Living donor liver transplantation (LDLT) is an alternative to conventional transplantation given its excellent results. The aim of this study is to evaluate long-term outcomes in LDLT recipients.
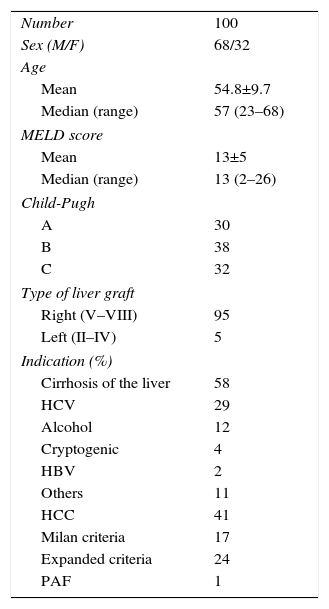
Methods100 consecutive THDV recipients from the Hospital Clínic of Barcelona from March 2000 to October 2015 were included. The main indication for transplantation was end-stage liver disease (58%) followed by hepatocellular carcinoma (41%). 95% of grafts consisted of the right liver of the donor and the 5% of the left liver.
ResultsAfter a median follow-up of 65.5 months, patient and graft survival at 1, 3, and 5 years was 93%, 80% and 74% and 90%, 76%, and 71%, respectively. The overall re-transplant rate was 9%. The most common long-term complication was biliary stenosis (40%) with an average time of onset of 13.5±12 months, with repeated admissions and an average of 1.9±2 endoscopic procedures and 3.5±3 Radiological procedures per patient. The definitive treatment was radiological dilation in 40% of cases, surgical intervention in 22.5% and re-transplantation in 7.5%.
ConclusionsGiven the long-term results, LDLT is confirmed as an alternative to conventional transplantation. However, the high rate of late biliary complications involves repeated admissions and invasive treatments that, while not compromising survival, can affect the patient's quality of life.
El trasplante hepático de donante vivo (THDV) es una alternativa al trasplante convencional dados sus excelentes resultados. El objetivo de este trabajo es la evaluación de los resultados a largo plazo en los receptores de THDV.
MétodosCien receptores consecutivos de THDV del Hospital Clínic de Barcelona desde marzo de 2000 hasta octubre de 2015. La indicación principal de trasplante fue hepatopatía terminal (58%) seguido de hepatocarcinoma (41%). Los injertos consistieron en un 95% del hígado derecho del donante y en un 5% del izquierdo.
ResultadosTras una mediana de seguimiento de 65,5 meses, la supervivencia global a uno, 3 y 5 años de los pacientes y de los injertos fue del 93%, 80% y 74% y del 90%, 76% y 71% respectivamente, con una tasa de retrasplante global del 9%. La complicación a largo plazo más frecuente fue una estenosis biliar (40%), con un tiempo medio de aparición de 13,5±12 meses, que comportó repetidos ingresos y una media de 1,9±2 abordajes endoscópicos y 3,5±3 abordajes radiológicos por paciente. El tratamiento definitivo fue dilatación radiológica en un 40% de los casos, intervención quirúrgica en un 22,5% y retrasplante en un 7,5%.
ConclusionesDados los resultados a largo plazo, el THDV se confirma como una alternativa al trasplante convencional. Sin embargo, la alta tasa de complicaciones biliares tardías conlleva repetidos ingresos y tratamientos invasivos que, si bien no comprometen la supervivencia, pueden afectar la calidad de vida del paciente.
After almost 2 decades since its introduction, living donor liver transplantation in adults (LDLTa) has been demonstrated a completely valid alternative to conventional transplantation from the standpoint of recipient and graft survival. Most studies focused on the recipients of living donor transplantation in adults (RLDLTa) emphasize the immediate postoperative period, centered on the postoperative course and highlighting long-term follow-up instead of graft and recipient survival. There are many potential complications that can appear and influence survival of the RLDLTa, such as hepatitis C virus (VHC) reinfection, the appearance of opportunistic infections, or the development of cancer. Additionally, once the initial transplantation period has passed, biliary tract complications are a real problem and, although they do not usually affect the overall survival of the recipient, they do have an important impact on quality of life.1 Likewise, if they are not satisfactorily resolved, the presence of these complications for a long period of time can cause irreversible changes in the graft that would unavoidably lead to re-transplantation.
The aim of this present study is to analyze the long-term results of the first 100 RLDLTa, with special emphasis on bile duct complications.
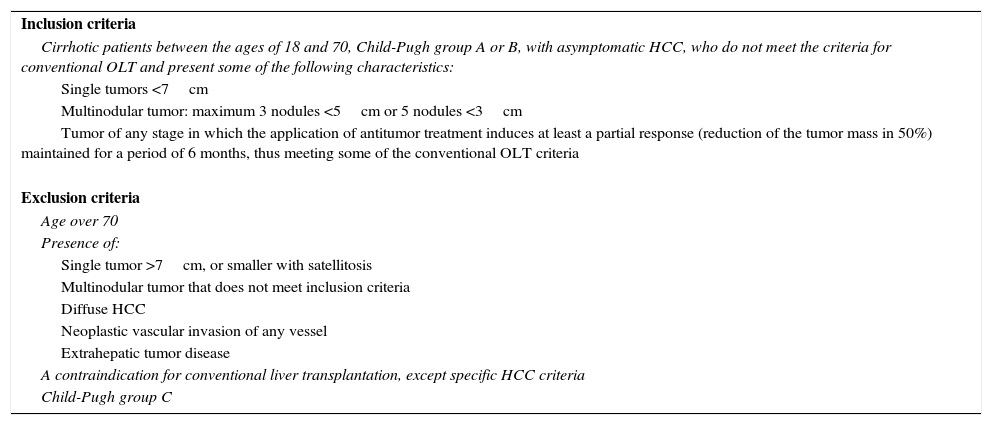
MethodsPatientsOurs is a retrospective study of 100 RLDLTa from 2000 and 2015. As demonstrated in Table 1, there were two indication groups for LDLTa: 58 patients (58%) with chronic hepatopathy in terminal liver failure, and 41 patients (41%) whose indication for transplantation was the presence of unresectable CHC. It is especially important to clarify that, in this latter group, in 24 out of the 41 patients (58.5%) the indication was the presence of CHC outside of the Milan criteria, but within the expanded criteria of our hospital (Table 2).
Demographic Data of RLDLTa Patients.
| Number | 100 |
| Sex (M/F) | 68/32 |
| Age | |
| Mean | 54.8±9.7 |
| Median (range) | 57 (23–68) |
| MELD score | |
| Mean | 13±5 |
| Median (range) | 13 (2–26) |
| Child-Pugh | |
| A | 30 |
| B | 38 |
| C | 32 |
| Type of liver graft | |
| Right (V–VIII) | 95 |
| Left (II–IV) | 5 |
| Indication (%) | |
| Cirrhosis of the liver | 58 |
| HCV | 29 |
| Alcohol | 12 |
| Cryptogenic | 4 |
| HBV | 2 |
| Others | 11 |
| HCC | 41 |
| Milan criteria | 17 |
| Expanded criteria | 24 |
| PAF | 1 |
Expanded LDLTa Inclusion and Exclusion Criteria for Recipients With Hepatocellular Carcinoma.
| Inclusion criteria |
| Cirrhotic patients between the ages of 18 and 70, Child-Pugh group A or B, with asymptomatic HCC, who do not meet the criteria for conventional OLT and present some of the following characteristics: |
| Single tumors <7cm |
| Multinodular tumor: maximum 3 nodules <5cm or 5 nodules <3cm |
| Tumor of any stage in which the application of antitumor treatment induces at least a partial response (reduction of the tumor mass in 50%) maintained for a period of 6 months, thus meeting some of the conventional OLT criteria |
| Exclusion criteria |
| Age over 70 |
| Presence of: |
| Single tumor >7cm, or smaller with satellitosis |
| Multinodular tumor that does not meet inclusion criteria |
| Diffuse HCC |
| Neoplastic vascular invasion of any vessel |
| Extrahepatic tumor disease |
| A contraindication for conventional liver transplantation, except specific HCC criteria |
| Child-Pugh group C |
Since 2003, and given the evidence available at that time of the influence of hepatic hemodynamics during graft implantation on postoperative results, we initiated a protocol for systematic hemodynamic monitoring in all RLDLTa, previously published by our group.2,3
Surgical Procedure and Immediate Post-opThe RLDLTa surgical procedure used in our group has already been previously published.3 Briefly, after the dissection of the elements of the hepatic hilum, a temporary portacaval anastomosis is routinely performed, and hepatectomy is subsequently completed by preservation of the native vena cava of the recipient. The hepatic graft is then implanted. In most cases, the graft consisted of the right liver lobe of the donor, so the implantation was initiated by anastomosis of the right hepatic vein of the graft with the right hepatic vein of the recipient, enlarging the ostium caudally to achieve the maximum possible diameter. Afterwards, anastomosis is completed of the right portal vein of the graft to that of the recipient, and the graft is reperfused. Then, the arterial anastomosis is performed between the right hepatic artery of the graft and the recipient before ligation of the gastroduodenal artery of the recipient. In 5 cases, the liver graft was the donor's left liver; in these cases, the left and middle hepatic veins of the graft are anastomosed with the vena cava of the recipient, and the portal and arterial anastomoses are between the portal vein and the hepatic artery of the recipient and the portal vein and the left hepatic artery of the graft. The last to be completed is the biliary anastomosis. Given the biliary anatomy of the graft, several types of anastomoses are performed, both duct-to-duct as well as hepaticojejunal.4 In most patients, the biliary anastomoses are guided by a Kehr drain tube. The patients are immediately transferred to the intensive care unit, where they are extubated in the following hours.
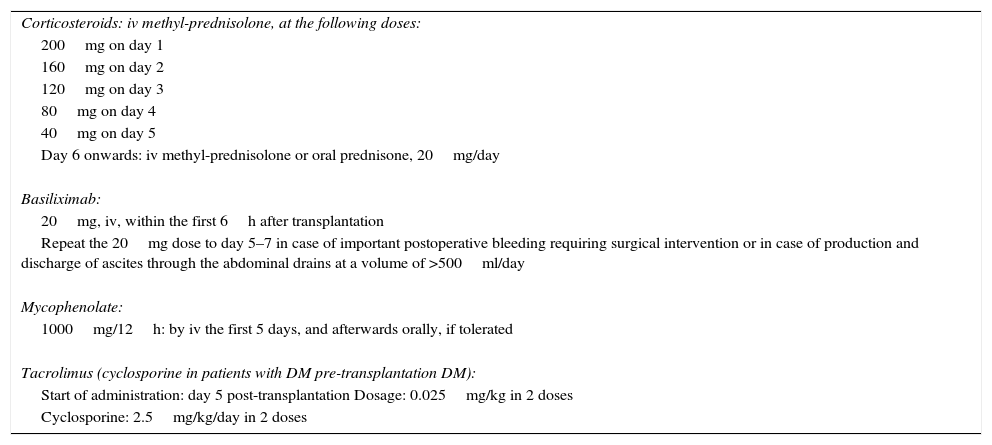
Patient immunosuppressant treatment is administered in accordance with our hospital's protocol (Table 3). Finally, once the patient is independent, presents no acute complications and has adequate levels of immunosuppressants, the patient is discharged.
Immunosuppression Protocol in RLDLTa.
| Corticosteroids: iv methyl-prednisolone, at the following doses: |
| 200mg on day 1 |
| 160mg on day 2 |
| 120mg on day 3 |
| 80mg on day 4 |
| 40mg on day 5 |
| Day 6 onwards: iv methyl-prednisolone or oral prednisone, 20mg/day |
| Basiliximab: |
| 20mg, iv, within the first 6h after transplantation |
| Repeat the 20mg dose to day 5–7 in case of important postoperative bleeding requiring surgical intervention or in case of production and discharge of ascites through the abdominal drains at a volume of >500ml/day |
| Mycophenolate: |
| 1000mg/12h: by iv the first 5 days, and afterwards orally, if tolerated |
| Tacrolimus (cyclosporine in patients with DM pre-transplantation DM): |
| Start of administration: day 5 post-transplantation Dosage: 0.025mg/kg in 2 doses |
| Cyclosporine: 2.5mg/kg/day in 2 doses |
After hospital discharge, the RLDLTa of our study were followed in the hospital outpatient setting, and postoperative liver function was strictly monitored during the postoperative period up to one year after transplantation. In the same way, all patients were systematically evaluated by hepatic magnetic resonance imaging 3 months after transplantation to evaluate the appearance of the graft and the possible appearance of complications, both vascular and biliary.
Biliary stenosis was defined as the presence of clinical symptoms (pruritus, jaundice) in a patient with elevated alkaline phosphatase and GGT levels as well as a significant decrease in the diameter of the biliary anastomosis, demonstrated radiologically. The treatment of each case was individualized depending on the location, and less aggressive treatment was initially used. Progressively, the therapeutic arsenal included endoscopic treatment, interventional radiology (simple dilatation or stent placement), or surgical treatment. Ultimately, and in the presence of irreversible liver damage as a consequence of chronic biliary obstruction, we decided on liver transplantation.
Statistical AnalysisUnless otherwise stated, the continuous variables are expressed as mean±standard deviation. Categorical variables were analyzed using the Chi squared test or Fisher's F test, and the difference between continuous variables using the Student's t test. The survival analysis was calculated with the Kaplan Meier method, and the differences between groups were analyzed using the Log Rank test. The P results >.05 were considered statistically significant. All statistical tests were performed using the SPSS Statistics 20 software for Windows (SPSS Inc., Chicago, Illinois, USA).
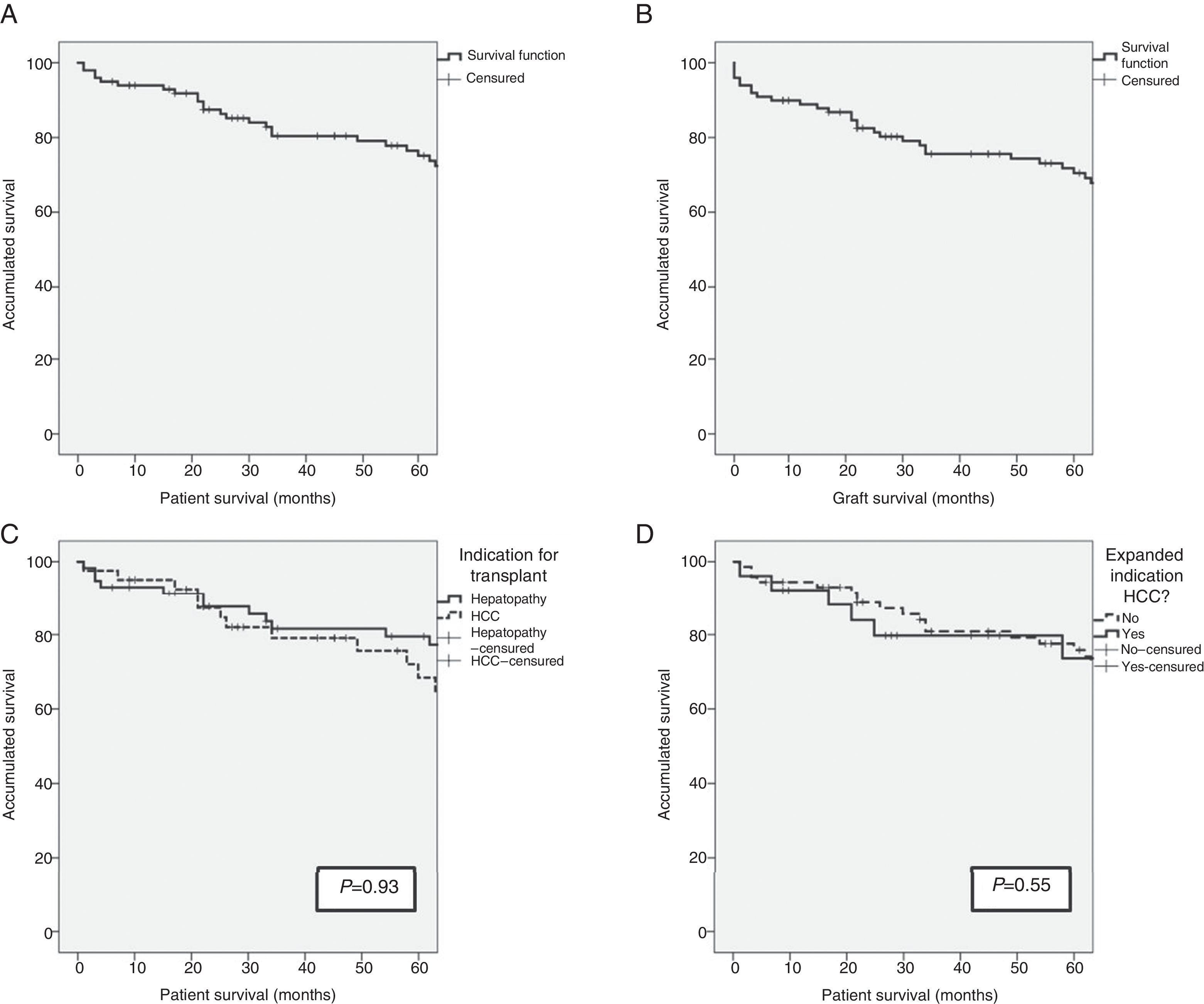
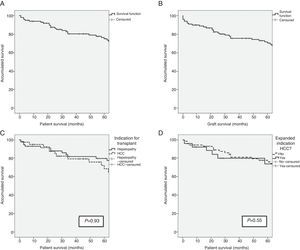
ResultsSurvival of the SeriesAfter a median patient follow-up of 65.5 months, overall patient survival one, 3 and 5 years after transplantation was 93%, 79.6% and 74.4%, respectively. The graft survival percentages were somewhat lower at 89%, 75.9% and 70.8% one, 3 and 5 years after transplantation, respectively.
The indication for transplantation did not play a determining role in patient survival. After having excluded the only patient whose indication was familial amyloid polyneuropathy, and despite the fact that the pre-transplant characteristics of the patients were different depending on the indication of transplantation (terminal hepatopathy vs hepatocellular carcinoma [HCC]), patient survival rates presented no statistically significant differences (93.1%, 81.6% and 79.5% vs 95.1%, 79.0% and 68.4%; P=.93) after one, 3 and 5 years, respectively. Patients whose indication for transplantation was HCC with expanded criteria presented survival rates of 92.3%, 79.7% and 73.6% after one, 3 and 5 years, respectively, with no differences presented with the rest of the patients (Fig. 1). The etiology of the hepatopathy in patients transplanted with HCC (41) was secondary to HCV infection in 80.5% (33 cases). These patients had a lower survival rate than the other HCV-free patients with HCC (P=.046). However, the proportion of HCV-positive patients in the two groups was 80.5% in patients transplanted due to HCC, and only 53.4% in patients transplanted due to terminal hepatopathy (P=.006). In addition, there were no differences in survival between the patients whose hepatopathy origin was HCV or other causes (P=.054).
(A) Survival in the first 5 years after transplantation of the LDLTa recipients; (B) survival in the first 5 years after transplantation of the living-donor graft; (C) comparison of survival rates between the LDLTa recipients whose transplant indication was chronic hepatopathy (solid line) or hepatocellular carcinoma (dashed line); (D) comparison of survival rates among the LDLTa recipients with hepatocellular carcinoma according to the indication having been based on the Milan or expanded criteria.
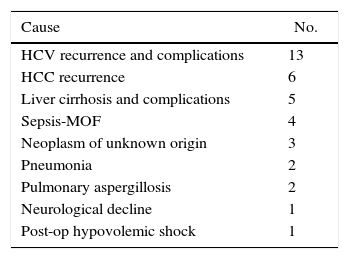
At the writing of this article, a total of 37 RLDLTa patients had deceased, 31 of which died more than 3 months after transplantation. Table 4 shows the causes of death of the RLDLTa.
Long-term ComplicationsLate-onset Bile Duct ComplicationsThe most frequent long-term complication was bile duct stenosis. Characteristically, and unlike in the case of biliary leakage, stenosis occurs a relatively long time after transplantation. Specifically, 40% of RLDLTa in the series presented biliary stenosis, whose presentation time was 13.5±12.3 months after transplantation (median 8.5 months, range 2–52).
In the analysis of the possible causes of biliary stenosis, a responsible risk factor could not be found. No differences were found in the incidence of stenosis between the presence of one single bile duct in the graft versus several (36.2% vs 45.2%, P=.41), between the need to perform one or 2 bile duct anastomoses (41.3% vs 35.0%, P=.79), between duct-to-duct or hepaticojejunal anastomosis (40.2% vs 38.9%, P=1) or between ductoplasty of the bile ducts or not during bench surgery (36.1% vs 50%, P=.26). Even in the long term, we did not find differences between the appearance of stenosis between the RLDLTa patients with or without biodegradable stents (41.2% vs 39.8%; P=1).
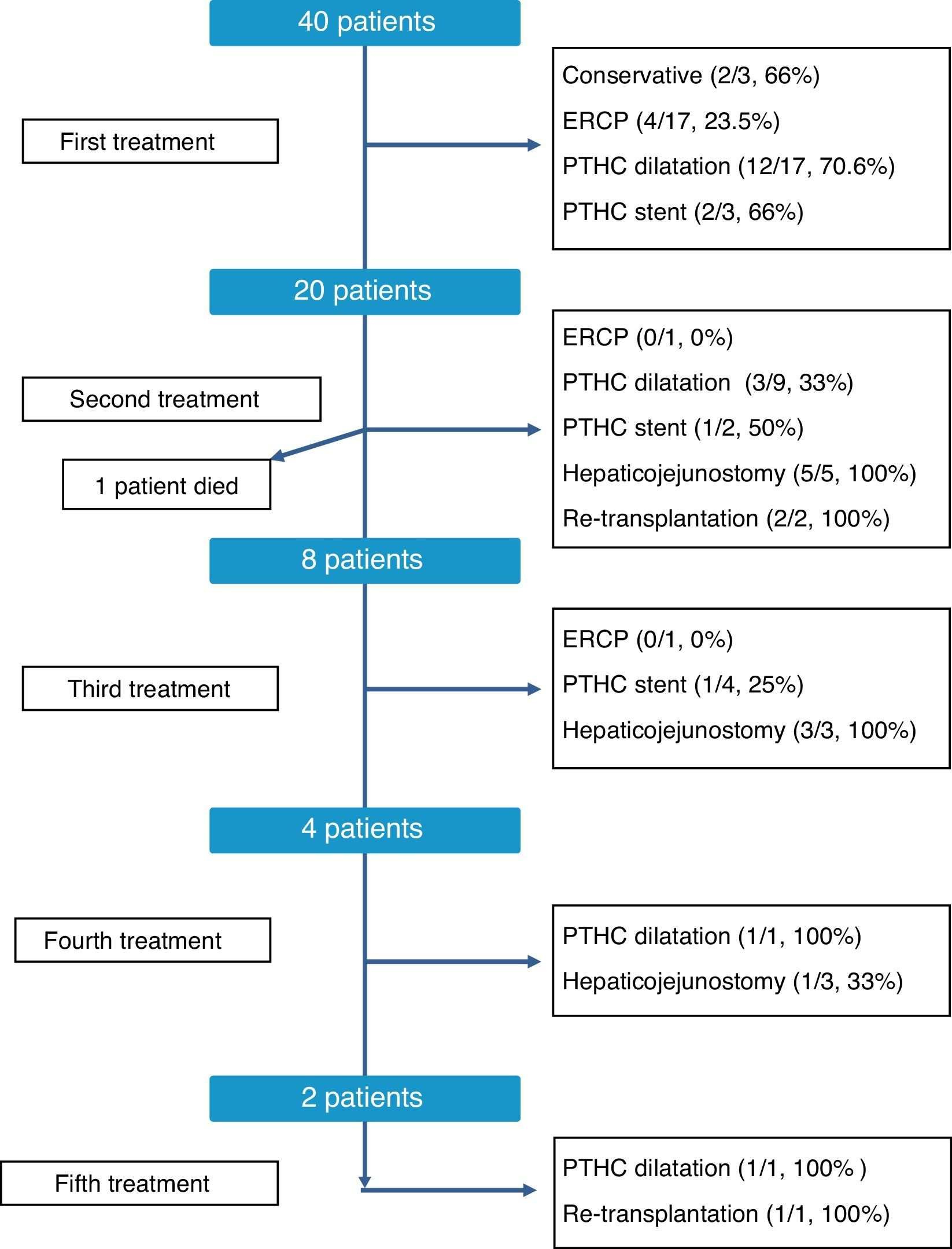
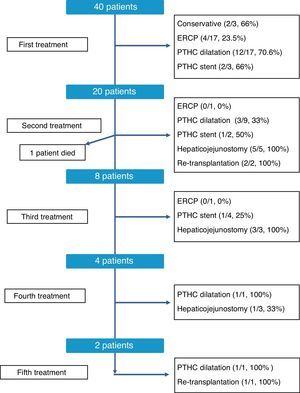
The therapeutic management of bile duct strictures in RLDLTa was individualized, as shown in Fig. 2. As can be observed, at the time of this writing all patients were satisfactorily treated, although certain patients required up to 5 different types of treatment. Overall, the most effective treatments to resolve the stenosis were conversion to hepaticojejunostomy, with an overall resolution rate of 81% (9/11 cases) and dilatation by percutaneous transhepatic cholangiography, with 60.7% of successes (17/28 cases). In contrast, endoscopic dilatation had a low success rate of 21% (4/19 cases). Finally, in 3 cases, we had to resort to liver transplantation as a consequence of the presence of refractory stenosis and the development of secondary biliary cirrhosis.
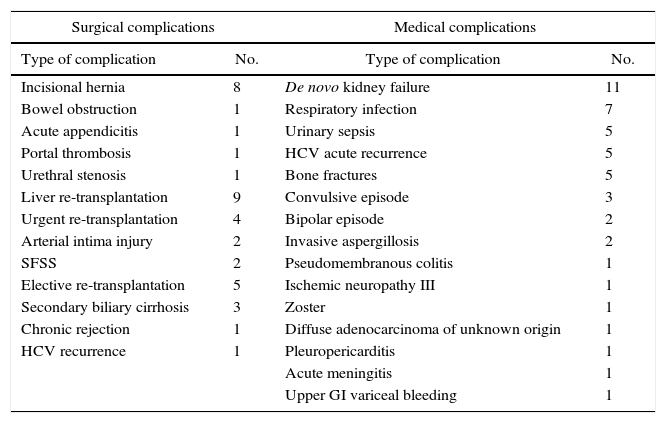
Other Late-onset ComplicationsAs can be observed in Table 5, the list of medical complications is headed by the appearance of de novo kidney disease in 11% of patients, probably influenced by the regimen of immunosuppressants. The most frequent surgical complications in the long term were the development of incisional hernias in 8% of RLDLTa, followed by other less frequent diseases, such as the presence of bowel obstruction.
Late-onset Complications Not Related With the Bile Duct.
| Surgical complications | Medical complications | ||
|---|---|---|---|
| Type of complication | No. | Type of complication | No. |
| Incisional hernia | 8 | De novo kidney failure | 11 |
| Bowel obstruction | 1 | Respiratory infection | 7 |
| Acute appendicitis | 1 | Urinary sepsis | 5 |
| Portal thrombosis | 1 | HCV acute recurrence | 5 |
| Urethral stenosis | 1 | Bone fractures | 5 |
| Liver re-transplantation | 9 | Convulsive episode | 3 |
| Urgent re-transplantation | 4 | Bipolar episode | 2 |
| Arterial intima injury | 2 | Invasive aspergillosis | 2 |
| SFSS | 2 | Pseudomembranous colitis | 1 |
| Elective re-transplantation | 5 | Ischemic neuropathy III | 1 |
| Secondary biliary cirrhosis | 3 | Zoster | 1 |
| Chronic rejection | 1 | Diffuse adenocarcinoma of unknown origin | 1 |
| HCV recurrence | 1 | Pleuropericarditis | 1 |
| Acute meningitis | 1 | ||
| Upper GI variceal bleeding | 1 | ||
In 2012, the first LDLTa was carried out with the use of the left liver as a graft, after the ample experience and good results published in the literature, especially from Asia. In total, 5 surgeries were conducted with this method. Without a doubt, the most notable aspect was the appearance of small-for-size syndrome in 2 out of the 5 patients, which required urgent re-transplantation, resulting in a re-transplantation rate of 40% in this patient group. The median follow-up of these patients was very short (19 months), and only one patient who had received this type of transplant died as a consequence of post-transplant lymphoproliferative syndrome.
DiscussionLDLTa has become a fully valid alternative to conventional transplantation because of its excellent short- and long-term results. In addition, in our setting, the expanded indication of CHC outside the Milan criteria has provided access to transplantation to patients who had no previous indication, with the resulting potential benefit in terms of quality of life and survival.
The survival rates of the recipients after a median follow-up of over 65 months were 93%, 79.6% and 74.4% per year after one, 3 and 5 years, respectively, regardless of the transplant indication. To put these data into perspective, the survival rates of conventional liver transplantation recipients published by the National Transplant Organization in 2015 were 85.8%, 78.3% and 73% one, 3 and 5 years after transplantation,5 and the rates published by the European liver transplant registry were 83%, 76% and 71% after one, 3 and 5 years, respectively.6 As for the survival of liver grafts, this dropped slightly to 89%, 75.9% and 70.8% after one, 3 and 5 years, respectively. At the time of writing, a total of 37 RLDLTa have died in our study, resulting in an overall survival of the series of 63%, with a median follow-up of more than 5 years.
Undoubtedly, the most significant complication in RLDLTa is the appearance of a bile duct complication. Specifically, a total of 40 RLDLTa presented long-term biliary stenosis. This phenomenon is well characterized in the literature, as it is a stenosis that typically appears late (in our series, after a median of 8.5 [2–52] months), with previously absolutely normal analytical and radiological studies. The origin appears to be multifactorial, involving factors such as bile duct ischemia, bile duct diameter, presence of more than one bile duct at the point of transection (43% of grafts had 2 or more bile ducts) and hypertrophy of the hepatic parenchyma around the bile duct. Despite this, the univariate analysis of the possible factors for biliary stenosis (number of bile ducts, number of biliary anastomoses, presence or absence of a biliary ductoplasty or type of anastomosis used [duct-to-duct vs hepaticojejunostomy]) has not shown a negative influence of any of them. In addition, in a preliminary study published by our group, we showed the positive influence that the placement of a biodegradable PDS biliary stent seemed to have during the procedure with the objective of reducing the long-term rate of bile stenosis by maintaining the maximum diameter of the anastomosis during the healing process. After a longer follow-up, the results have shown a rate of bile stenosis in RLDLTa patients with bioresorbable stents of 41.2%, which is comparable to the remaining patients without stents, with a rate of 39.76% (P=1). The topic of late stenosis of living donor transplant recipients is one of the most interesting aspects of this transplantation method. In 2 recent reviews of the literature, biliary complications after LDLTa have been shown to be repeatedly high, with published figures from 5 to 40.6% and biliary anastomosis stricture accounting for the complication in 3.7%–24.3% of cases.7,8 Likewise, in a review published in 2013 by the A2ALL consortium including only US hospitals, 39.6% of RLDLTa presented biliary complications, 32.6% of which were biliary stenoses,9 with a mean time at onset of 107 Days (range 55–278 days) after transplantation, which concurs with our previous experience.1
The treatment of stenosis should be immediate, since it can cause problems of greater magnitude in the short term (acute cholangitis, renal insufficiency) as well as the long term, with the development of irreversible changes in the liver parenchyma. In our hospital, bile stenosis treatments were decided by a multidisciplinary committee in an individualized manner for each patient. The analysis of the results shows us data to consider. First, the definitive resolution rate of biliary stenosis was high; at the time of writing of this present article, all patients presented resolution of their biliary stenosis. Second, there is a significant disparity between the success rates based on the studies used. Although each case was individualized, the resolution rate of endoscopic dilatation was clearly lower than percutaneous dilatation (21% vs 60.7% of cases). Unlike the bile stenosis in conventional liver transplant recipients, the endoscopic approach in RLDLTa has not shown the same effectiveness. Balderramo et al. report that endoscopic management in RLDLTa may be quite difficult because of the complex nature of duct-to-duct reconstruction, and that patients often require frequent ERCP with the use of smaller stents (7–8.5 Fr), which is successful in up to 65% of patients. However, the stenosis relapse rate may be up to 30%.10 Although surgical reoperation was not initially used in any of the cases, it is the most successful modality, even though it can also be considered the most aggressive. Its rate of resolution of stenosis, especially when it comes to patients with months of ineffective treatment, suggests that it could be used earlier in therapeutic management and thereby eliminate the need for multiple treatment sessions and hospitalization. Nonetheless, more data are needed to support this hypothesis.
A total of 9 re-transplantations were performed. The urgent cases were caused by arterial intima injury in 2 patients and small-for-size syndrome in 2 patients, while the causes of elective re-transplantation were led by secondary biliary cirrhosis (3 cases), chronic rejection (one case), and complications of HCV reinfection (one case). There are causes of re-transplantation that can be presented in much the same way in conventional transplantation, but the rate of re-transplantation caused by biliary tract problems is undoubtedly one of the most controversial points of LDLTa. A recent study published by Bittermann et al. analyzes the results after liver re-transplantation in 209 RLDLTa registered in the UNOS database and, while the most frequent cause of re-transplantation in the first 14 days was vascular complications (39.7%), biliary complications were the cause of re-transplantation in 8.1%, and in the multivariate analysis they were not a risk factor for increased mortality.11
The other late-onset complications experienced by RLDLTa are specified in Table 5. Clearly, a frequent complication, and one that may have important consequences for RLDLTa, is clinically significant reinfection by HCV. It should be noted that in the timeframe of this series, the fundamental treatment for HCV infection consisted basically of the combination of interferon and ribavirin; nonetheless, in spite of having treated the infection prior to transplantation, the graft reinfection rate was practically universal, and a significant number of RLDLTa had recurrence of hepatic cirrhosis. Another common medical complication was the appearance of de novo renal failure, which was probably caused by multiple factors, in spite of the prominent influence of immunosuppressants and the presence of respiratory infections. As can be observed, the surgical complications were headed by the appearance of an incisional hernia in 8% of cases. This is a relatively frequent complication in liver transplant recipients as a result of the incision used as well as other factors, such as immunosuppressants and corticosteroid therapy, or the relatively high frequency of hypoproteinemia in the recent postoperative period or once patients developed recurrent HCV infection.
Finally, and given the importance of the results, it is worth mentioning in the analysis of the first 100 cases the introduction of LDLTa using the left liver of the donor as a graft. In our experience, despite the careful selection of the donor-recipient pairing and close hemodynamic monitoring prior to, during and after transplantation, the results were not satisfactory. Specifically, out of the 5 RLDLTa recipients of a left liver, there were 2 urgent re-transplantations due to the so-called “small-for-size syndrome”. Obviously, the numbers are small, but the rate of re-transplantation is unusually high compared to the rate of re-transplantation in RLDLTa with the right liver (40% vs 6.3%, P=.05, RR=9.89 [95% CI: 1.38–70.98]).
In conclusion, the long-term results of living donor liver transplantation are equivalent to those obtained using conventional transplantation, from the standpoint of overall and graft survival. However, this transplantation technique entails a high number of biliary tract complications, which, despite being able to be treated satisfactorily, frequently require surgery for complete resolution.
Authorship- -
Santiago Sánchez Cabús: study design, data collection, analysis and interpretation of the results, article composition, critical review and approval of the final version.
- -
Laia Estalella: data collection, critical review and approval of the final version.
- -
Mihai Pavel: data collection, critical review and approval of the final version.
- -
David Calatayud: critical review and approval of the final version.
- -
Víctor Molina: critical review and approval of the final version.
- -
Joana Ferrer: critical review and approval of the final version.
- -
Constantino Fondevila: critical review and approval of the final version.
- -
Josep Fuster: critical review and approval of the final version.
- -
Juan Carlos García-Valdecasas: study design, critical review and approval of the final version.
The authors have no conflict of interests to declare.
Please cite this article as: Sánchez Cabús S, Estalella L, Pavel M, Calatayud D, Molina V, Ferrer J, et al. Análisis de los resultados a largo plazo del trasplante hepático de donante vivo en adulto. Cir Esp. 2017;95:313–320.