The spleen is one of the most frequently injured organs in blunt abdominal trauma. In the past decades, the treatment of patients with blunt splenic injury has shifted from operative to non-operative management. The knowledge of physiology and immunology of the spleen have been the main reasons to develop techniques for splenic salvage. The advances in high-resolution imaging techniques, as well as less invasive procedures, including angiography and angioembolization, have allowed a higher rate of success in the non-operative management. Non-operative management has showed a decrease in overall mortality and morbidity. The aim of this article is to analyze the current management of splenic injury based on a literature review of the last 30 years, from we have identified 63205 patients. This would enable the surgeons to provide the best care possible in every case.
El bazo es uno de los órganos más frecuentemente dañado en el traumatismo abdominal cerrado. El manejo de la lesión esplénica ha evolucionado en los últimos años, con cada vez mayor tendencia al manejo conservador. El conocimiento de su función inmunológica ha sido el motor inicial para impulsar el desarrollo de técnicas de preservación del bazo. El mayor acceso a pruebas de imagen de alta resolución, así como a técnicas terapéuticas poco agresivas, como la angioembolización, ha permitido una mayor tasa de éxito en el manejo no quirúrgico de estos casos, con una disminución en la morbimortalidad global asociada a estos pacientes. El objetivo de esta revisión es dar a conocer el manejo actual de traumatismo esplénico basado en la bibliografía internacional de los últimos 30 años —se han identificado 63.205 pacientes—y, así, ofrecer al cirujano mejores herramientas a la hora de decidir el tratamiento recomendable en cada caso.
The spleen is the most frequently affected organ in blunt abdominal trauma, occurring in up to 60% of cases according to reports in the literature.1 In recent decades, the management of this situation has changed. The first case of traumatic rupture of the spleen was published by Eisendrath2 in 1902. At the beginning of the nineteenth century, the surgical treatment consisted of splenectomy, which was performed to avoid exsanguination, with a postoperative mortality rate of 40% compared to that associated with non-intervention, which could reach up to 90%.1
Over the years, our understanding of splenic immune function and the importance of the mononuclear-phagocytic system to combat infections by encapsulated microorganisms have identified the high mortality rate associated with post-splenectomy sepsis, which is present in up to 50% of cases.3
Studies in pediatric patients initiated the development of spleen preservation techniques. Splenorrhaphy, packing or the use of hemostatic agents,4 as well as the development of imaging tests and the use of non-invasive measures such as embolotherapy, has meant that conservative treatment has been slowly adopted to become the current gold standard treatment in this age group.5
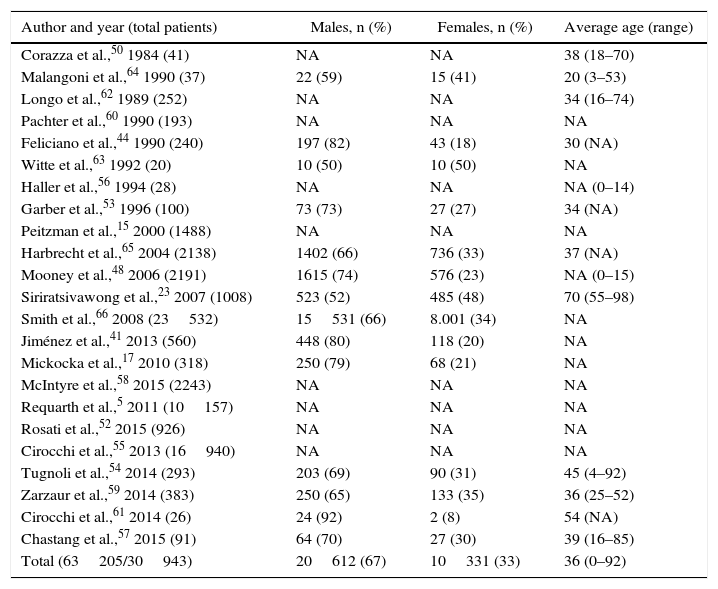
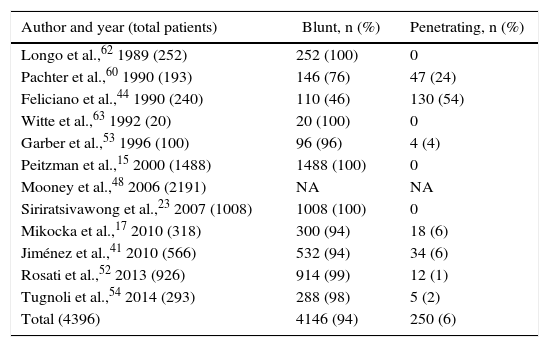
The objective of the present review is to explore the current management based on the international bibliography published over the last 30 years. A literature search was conducted through Pubmed, selecting articles published in English and Spanish between 1987 and 2017, with no age exclusion. We identified 23 series with a total of 63205 patients (Table 1), 90% of which were affected by a blunt mechanism of injury (Table 2).
Incidence by Author, Sex and Age.
| Author and year (total patients) | Males, n (%) | Females, n (%) | Average age (range) |
|---|---|---|---|
| Corazza et al.,50 1984 (41) | NA | NA | 38 (18–70) |
| Malangoni et al.,64 1990 (37) | 22 (59) | 15 (41) | 20 (3–53) |
| Longo et al.,62 1989 (252) | NA | NA | 34 (16–74) |
| Pachter et al.,60 1990 (193) | NA | NA | NA |
| Feliciano et al.,44 1990 (240) | 197 (82) | 43 (18) | 30 (NA) |
| Witte et al.,63 1992 (20) | 10 (50) | 10 (50) | NA |
| Haller et al.,56 1994 (28) | NA | NA | NA (0–14) |
| Garber et al.,53 1996 (100) | 73 (73) | 27 (27) | 34 (NA) |
| Peitzman et al.,15 2000 (1488) | NA | NA | NA |
| Harbrecht et al.,65 2004 (2138) | 1402 (66) | 736 (33) | 37 (NA) |
| Mooney et al.,48 2006 (2191) | 1615 (74) | 576 (23) | NA (0–15) |
| Siriratsivawong et al.,23 2007 (1008) | 523 (52) | 485 (48) | 70 (55–98) |
| Smith et al.,66 2008 (23532) | 15531 (66) | 8.001 (34) | NA |
| Jiménez et al.,41 2013 (560) | 448 (80) | 118 (20) | NA |
| Mickocka et al.,17 2010 (318) | 250 (79) | 68 (21) | NA |
| McIntyre et al.,58 2015 (2243) | NA | NA | NA |
| Requarth et al.,5 2011 (10157) | NA | NA | NA |
| Rosati et al.,52 2015 (926) | NA | NA | NA |
| Cirocchi et al.,55 2013 (16940) | NA | NA | NA |
| Tugnoli et al.,54 2014 (293) | 203 (69) | 90 (31) | 45 (4–92) |
| Zarzaur et al.,59 2014 (383) | 250 (65) | 133 (35) | 36 (25–52) |
| Cirocchi et al.,61 2014 (26) | 24 (92) | 2 (8) | 54 (NA) |
| Chastang et al.,57 2015 (91) | 64 (70) | 27 (30) | 39 (16–85) |
| Total (63205/30943) | 20612 (67) | 10331 (33) | 36 (0–92) |
The total number of patients found was 63848; out of this total, sex was defined in only 31586.
NA: not available.
Mechanism of Splenic Injury.
| Author and year (total patients) | Blunt, n (%) | Penetrating, n (%) |
|---|---|---|
| Longo et al.,62 1989 (252) | 252 (100) | 0 |
| Pachter et al.,60 1990 (193) | 146 (76) | 47 (24) |
| Feliciano et al.,44 1990 (240) | 110 (46) | 130 (54) |
| Witte et al.,63 1992 (20) | 20 (100) | 0 |
| Garber et al.,53 1996 (100) | 96 (96) | 4 (4) |
| Peitzman et al.,15 2000 (1488) | 1488 (100) | 0 |
| Mooney et al.,48 2006 (2191) | NA | NA |
| Siriratsivawong et al.,23 2007 (1008) | 1008 (100) | 0 |
| Mikocka et al.,17 2010 (318) | 300 (94) | 18 (6) |
| Jiménez et al.,41 2010 (566) | 532 (94) | 34 (6) |
| Rosati et al.,52 2013 (926) | 914 (99) | 12 (1) |
| Tugnoli et al.,54 2014 (293) | 288 (98) | 5 (2) |
| Total (4396) | 4146 (94) | 250 (6) |
If a possible splenic lesion is suspected, the next step is to assess the degree of spleen involvement. The diagnostic method of choice is computed tomography (CT), provided the patient is hemodynamically stable. The use of CT has contributed to the development of non-operative management (NOM) of the spleen: some series have reported an increase in NOM from 11 to 71% for the same degree of injury.6,7 The dual use of arterial and venous phases has a sensitivity of 90% for the identification of pseudoaneurysm, 97% for active bleeding and 99% for perisplenic hematoma.8
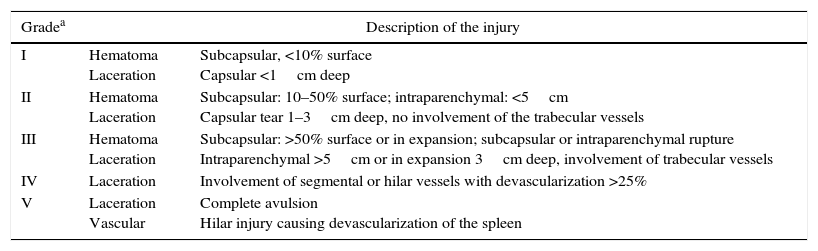
The most commonly used scale for determining the degree of splenic injury was established by the American Association for the Surgery of Trauma (AAST) (Table 3).9,10 According to the literature reviewed, the most frequent grades of injury are II and III, followed by grades I, IV and V (Table 4).
American Association for the Surgery of Trauma (AAST) Spleen Injury Grading System.10
| Gradea | Description of the injury | |
|---|---|---|
| I | Hematoma Laceration | Subcapsular, <10% surface Capsular <1cm deep |
| II | Hematoma Laceration | Subcapsular: 10–50% surface; intraparenchymal: <5cm Capsular tear 1–3cm deep, no involvement of the trabecular vessels |
| III | Hematoma Laceration | Subcapsular: >50% surface or in expansion; subcapsular or intraparenchymal rupture Intraparenchymal >5cm or in expansion 3cm deep, involvement of trabecular vessels |
| IV | Laceration | Involvement of segmental or hilar vessels with devascularization >25% |
| V | Laceration Vascular | Complete avulsion Hilar injury causing devascularization of the spleen |
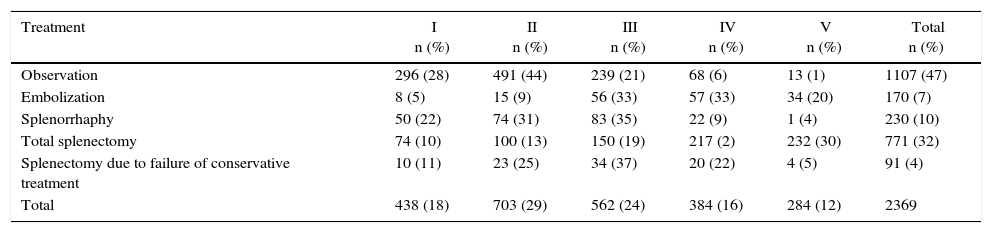
Treatment by Grade of Splenic Injury According to the American Association for the Surgery of Trauma (Organ Injury Scale).
| Treatment | I n (%) | II n (%) | III n (%) | IV n (%) | V n (%) | Total n (%) |
|---|---|---|---|---|---|---|
| Observation | 296 (28) | 491 (44) | 239 (21) | 68 (6) | 13 (1) | 1107 (47) |
| Embolization | 8 (5) | 15 (9) | 56 (33) | 57 (33) | 34 (20) | 170 (7) |
| Splenorrhaphy | 50 (22) | 74 (31) | 83 (35) | 22 (9) | 1 (4) | 230 (10) |
| Total splenectomy | 74 (10) | 100 (13) | 150 (19) | 217 (2) | 232 (30) | 771 (32) |
| Splenectomy due to failure of conservative treatment | 10 (11) | 23 (25) | 34 (37) | 20 (22) | 4 (5) | 91 (4) |
| Total | 438 (18) | 703 (29) | 562 (24) | 384 (16) | 284 (12) | 2369 |
The percentages for each grade are based on the total of each type of treatment.
Once the degree of splenic lesion has been established, management will depend on the hemodynamic status of the patient, the presence of associated lesions, and the availability of resources at each medical center. Hemodynamically unstable patients with positive focused abdominal sonography for trauma will require surgical intervention with a high probability of splenectomy.9
Non-Operative ManagementThe aim of non-surgical management in splenic trauma is to preserve function and reduce the morbidity and mortality associated with surgery. It is associated with a lower rate of non-therapeutic laparotomies, lower rate of blood transfusion, reduced overall morbidity and mortality rates, and lower hospital costs.9,11–13
Nonsurgical management should include: hospitalization in intermediate or intensive care units with continuous monitoring of vital signs, relative rest, control of hemoglobin levels and a follow-up series of abdominal explorations. There should also be blood transfusions available at the hospital, accessibility to CT and the continued presence of surgeons and interventional radiologists.14 Depending on the series analyzed, arteriography and embolization may be included in the non-surgical treatment. Conservative treatment should be considered a therapeutic option in patients who are hemodynamically stable, regardless of the degree of splenic injury. However, there is a direct correlation between the degree of injury and the rate of failure of non-surgical treatment.15–18
The guidelines of the Eastern Association for the Surgery of Trauma (EAST) do not contraindicate conservative treatment in patients with severe splenic injury diagnosed by CT, as long as they are hemodynamically stable.11,19,20
Peitzman et al.15 report a failure rate with this treatment of 4.8% for grade I and 75% for grade V. In a meta-analysis published by Requart et al.16 in 2011 with a total of 10157 patients, the non-surgical treatment failure rate was 8.3%. When non-surgical treatment was analyzed without embolization, the failure rate ranged from 4.7 to 83.1% for grades I and V, respectively.
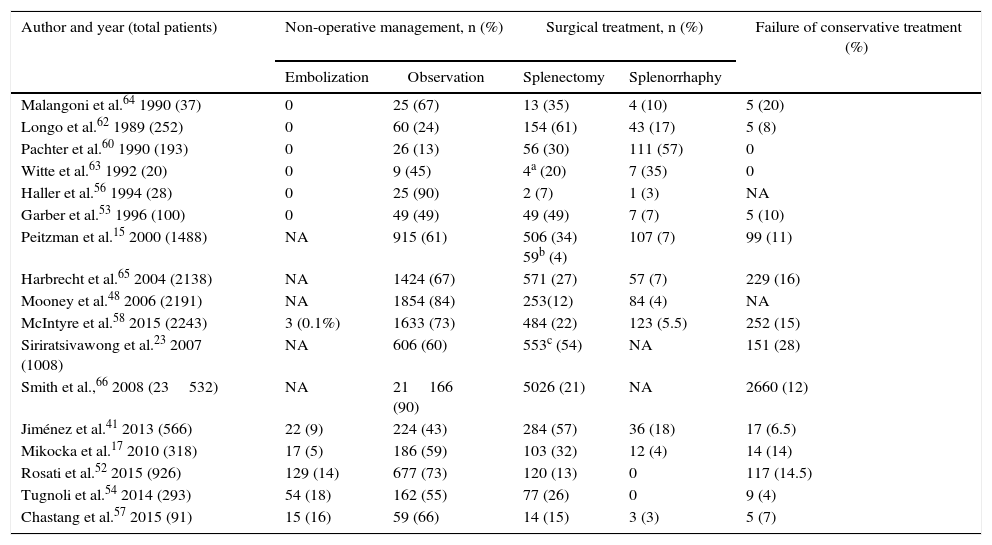
In recent years, there has been a certain downwards trend in NOM failure due to the introduction of arteriography and embolization, especially for grades IV and V. Table 5 demonstrates the results analyzed; the number of splenectomies includes both those done during the initial surgical management as well as those performed due to the failure of conservative treatment.
Treatment of Splenic Trauma.
| Author and year (total patients) | Non-operative management, n (%) | Surgical treatment, n (%) | Failure of conservative treatment (%) | ||
|---|---|---|---|---|---|
| Embolization | Observation | Splenectomy | Splenorrhaphy | ||
| Malangoni et al.64 1990 (37) | 0 | 25 (67) | 13 (35) | 4 (10) | 5 (20) |
| Longo et al.62 1989 (252) | 0 | 60 (24) | 154 (61) | 43 (17) | 5 (8) |
| Pachter et al.60 1990 (193) | 0 | 26 (13) | 56 (30) | 111 (57) | 0 |
| Witte et al.63 1992 (20) | 0 | 9 (45) | 4a (20) | 7 (35) | 0 |
| Haller et al.56 1994 (28) | 0 | 25 (90) | 2 (7) | 1 (3) | NA |
| Garber et al.53 1996 (100) | 0 | 49 (49) | 49 (49) | 7 (7) | 5 (10) |
| Peitzman et al.15 2000 (1488) | NA | 915 (61) | 506 (34) 59b (4) | 107 (7) | 99 (11) |
| Harbrecht et al.65 2004 (2138) | NA | 1424 (67) | 571 (27) | 57 (7) | 229 (16) |
| Mooney et al.48 2006 (2191) | NA | 1854 (84) | 253(12) | 84 (4) | NA |
| McIntyre et al.58 2015 (2243) | 3 (0.1%) | 1633 (73) | 484 (22) | 123 (5.5) | 252 (15) |
| Siriratsivawong et al.23 2007 (1008) | NA | 606 (60) | 553c (54) | NA | 151 (28) |
| Smith et al.,66 2008 (23532) | NA | 21166 (90) | 5026 (21) | NA | 2660 (12) |
| Jiménez et al.41 2013 (566) | 22 (9) | 224 (43) | 284 (57) | 36 (18) | 17 (6.5) |
| Mikocka et al.17 2010 (318) | 17 (5) | 186 (59) | 103 (32) | 12 (4) | 14 (14) |
| Rosati et al.52 2015 (926) | 129 (14) | 677 (73) | 120 (13) | 0 | 117 (14.5) |
| Tugnoli et al.54 2014 (293) | 54 (18) | 162 (55) | 77 (26) | 0 | 9 (4) |
| Chastang et al.57 2015 (91) | 15 (16) | 59 (66) | 14 (15) | 3 (3) | 5 (7) |
Currently, the overall rate of failure of NOM is estimated at 10%. Several risk factors associated with failure have been described: injury grade, presence of important hemoperitoneum, contrast extravasation on imaging studies, arterial hypotension upon admittance, associated brain injury and the need for blood transfusion.14 The latter is considered an independent risk factor for mortality in polytrauma patients.11,21 None of these factors is considered an absolute contraindication to initiate non-surgical treatment, except for the fact that some authors recommend not indicating conservative treatment if more than 5 units of red blood cells have been transfused in the first 24h.11,22 In patients with pre-existing anticoagulant treatment, this is reversed; therefore, it is not a NOM exclusion criterion.11,22
Age is also not considered an exclusion criterion. In a study carried out with 1008 patients over the age of 55 and blunt splenic trauma, the NOM success rate was 75.3%. Mortality was higher in the patient group requiring surgery (35 vs 16.7%). The study concluded that the majority of the patients over the age of 55 may benefit from conservative treatment.23
When determining appropriate management, the risk/benefit relationship should always be considered and the associated risk factors should be assessed for each case. It is essential for surgeons to be experienced and for there to be a multidisciplinary team for correct management.6
There is no unanimous consensus about what follow-up regimen is required. Some authors recommend follow-up CT scans for the diagnosis of non-hemorrhagic vascular lesions, such as pseudoaneurysm. In any event, CT would be indicated in the persistence of elevated inflammatory markers and abdominal pain, suspicion of intestinal injury, sudden drop in hemoglobin and hematocrit levels, or decline in general condition.11,12,20,22
With regards to blood count, a series of controls is recommended every 6h during the first 24h, every 12h until the third day and every 24h until discharge.11,24 There is no consensus about when to initiate prophylaxis for deep vein thrombosis. In a retrospective study done with 328 patients, early administration of low-molecular weight heparin between 48 and 72h after admittance is not associated with increased bleeding or greater failure of non-operative treatment.11,20
As for hospital discharge, most experts agree that the most important determining factors are symptoms and the stability of hemoglobin levels.11,22 The main risks of conservative management are late-onset bleeding, the need for transfusion and undetected intraabdominal injuries. According to a review of the literature, the risk for developing pseudoaneurysms, pseudocysts and splenic abscesses is higher in children, with a rate of 7.5%.9,25,26
Angiography and EmbolotherapyIn 1975, Chuang et al.27 completed an experimental study in dogs with splenic bleeding that was controlled by injecting embolic material in the splenic artery. They concluded that selective embolization of the artery was a method that could be used in humans who were not candidates for splenectomy.
In the 1970s, several studies were published with hopeful results. Sclafani et al.28,29 introduced the concept of proximal embolization in patients with blunt splenic trauma, with a success rate of 97%.
Angiographic procedures are diagnostic as well as therapeutic. Their objective is to embolize the artery and stop bleeding, which is the basis of conservative treatment in splenic trauma.30,31 These procedures are used in hemodynamically stable patients, under strict surgical supervision, and they provide the possibility to perform emergency splenectomy, if necessary. The percentage of urgent surgical interventions with these methods has decreased from 33.3 to 11.9%.32 However, only 5 to 7% of patients require embolization.19
The advantage of this technique is that other associated vascular lesions can be treated concomitantly. The disadvantages are the need for an interventional radiologist, the consumption of healthcare resources and the need to use large amounts of intravenous contrast.19
The types of vascular injuries include pseudoaneurysm, vascular lesion, and arteriovenous fistula. The identification of a fistula is associated with failure of conservative treatment in up to 60%.33 Endovascular embolization should be performed as soon as there is evidence of extravasation on CT, before there is hemodynamic deterioration.34,35
The indications of embolization36 include:
Absolute:
- •
Grades IV and V in the absence of other injuries in a stable patient.
- •
Extravasation of perisplenic contrast.
Relative:
- •
Grades I, II and III in the presence of signs of contrast extravasation on CT.
- •
Associated splenic intravascular injury (pseudoaneurysm or arteriovenous fistula).
- •
Moderate hemoperitoneum.
- •
Decreased hemoglobin levels during conservative management.
Endovascular embolization can be either proximal or distal. According to the consulted literature, the proximal approach is faster, simpler and associated with less failure of conservative treatment; additionally, it presents fewer complications than the distal approach.37
At the same time, there are two categories of complications:
Major complications include bleeding of up to 9%,38 associated with undiagnosed pseudoaneurysms or re-bleeding, undetected associated injuries (for instance, pancreatic or diaphragmatic), sepsis or splenic abscesses (4%), splenic atrophy or infarction, acute pancreatitis (especially associated with proximal angioembolization39), arterial iatrogenic injury (3%) (more common in children), nephropathy secondary to the contrast medium and deep vein thrombosis.
Minor complications include splenic infarction associated with the proximal technique in 20% and distal in 27% (which are usually asymptomatic), migration of the embolic material with a currently unknown incidence, vascular damage related to the catheter, hematoma, paralytic ileus and thrombocytosis.40
Routine immunization is not indicated in patients with conservative management. Even in patients with endovascular embolization and subsequent splenic infarction, the phagocytic function is maintained in most cases.11
Surgical ManagementAccording to Jiménez et al.,41 in a study of data from 6 hospitals in Spain published in 2013 with a total of 566 patients, management was surgical in 56.6% of cases. Surgical treatment is indicated in all hemodynamically unstable patients, those with suspected associated lesions requiring surgical treatment, or in cases of failed conservative treatment.9
The choice of surgical treatment does not always entail splenectomy. There is the option of spleen-preserving surgery, provided the patient is hemodynamically stable and laparotomy is indicated for other associated injuries.6,9
There are different intraoperative options for hemorrhagic control of splenic injuries, but the most classic (and usually the fastest) is splenectomy. However, due to the immunological function of the spleen, other techniques have been developed, such as splenorrhaphy, partial splenectomy and the use of commercial hemostatic agents, as well as absorbable polyglycolic acid meshes for the control of bleeding.42,43 For grade IV and V lesions, splenectomy continues to be the first option. However, due to the hemodynamic instability of the patient and damage control surgery, splenectomy would be the indication in these patients regardless of the degree of injury.6,9,14 In the review of the literature, out of the 771 splenectomies performed, 58% corresponded with grades IV and V injuries (Table 4).
In a study published by Feliciano et al.44 with a total of 240 patients, 86.2% of the splenorrhaphies were performed in grades I and III injuries. Adequate mobilization and visualization of the spleen is recommended during surgery so as not to overlook potentially unnoticed lesions.44
The Society of American Gastrointestinal and Endoscopy Surgeons (SAGES) accept diagnostic laparoscopy as a feasible and safe method if applied to selected trauma patients. This includes patients who are hemodynamically stable with injuries in the left thoracoabdominal region and suspicion of intra-abdominal injury not diagnosed by imaging techniques.6,45 Experience in this technique is essential to avoid overlooking injuries. More studies are required to define the role of laparoscopy in the treatment of polytrauma patients.
There is a risk of developing post-splenectomy fulminant sepsis. The period of maximum risk of severe infection is in the first 3–5 years after surgery and remains at 5% for the rest of the patient's life.46 In children, the reported incidences are between 2.4 and 11%.9,47 The remaining complications associated with surgery are more related with the presence of associated lesions than with splenectomy per se. For prevention, vaccination is recommended against pneumococcus and influenza 2 weeks after splenectomy (based on levels of evidence 2 and 3) as well as in those patients who develop hyposplenism after conservative treatment. These patients should know that they are more susceptible to malaria than the rest of the population, so they should strictly follow antimalarial prophylaxis corresponding to endemic areas during their travels.46 There is no agreement about the widespread use of antibiotic prophylaxis in all splenectomized patients. Given the increased risk of sepsis in children and adolescents, prophylaxis is recommended in all children under 16 years of age, as well as in patients over 50 years of age and in those with poor serological response to the vaccine.6,9,46
Follow-up After Splenic TraumaThere is currently no scientific evidence on the need for follow-up imaging studies after NOM. Similarly, there is no established consensus regarding restrictions for resuming physical activity.6,19 In the review of the literature, it is recommended to avoid contact and high-risk sports during the first 3 months after discharge, although there are no clinical studies that support this duration.6,48
The review by Carlotto et al.11 recommends discharge between the 3rd and the 7th day after hospital admission if the patient progresses adequately with NOM. Likewise, they establish a rest period depending on the injury grade and the type of physical activity, ranging from 2 weeks for daily activities in grades I–V and up to 12 months in contact sports with grade IV and V injuries.
Juyia and Kerr49 report that 84% of injuries, regardless of the degree of severity, show radiological improvement 10 weeks after the trauma, ranging from 3 weeks for grade I and 21 weeks for grade IV.
Splenic Injuries in Pediatric PatientsThe spleen is the most frequently injured organ in abdominal trauma in children. It is more vulnerable due to its position below the ribcage and the elasticity of the supporting ligaments.50
As mentioned above, this age group has a higher risk of developing post-splenectomy fulminant sepsis, with a risk of death almost 600 times higher than the general population.51 Due to this, NOM was first initiated in the pediatric population with splenic trauma, and today it is the gold standard treatment in this age group. Success rates have been reported between 85 and 90%, depending on the series.46,50,52
As in adult patients, NOM is an option, as long as the patient is hemodynamically stable. Laparotomy is indicated if, at admittance, more than 40ml of blood is needed per kg of weight, representing half of their blood volumen.52
ConclusionNon-surgical management requires a series of criteria to guarantee success. Patients should be properly selected and always under the supervision of a multidisciplinary team at a hospital that meets the requirements for conservative management.
In a review of the literature, the success rate is around 80–90%, and certain risk factors have been described for failure. The use of angiography and endovascular embolization may be a less invasive therapeutic option in hemodynamically stable patients, in patients with evident contrast extravasation and grade IV and V injuries. This technique may be an alternative for splenic preservation that ensures a higher percentage of success in conservative management.
Splenectomy remains the technique of choice for patients who are hemodynamically unstable or in cases of failed conservative treatment. Spleen-preserving surgery, such as splenorrhaphy, partial splenectomy, or hemostatic techniques, may be an alternative in patients requiring laparotomy due to other associated injuries, which are hemodynamically stable and have no acidosis, coagulopathy, or hypothermia, and are not candidates for damage-control surgery, for which splenectomy should be the norm.
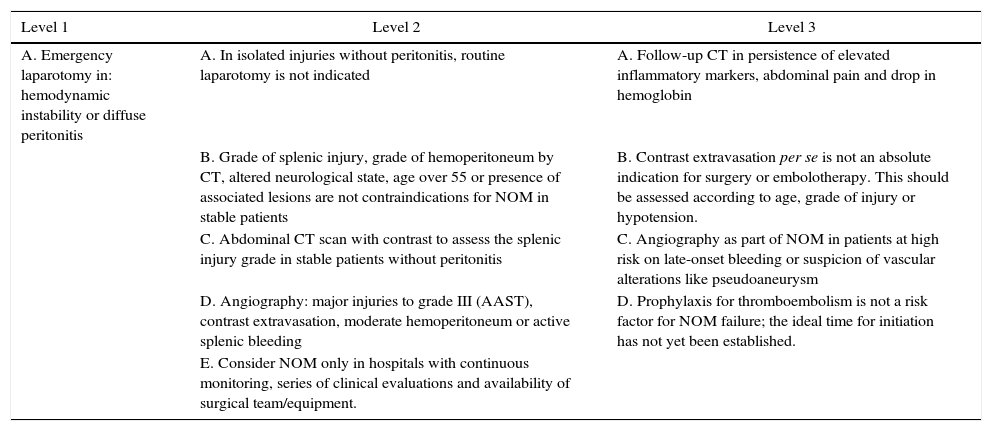
Table 6 shows a series of recommendations20 based on different levels of evidence. Further studies are recommended with the participation of various medical centers in order to unify evidence-based criteria for decision making in the treatment of this trauma injury.
Levels of Recommendation After Blunt Splenic Trauma According to EAST.20
| Level 1 | Level 2 | Level 3 |
|---|---|---|
| A. Emergency laparotomy in: hemodynamic instability or diffuse peritonitis | A. In isolated injuries without peritonitis, routine laparotomy is not indicated | A. Follow-up CT in persistence of elevated inflammatory markers, abdominal pain and drop in hemoglobin |
| B. Grade of splenic injury, grade of hemoperitoneum by CT, altered neurological state, age over 55 or presence of associated lesions are not contraindications for NOM in stable patients | B. Contrast extravasation per se is not an absolute indication for surgery or embolotherapy. This should be assessed according to age, grade of injury or hypotension. | |
| C. Abdominal CT scan with contrast to assess the splenic injury grade in stable patients without peritonitis | C. Angiography as part of NOM in patients at high risk on late-onset bleeding or suspicion of vascular alterations like pseudoaneurysm | |
| D. Angiography: major injuries to grade III (AAST), contrast extravasation, moderate hemoperitoneum or active splenic bleeding | D. Prophylaxis for thromboembolism is not a risk factor for NOM failure; the ideal time for initiation has not yet been established. | |
| E. Consider NOM only in hospitals with continuous monitoring, series of clinical evaluations and availability of surgical team/equipment. |
AAST: American Association for the Surgery of Trauma; CT: computed tomography; NOM: non-operative management.
The authors have no conflict of interests to declare.
Please cite this article as: Petrone P, Anduaga Peña MF, Servide Staffolani MJ, Brathwaite C, Axelrad A, Ceballos Esparragón J. Evolución en el tratamiento conservador del traumatismo esplénico contuso. Cir Esp. 2017;95:420–427.