous blood vessels (arrowheads). Immunohistochemistry ABC anti-ECGF×200; (B) expression of ECGF in the esophagus of the control animals 8 days after the intervention. A low number of positive cells are observed (arrowheads) in the inflammatory infiltrate, proximal to the area of cellular debris (asterisk). Inmunohistochemistry ABC anti-ECGF×200.
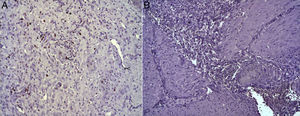
(A) Representative image of the most relevant histopathologic changes observed after the application of the collagen dressing 30 days after the intervention. Complete reconstitution of the entire esophageal wall is observed, including the lamina muscularis (asterisk). Hematoxylin–eosin×100; (B) representative image of the most relevant histopathologic changes observed in the group of control animals 30 days after the intervention. Observe the line of connective tissue that passes through all the layers of the esophageal wall (arrowheads). Hematoxylin–eosin×100.
The aim of the present study was to study the physiopathologic and histologic mechanisms that occur in esophagogastric anastomoses after the application of collagen sponges in an experimental rat model. In our study, the dehiscence pressure measured by manometry was significantly higher in those cases in which the fibrin patches were applied versus a control group, results which concur with previous studies.6
Eight days after surgery, we have observed a profuse foreign body inflammatory reaction associated with the use of collagen sponges, with signs of cell proliferation and expression of ECGF, FGF1 and FGF2, cytokines involved in the phenomena of tissue regeneration8 and signs of restitutio ad integrum of the esophageal wall on day 30. Previous studies6 have demonstrated the presence of a marked increase in fibroblastic proliferation on the 7th postoperative day and increase in the amount of connective tissue associated with the application of collagen dressing on the anastomoses.5,6 Our results support these studies, also showing the presence of signs of vascular proliferation associated with the inflammatory phenomenon, in comparison with the group in which the collagen dressing sponge was not applied. Likewise, we observed the higher expression of VEFG in macrophages of the inflammatory infiltrate of the group in which the collagen dressing was applied. Previous studies9,10 suggest an important role of ECGF in the phenomena of tissue repair regeneration, so this abundant expression of ECGF by this cellular subpopulation in animals in which a collagen dressing has been applied could collaborate in the increase of the connective matrix observed. Furthermore, we have also observed an abundant expression of FGF-1 and FGF-2 in these animals compared to the control group. FGF is a cytokine secreted by an abundant number of cellular subpopulations, directly involved in healing phenomena,11 so again the secretion of these cytokines would collaborate in the phenomena of tissue regeneration, which globally is very effective after observing the complete restitution of the tissue architecture of the organ 30 days after surgery. In contrast, the presence of connective scarring was observed in the animals of the control group, with possible loss of functionality. There are no previous studies that analyze the physiological mechanisms that occur after conducting esophagogastric anastomosis reinforced with a collagen dressing.
There could be doubt about whether the greater resistance of the anastomoses in the experimental group is due more to the inflammatory process that develops than to the repairing effect of the applied device, or both. In any case, these results indicate that the application of the collagen sponge facilitates the phenomena of tissue repair, so it could be very useful to reinforce esophagogastric anastomoses for the prevention of dehiscence.
AuthorshipRocío García Pérez: study design, data collection, analysis and interpretation of the results and article composition
Vicente Munitiz: study design, data collection, analysis and interpretation of the results, article composition, critical review and approval of the final version
Carlos Manuel Martínez Cáceres: data collection, analysis and interpretation of the results and article composition
David Ruiz de Angulo: data collection and article composition
Angeles Ortiz: study design, critical review and approval of the final version
Luisa F. Martínez de Haro: study design, critical review and approval of the final version
Diana Navas: data collection, analysis and interpretation of the results
Pascual Parrilla: study design, critical review and approval of the final version
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Garcia-Perez R, Munitiz V, Martinez-Caceres CM, Ruiz de Angulo D, Ortiz A, Martinez de Haro LF, et al. Análisis histopatológico e inmunohistoquímico del uso del apósito de colágeno como refuerzo de anastomosis esofágica en un modelo experimental en rata. Cir Esp. 2017;95:588–593.
Presented as a poster at the 29th National Conference of the AEC (Madrid 2012).